Systemic lupus erythematosus is a multisystemic autoimmune disorder that predominantly affects women in reproductive years. Pregnancy in women with SLE is still considered a high-risk condition although several strategies may improve maternal and fetal outcomes. Preconception counseling is fundamental and should include identification of risk factors for adverse pregnancy outcomes, explanation of potential maternal and obstetric complications and timely planning of pregnancy. Risk stratification must consider end-organ damage, comorbidities, disease activity and autoantibodies profile in order to implement an individual-risk pregnancy monitoring plan by a multidisciplinary team. Hydroxychloroquine and low dose aspirin have shown to lower the risk of disease flares and preeclampsia with a good safety profile, so its use during pregnancy in all SLE patients is recommended. Lupus nephritis and preeclampsia share clinical and laboratory features hindering differentiation between both entities. Novel angiogenic markers and fetal ultrasound findings could be helpful in the differential diagnosis, especially after 20 weeks of gestation. Antiphospholipid antibodies, particularly lupus anticoagulant, are closely associated with obstetric complications. Therapy with low dose aspirin and heparin, according to risk profile, may improve live birth rates. Anti-Ro/La antibodies confer risk for neonatal lupus, and therefore preventive therapy and special fetal surveillance should be instituted.
El lupus eritematoso sistémico es un trastorno autoinmune multisistémico que afecta primordialmente a mujeres en edad reproductiva. El embarazo en mujeres con LES aún se considera una condición de alto riesgo, a pesar de que diversas estrategias pueden mejorar los desenlaces maternos y fetales. La asesoría preconcepción es fundamental, y debe incluir la identificación de factores de riesgo de desenlaces adversos del embarazo, una explicación de las posibles complicaciones maternas y obstétricas, así como la planificación oportuna del embarazo. La estratificación de riesgos debe considerar el daño orgánico terminal, las comorbilidades, la actividad de la enfermedad y el perfil de autoanticuerpos, a fin de llevar a cabo un plan de monitoreo de los riesgos individuales del embarazo por parte de un equipo multidisciplinario. La hidroxicloroquina y la aspirina a bajas dosis han demostrado reducir el riesgo de exacerbaciones de la enfermedad y de preeclampsia, con un buen perfil de seguridad, por lo cual se recomienda su uso en todas las pacientes con LES durante el embarazo. La nefritis lúpica y la preeclampsia comparten características clínicas y de laboratorio, obstaculizando la diferenciación entre las 2 entidades. Nuevos marcadores angiogénicos y hallazgos ecográficos fetales pudieran ser de utilidad para el diagnóstico diferencial, especialmente después de las 20 semanas de gestación. Los anticuerpos antifosfolípidos, en particular el anticoagulante lúpico, tiene una estrecha asociación con las complicaciones obstétricas. El tratamiento con aspirina a bajas dosis y heparina, según el perfil de riesgos, puede mejorar las tasas de nacimientos vivos. Los anticuerpos anti-Ro/La representan un riesgo de lupus neonatal, por lo cual debe instituirse tratamiento preventivo y vigilancia fetal especial.
Systemic lupus erythematosus (SLE) is a chronic multisystemic autoimmune disease, with a remitting and relapsing course. It mainly affects young women of reproductive age, so addressing issues such as pregnancy is an essential part of the comprehensive management of these patients.
Pregnancy represents a critical period in women's life due to profound immunological and hormonal changes that must occur to tolerate the fetus. The interaction of SLE and the immunologic adaptations of pregnancy lead to unique challenges in this setting, as alterations in immune mechanisms can have consequences both for the fetus and for the mother.
Previously, pregnancy in SLE women was discouraged due to concerns of disease flares or adverse pregnancy outcomes (APOs). Nowadays, a better understanding of the relationship between disease and pregnancy has resulted in individual risk-based monitoring and management to achieve successful pregnancy outcomes in SLE patients.
This review will address the relationship between lupus activity and pregnancy and the impact of SLE on pregnancy outcomes. Strategies before, during and after pregnancy to improve its outcomes will be discussed. High risk scenarios during pregnancy in SLE patients including lupus nephritis (LN), presence of anti-Ro/SSA and/or anti-La/SSB antibodies and antiphospholipid (aPL) positivity or SLE-associated antiphospholipid syndrome (APS) deserve specific monitoring and management; hence, they will be reviewed in an individual basis.
MethodologyA non-systematic literature review was conducted searching in MEDLINE and Embase, using the MeSH terms: “Lupus Erythematosus, Systemic” AND “Pregnancy outcomes” AND “Flares” AND “Medications” OR “Systemic lupus erythematosus pregnancy”) OR “Lupus nephritis in pregnancy” OR “Neonatal Systemic Lupus Erythematosus”. The search was restricted to papers published in Spanish or English, from 1990 to 2020.
ResultsInfluence of SLE on pregnancy outcomesDespite diagnostic and therapeutic advances, pregnancies in SLE patients are still considered a high risk condition due to an elevated risk of major obstetric and neonatal complications. A population-based study from 2000 to 2003 found that maternal mortality was 20-fold higher among women with SLE. The risk for serious medical and pregnancy-assocaited complications was also 3 to 7-fold higher for SLE women compared to the general population.1
In recent years, outcomes during pregnancy in patients with SLE have improved as a result of preconceptional counseling, close monitoring during pregnancy and postpartum and multidisciplinary management.2 However, according to a recent meta-analysis, maternal and fetal morbidity is still higher in pregnancies of women with SLE.3 Additionally, it has been estimated that women with SLE have fewer live births compared to the general population.4
Impact of pregnancy on disease: SLE activity and flaresImmunologic adaptations during pregnancy and postpartum can influence maternal autoimmune disease in several ways. Since SLE is considered mainly a Th2-mediated disease, immune pregnancy-related changes could theoretically trigger the onset of the disease or increase the risk of disease flares during this period.5
The risk of SLE flares during pregnancy has been a matter of debate. Most of prospective studies in SLE pregnancies have shown that the risk of disease flare is higher during pregnancy, although there are some discrepancies due to heterogeneity of lupus flare definition and tools used to assess lupus activity.6 Newer studies using validated instruments for disease activity assessment have found a 2–3 fold increase in SLE activity during pregnancy.7,8 The majority of these flares are considered mild to moderate and may include renal, hematological and musculoskeletal systems. Likewise, previous organ involvement seems to predict the same type of condition during pregnancy.
Disease activity at conception and in the previous 6 months, both clinical and serological, is a key predictor, not only for obstetrical complications, but also of SLE flares during pregnancy. Prospective studies of pregnant lupus patients have reported some risk factors for SLE activity during pregnancy: a higher number of flares prior to pregnancy, high SLEDAI index before pregnancy and preconception SLE activity.9,10 In fact, there is around a seven-fold risk of severe lupus flare in patients with active SLE at conception.11 Moreover, SLE disease activity immediately prior to pregnancy also impacts damage accrual after pregnancy.12
On the other hand, SLE activity during or prior to pregnancy is associated with several maternal and fetal complications such as fetal loss, preterm birth, intrauterine growth retardation (IUGR) and hypertensive complications. Therefore, early identification and prompt treatment in pregnant women with lupus activity is essential to improve pregnancy outcomes.10
Strategies to improve pregnancy outcomesBefore pregnancy: preconception counselingPreconception counseling is essential to identify risks factors for APO in women with SLE. This assessment is important for the timely implementation of preventive strategies and to establish a patient-tailored multidisciplinary monitoring plan before and during pregnancy.13
Current recommendations emphasize the importance of preconceptional counseling in women with lupus, although several barriers to family planning counseling have been identified.13,14 Anxiety about managing high-risk pregnancies in SLE women and lack of consensus recommendations regarding medication safety during pregnancy were difficulties expressed by rheumatologist about family planning counseling in a semi-structured interviews study.15 Open and accurate conversations about pregnancy planning and management between the rheumatologist and the SLE female patient in childbearing-age should be encouraged. A strategy consists of a simple single question that directly addresses the issue: do you want to get pregnant in the next year? This one-question based approach could help rheumatologist or physicians taking care of SLE patients to address reproductive desire effectively during consultation.16
Given a very high risk of maternal complications, pregnancy should be discouraged in some clinical scenarios such as moderate to severe SLE activity, stroke in the past 6 months, severe pulmonary arterial hypertension, moderate to severe heart failure (LVEF<40%), end-stage chronic kidney disease, history of early preeclampsia (<28 weeks) and HELLP syndrome despite preventive therapy.2
Risk stratification should be individualized according to several factors including comorbidities, disease activity, disease-related organ damage, and autoantibody profile (aPL, anti-Ro/SSA and anti-La/SSB). In non-primigravida women, the history of adverse outcomes in previous pregnancies is very relevant to determine the likelihood of complications in future pregnancies.
As mentioned above, disease activity at conception and in the previous 6 months is a main predictor for obstetrical complications and SLE flares, so SLE women should conceive during a period of stable or quiescent disease of at least 6 months for maternal safety and optimal pregnancy outcome. If disease is active, pregnancy should be differed and aggressive treatment initiated. Planned pregnancies during stable or low disease activity are associated with better pregnancy outcomes, including higher live-birth rates as compared to unintended pregnancies in SLE women.17,18
Assessing autoantibody status helps determine specific pregnancy risks and establish a monitoring plan for both mother and fetus and need for additional therapy. Every woman with SLE should be evaluated for the presence of aPL antibodies and anti-Ro/SSA and anti-La/SSB prior to, or early in pregnancy, to ascertain the risk of miscarriage and neonatal lupus, respectively.14,19
Besides disease-related risk factors, SLE women are more likely to have other medical conditions like diabetes mellitus, hypertension and thrombophilia, that significantly increase the risk for APO.1 Arterial hypertension results in higher in risk of pregnancy loss (OR 2.4, RR 2.9), preterm birth and IUGR (OR 6.8), so optimal blood pressure control with pregnancy compatible antihypertensives before and through pregnancy is essential.32,33
The preconceptional period is the most appropriate time to assess current SLE medication and, if pregnancy contraindicated drugs are being used, to switch to pregnancy-compatible drugs for disease control in order to minimize the risks for the mother and the fetus. Moreover, pregnancy planning allows for checking disease stability after treatment modifications and ensures adequate washout of teratogenic drugs. Although evidence-based information regarding safety of disease modifying antirheumatic drugs in pregnancy is scarce, rheumatology organizations have conducted their own analysis of medication use during pregnancy and lactation, to facilitate therapeutic decisions as summarized in Table 1.13,14,20
Medications compatible with pregnancy and lactation. Adapted from Refs. 13, 14, 20.
| Medication | Pregnancy | Breastfeeding |
|---|---|---|
| Corticosteroids | Compatible. Optimally less 20mg/dy; potential increased risk of preterm birth and low birth weight at higher doses. | Compatible. Ideally wait 2h after dose to breastfeed. |
| Methotrexate | Contraindicated; teratogenic.Discontinue 6 months before. | Not recommended |
| Leflunomide | Contraindicated. In case of unplanned pregnancy while taking the pedication, administer cholestyramine. | Not recommended |
| Sulfasalazine | Compatible. Folate supplementation needed | Compatible |
| Hydroxychloroquine | Compatible. Reduces risk of SLE flare in pregnancy; may improve pregnancy outcomes in SLE and recurrence of CHB. | Compatible |
| Azathioprinea | Compatible. Crosses the placenta but fetal liver lacks the enzyme to convert to the active metabolite | Compatible |
| Mycophenolate mofetil | Contraindicated. Increased risk of first trimester pregnancy loss and midline malformations | Not recommended |
| Anti-TNF | Compatible. If used during pregnancy, consider discontinuation during third the trimester when placental transfer occurs. | Compatible |
| Cyclophosphamidea | Contraindicated. | Contraindicated |
| Cyclosporine and tacrolimusa | Compatible | Compatible |
| Nonsteroideal antiinflammatory drugs | Risk of miscarriage during the first trimester | Compatible |
| Angiotensin converting enzyme inhibitor | Contraindicated in second and third trimester due to fetal renal effects | Insufficient data |
| Rituximaba | Insufficient data | Safe |
Patients with SLE should be managed by a multidisciplinary team, including a rheumatologist, obstetrician, a maternal–fetal medicine physician and other specialists depending on organ involvement. Close obstetric and rheumatologic monitoring involving baseline and regular clinical, laboratory and obstetric ultrasound evaluations is recomended.14,21 Disease activity assessment by a rheumatologist should be performed at baseline and every 4–6 weeks, according to disease status and risk stratification, to early recognize signs of disease flare or pregnancy complications. At baseline, predictive factors for APOs must be identified. Particular attention to blood pressure, blood count, renal and hepatic function, urinalysis and proteinuria is suggested at follow-up visits. Anti-dsDNA antibodies and complement C3 and C4 should be measured every trimester.14,22
Disease activity assessment and SLE flaresRecognition of disease flares during pregnancy can be challenging due to the physiological changes that occur which can overlap with clinical and laboratory features of active SLE.9 Thus clinical data and laboratory findings in pregnant patients with SLE should be interpreted with caution. Thrombocytopenia, mild anemia and increased erythrocyte sedimentation rate often occur during normal pregnancy. Complement levels are less reliable to identify or support the suspicion of disease activity due to its physiological increase during pregnancy, although a decrease ≥25% in C3 and C4 levels relative to baseline and increase in anti-dsDNA antibodies may be useful to differentiate complications such as preeclampsia and SLE activity.23
Modified pregnancy-scores have been suggested to measure disease activity during pregnancy, taking into account physiological gestational changes and morbidities that can mimic SLE.24–27 In clinical practice, these tools are not used routinely by rheumatologists; in contrast, indicators such as new organ involvement, an increase in known disease manifestations, or switching the immunosuppressive medication, are considered suggestive of SLE flare.19
The primary goal of managing SLE patients during pregnancy is to maintain disease remission and treating disease flares to minimize the effects of maternal disease on pregnancy outcomes without harming the fetus. However, even when lupus activity is under control, unfavorable perinatal outcomes can still occur.28
Disease flares are managed with non-fluorinated glucocorticoids which are inactivated by placental 11β-hydroxysteroid dehydrogenase thus limiting fetal exposure.29 In case of severe activity, methylprednisolone pulses can be administered. Although glucocorticoids are considered safe in pregnancy, preterm births and orofacial clefts have been reported in pregnancies exposed to prednisone-equivalent doses >20mg/day; tapering to lower doses if possible is recommended.14,30,31 Early introduction or increasing dose of pregnancy-compatible immunosuppressive agents such as azathioprine and tacrolimus is a strategy to control disease activity and avoid exposure to high-dose steroids. Methotrexate, leflunomide and mycophenolic acid should be avoided due to their known or potential teratogenicity. Cyclophosphamide is associated with high risk or fetal loss, so it should be avoided during the first trimester and reserved only for life-threatening diseases during the second or third trimester.13,14 Rituximab has not been associated to any specific fetus malformations in mothers exposed preconceptionally or early in pregnancy, although its use in late pregnancy increases the risk for B cells depletion in neonates exposed in utero.34 Limited data is available on the safety of belimumab during pregnancy.20
Hydroxychloroquine for all SLE pregnant womenHydroxychloroquine is an antimalarial widely used in pregnancy with a good safety profile. No malformations, growth restriction or ocular toxicity have been reported in in-utero exposed fetus so far.35 Recent studies have shown that the use of hydroxychloroquine (HCQ) is beneficial for both mother and neonate; the recommendation is that all women should start or continue using hydroxychloroquine throughout pregnancy.13 A lower average dose of prednisone and reduced risk of flares throughout pregnancy has been observed in SLE pregnant women taking HCQ.36 Discontinuation of HCQ has been associated with a higher level of lupus activity and increased flare rates during pregnancy.37
Besides flare prevention, a beneficial effect over preterm delivery and IUGR has been reported in SLE pregnancies exposed to HCQ.38 Furthermore, a retrospective single center study including 151 pregnancies reported lower rates of preeclampsia among SLE pregnancies receiving HCQ therapy compared to the non-treatment group (7.5 vs 19.7%, p=0.032). Additionally, HCQ crosses the placenta and hence provides additional benefits by preventing specific neonatal complications such as congenital heart block.
However, HCQ serum concentrations vary widely each trimester due to physiological changes in pregnancy and this variation may impact pregnancy outcomes. A recent observational study that examined the levels of HCQ in 50 pregnant patients with autoimmune diseases showed that women with average HCQ levels of 100 ng/ml or less delivered prematurely more frequently (83% vs 21%, p=0.01).39
Low dose aspirin (LDA) and preeclampsia riskPreeclampsia occurs in 2–8% of pregnancies in the general population. Lupus nephritis, SLE and aPL/APS are risk factors for preeclampsia, with a 14% increased risk as compared to healthy women.1,40 A meta-analysis of randomized controlled trials showed that LDA prior to 16 weeks of gestation was associated with a major reduction in the risk of preterm preeclampsia (RR 0.11, CI 0.04–0.33) among high-risk women.41 In a subsequent meta-analysis including pregnancies with abnormal uterine artery Doppler flow velocimetry, the administration of LDA before 16 weeks of gestation resulted in a lower risk for preeclampsia (RR 0.6, CI 0.27–0.83) and for severe preeclampsia (RR 0.3, CI 0.11–0.69).42 Based on this evidence, early initiation of LDA (81–100mg daily) is recommended for women with an absolute risk for preeclampsia>8%; LDA use should be encouraged in all SLE and/or APS pregnancies as an effective therapy to prevent preeclampsia.43 LDA seems to be safe for both mother and fetus, as no significant risk of maternal or fetal bleeding and no association with premature ductus arteriosus closure has been observed.43,44 Despite its potential benefits and safety, LDA is underused in SLE pregnancies.45
Fetal monitoringThe use of obstetric ultrasound at specific intervals is important for assessing fetal anatomy and growth, amniotic fluid and placental flow. Doppler ultrasonographic assessment of the umbilical and uterine arteries early in the second trimester (20–24 weeks of gestation) is helpful for screening of placental insufficiency problems such as IUGR and preeclampsia.46 Uterine artery pulsatility during this period is a sensitive and specific test for preeclampsia and small-for-gestational age in SLE women.47,48 Umbilical Doppler ultrasound is more accurate to assess placental function, showing various levels of impairment such as absent or even reverse diastolic flow or increased resistance.22 The frequency of fetal surveillance using Doppler ultrasound and biometrics over the third trimester must be tailored according to the fetal status to determine adequate time to delivery and reduce perinatal deaths.13,49
After pregnancy: postpartum surveillance, lactation and contraceptionPuerperium is considered a period of high risk for lupus flares. An increased rate of flares in the initial 3 months postpartum compared to non-pregnant patients (HR 1.48; CI 1.07–1.95) was recently described in a retrospective analysis of 398 SLE pregnancies of Hopkins Lupus Cohort. Hydroxycloroquine therapy mitigated the risk of flares during pregnancy and postpartum to similar rates as non-pregnant SLE patients.37 Similar observations at 6 weeks postpartum have been previously reported by Ruiz-Irastorza.50 A higher disease activity at 6 and 12 months postpartum compared to third trimester and 6 weeks postpartum was reported in a prospective cohort of 145 pregnancies, highlighting the importance of postpartum surveillance.51
Therefore, rheumatology follow-up and continuation of HCQ therapy during postpartum is advised. Rheumatologist should ensure medication compatibility with breastfeeding and encourage treatment compliance to control the disease. Contraception should be discussed late in pregnancy and/or postpartum with every patient. Highly-effective contraceptive methods must be preferred to reduce the risk of unplanned pregnancies. Specific contraceptive measures should be adopted based on disease activity and thrombotic risk.13,14
Special high risk scenariosLupus nephritis and pregnancyLupus nephritis (LN) is among the conditions that most often result in increased morbidity and mortality during pregnancy. LN may have an adverse impact on pregnancy and pregnancy itself may increase the risk of renal flare. During pregnancy, 26% of SLE women experience a lupus flare and 16% a renal flare.52 Active renal disease at conception is the most important predictor for renal flare, although the risk for LN persists in women with inactive disease within 1 year prior to conception.53 Moreover, at least half of the women with LN will develop chronic kidney disease over the next 10 years.54
Perinatal outcomes in lupus nephritisA higher incidence of maternal complications and preterm delivery in SLE women with lupus nephritis has been reported, as compared to patients with no history of renal involvement.55 However, recent data have shown that the risk is related to LN activity at onset of pregnancy, not to lupus nephritis per se. A prospective cohort study of 119 lupus pregnancies reported a higher rate of maternal complications, specifically renal flares, in patients with a history of lupus nephritis (50% vs 27.7%, p=0.015), but no differences were seen after excluding patients with renal flares during the 6 months preceding pregnancy.56 Wagner found that active LN at the beginning of conception is a high risk factor for maternal complications such as preeclampsia, eclampsia and HELLP syndrome. For the baby, the most common complications included miscarriage, small for gestational age, IUGR, preterm delivery and stillbirth.55 A prospective multicenter study including 71 pregnancies (78.9% with complete renal remission before pregnancy) did not find an increased risk of renal flares during pregnancy in patients with stable lupus nephritis who received prepregnancy counseling.57 Thus, active, but not quiescent, LN is the main risk factor for poor maternal–fetal outcomes. Prepregancy counseling is essential to advise SLE patients to become pregnant, as long as the LN is inactive and receiving pregnancy compatible treatment.
Importantly, lupus nephritis is a risk factor for pregnancy hypertensive complications, so preconceptional counseling guided by an experienced multidisciplinary team is advised.58
Active lupus nephritis and/or preeclampsia: differential diagnosisPreeclampsia is a pregnancy specific syndrome characterized by hypertension and proteinuria, with onset in the second half of pregnancy. Dysfunctional angiogenesis leading to impaired placental development is implicated in the pathogenesis of preeclampsia, supporting a central role of the placenta in preeclampsia development. A meta-analysis of lupus pregnancies showed a preeclampsia rate of 7.8%, but other studies suggest that it can be twice as high, particularly in women with nephritis.52,59
Diagnosis of LN during pregnancy can be difficult because it shares overlapping features with pre-eclampsia including hypertension, proteinuria, thrombocytopenia and renal impairment.60 Accurate diagnosis is critical as management differs significantly between both entities; while LN requires immunosuppressive treatment, in severe preeclampsia delivery may be indicated.
Prior to 20 weeks of gestation, SLE women who present with hypertension and increased proteinuria, PE is an unlikely diagnosis and LN should be strongly considered. However, after 20 weeks of gestation the distinction between preeclampsia and LN can be a difficult task for rheumatologists and nephrologists. Clinical and biochemical markers such as high blood pressure, increased uric acid and elevated liver enzymes favor preeclampsia diagnosis while hypocomplementemia, increased anti-dsDNA titer, hematuria, active urinary sediment, and the presence of extra-renal SLE symptoms suggests active LN.61 A clinical setting in which hypertension dominates, severe proteinuria without hematuria may suggest preeclampsia (see Fig. 1).62
Algorithm for differential diagnosis between lupus nephritis flare and preeclampsia before 20 weeks. New onset or worsening of proteinuria and hypertensión before 20 weeks will almost probably represent a flare of lupus nefritis. *Angiogenic and antiangiogenic factors could be helpful to differentiate between a flare vs preeclampsia.
From the middle of the second to third trimester, new onset or worsening proteinuria, hypertension and impaired kidney function may be due to 3 possibilities: preeclampsia, LN flare with superimposed preeclampsia or only a flare of LN (see Fig. 2).
Algorithm for differential diagnosis between lupus nephritis flare and preeclampsia after 26 weeks. New or worsening proteinuria, hypertension and impaired renal function occurring between 26 and 40 weeks requires considering 3 options: 1. Altered ratio of low placental growth factor and hight sFlt1 (>1872pg/ml) and PIGF (<70.3pg/ml) predict the onset of preeclampsia. 2. Active urinary sediment plus altered angiogenic factors predicts the presence of both preeclampsia and lupus nephritis flare. 3. Active urinary sediment with hematuria and lower complement levels compared to baseline suggests lupus nephritis flare. IUGR: intrauterine growth restriction; SGA: small for gestational age; sFLT-1: soluble fms-like tyrosine kinase-1; PlGF: placental growth factor. *Always correlate with fetal ultrasound findings.
Novel angiogenic markers like soluble tyrosine kinase-like factor (s-FLT-1), soluble endoglin, and placental growth factor (PlGF) can be helpful in the differential diagnosis. In a longitudinal observational study of 276 pregnant women with chronic hypertension and chronic kidney disease, lower maternal PlGF concentrations after 22 weeks of gestation were found to have a high diagnostic accuracy for superimposed PE.63
The PROMISSE study assessed the usefulness of circulating angiogenic factors for predicting APO (PE<34 weeks, fetal/neonatal death and preterm delivery<30 weeks) in 492 pregnant women with aPL and/or stable SLE. At 12–15 weeks of gestation, the strongest predictor of severe APOs was sFLT-1 levels (OR 12.3, 95% CI 3.5–84.8), while sFlt-1/PlGF ratio at 16–19 weeks was most predictive of severe APO (OR 31.3, 95% CI 8.0–121.9). The highest risk was for women with both PlGF in the lowest quartile and sFLT1 in the highest quartile (OR 31.1, 95% CI 8.0–121.9; PPV 58%; NPV 95%).64 A subsequent study confirmed the value of the sFLT-1/PlGF ratio to predict preeclampsia and IUGR in 44 SLE pregnancies.65
Likewise, a higher sFLT-1/PIGF ratio during the third trimester has been reported in women with preeclampsia versus patients with chronic kidney disease.66 Therefore, measuring the sFlt-1/PIGF ratio may be clinically useful to rule out preeclampsia not just in new-onset LN but also other forms of glomerulonephritis with hypertension.
Doppler ultrasound findings can also be helpful in making a differential diagnosis between PE and SLE flares. In a prospective cohort study, mean pulsatility index of uterine arteries at 32–34 weeks was higher in patients with PE and/or IUGR compared to LN flares.65
In general, the diagnosis of preeclampsia is clinical. However, kidney biopsy should be considered when LN or other primary glomerular disease are suspected. Although, kidney biopsy during pregnancy is controversial, it should be done in cases where treatment decisions may be dictated by histopathological findings, especially in presumptive LN. In a case series including 11 pregnant women who underwent kidney biopsy at 16 weeks for LN flare suspicion, the renal biopsy findings changed their management in all but one patient, with no apparent complications for the mother or the fetus.67 These observations highlight the potential impact of renal biopsy on therapeutic decisions in pregnant women with LN.
During the first trimester, kidney biopsy is considered low risk as the frequency of complications is similar to non-pregnant women. The highest risk is seen at 20–32 weeks because any intervention could trigger preterm labor.
Management of lupus nephritis in pregnancyManagement and monitoring of pregnancy in SLE women with active LN and preeclampsia represents a challenge even for the most trained medical team. Rheumatologists and nephrologists should work together to manage these patients in order to improve pregnancy outcomes.
Depending on the gestational age at which LN occurs, 3 possible scenarios should be considered: kidney biopsy-guided treatment, initiation of empirical management to prolong pregnancy, or termination of pregnancy. Decision should be aimed at reducing morbidity and mortality of the mother-baby dyad.
Since 30–50% of pregnancies are unintended, an important question is how to manage pregnancies in women inadvertently exposed to teratogenic drugs. Some patients choose immediate termination, while others decide to continue with the pregnancy. The date of exposure must be defined for adequate risk assessment.
Treating pregnant women with LN is challenging as the well-being of two individuals must be considered. Potential harm to the fetus must always be weighed against the risk of treatment discontinuation and the potential to favor the development of flares.
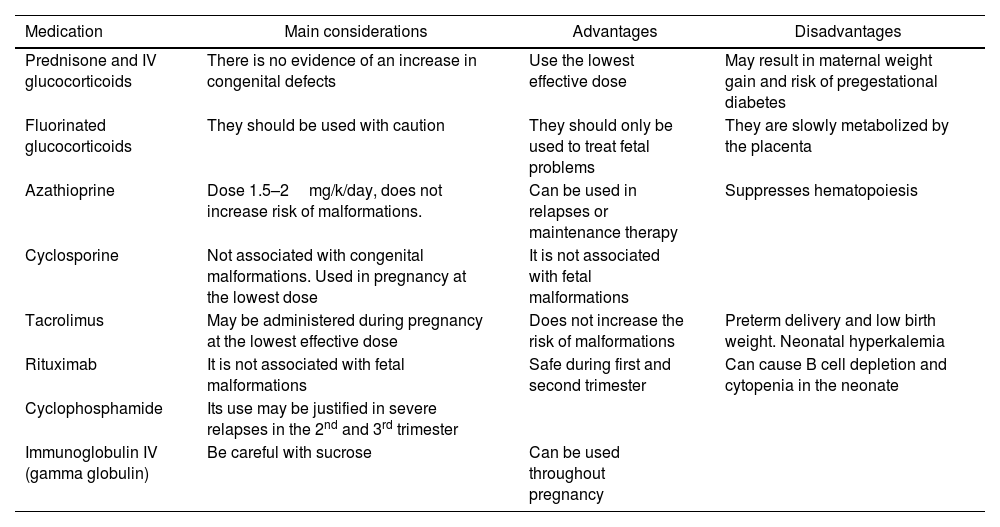
The list of medications used to treat LN is long, but the information on their use during pregnancy is limited. Table 2 shows the medications commonly used for LN treatment during pregnancy.
Immunosuppressive drugs for the treatment of lupus nephritis during pregnancy.13,14,68,69,70
| Medication | Main considerations | Advantages | Disadvantages |
|---|---|---|---|
| Prednisone and IV glucocorticoids | There is no evidence of an increase in congenital defects | Use the lowest effective dose | May result in maternal weight gain and risk of pregestational diabetes |
| Fluorinated glucocorticoids | They should be used with caution | They should only be used to treat fetal problems | They are slowly metabolized by the placenta |
| Azathioprine | Dose 1.5–2mg/k/day, does not increase risk of malformations. | Can be used in relapses or maintenance therapy | Suppresses hematopoiesis |
| Cyclosporine | Not associated with congenital malformations. Used in pregnancy at the lowest dose | It is not associated with fetal malformations | |
| Tacrolimus | May be administered during pregnancy at the lowest effective dose | Does not increase the risk of malformations | Preterm delivery and low birth weight. Neonatal hyperkalemia |
| Rituximab | It is not associated with fetal malformations | Safe during first and second trimester | Can cause B cell depletion and cytopenia in the neonate |
| Cyclophosphamide | Its use may be justified in severe relapses in the 2nd and 3rd trimester | ||
| Immunoglobulin IV (gamma globulin) | Be careful with sucrose | Can be used throughout pregnancy |
Antiphospholipid syndrome is one of the major contributors to pregnancy loss in SLE women; it manifests as recurrent miscarriages, fetal demise or stillbirth.59 In addition, APS predisposes pregnant women to late gestational complications associated with placental insufficiency, such as PE and IUGR. Serious complications have been reported in up to 12% of pregnancies in lupus patients. Interestingly, adverse outcomes in pregnancies of SLE women with aPL antibodies may occur even during remission or mild activity of the disease.71
Antiphospholipid antibodies target the placenta by binding β2 glycoprotein I (β2GPI) constitutively expressed on trophoblast cell surface, disrupting the secretion of trophoblast angiogenic factors early in gestation and impairing placental development favoring adverse outcomes.72
The prevalence of aPL antibodies in SLE is variable and depends on the type and isotype of antibodies. A prevalence of 12–44% of anticardiolipin antibodies (aCL), 15–34% for lupus anticoagulant (LA) and 10–19% for anti-β2glycoprotein I (aβ2GPI) have been reported.73 A higher frequency of thrombosis and pregnancy loss in SLE-associated APS than in primary APS has been described. Moreover, the Hopkins Lupus cohort diagnosis of SLE-associated APS reported a 3-fold increase in miscarriages especially after 20 weeks, and was an independent risk factor for further pregnancy losses.74
The association of aPL with APOs differs among the various aPL antibodies. Specific serological profiles have been defined as high risk due to a stronger association with APOs. Lupus anticoagulant has been identified as the primary predictor of APOs and triple positivity for all three antibodies confers a specially high risk for thrombosis and pregnancy complications.75 In the PROMISSE study, a large-scale multicenter prospective study of pregnant women with aPL and/or underlying stable SLE, a higher rate of APOs in patients with aPL (43.8%) compared to 15.4% of patients without aPL was observed, while poor pregnancy outcomes were mainly associated with LA positive patients. The presence of LA was identified as a baseline independent predictor of APOs (OR 8.32) while no other aPL antibody independently predicted APO.76 The EUROAPS registry also reported that LA, isolated or in combination with aCL and/or aβ2GPI was the strongest marker for poor obstetric outcomes.77
The treatment of pregnant patients with aPL depends on the risk profile and history of adverse obstetric events or thrombosis. In women with obstetric aPS, combination therapy with LDA and prophylactic-dose heparin is recommended. In case of previous thrombosis, therapeutic-dose heparin in addition to LDA must be administered during pregnancy as vitamin K antagonists are teratogenic.13,14
Despite optimal standard treatment, 15–20% of pregnancies in aPL positive women result in fetal demise.78 Adding hydroxychloroquine to the standard treatment has been recently suggested in obstetric aPS, based on evidence that HCQ seems to dampen the deleterious effects of aPL on the trophoblast.79,80 Two currently ongoing randomized controlled trials will assess the HCQ effect in pregnancies of women with aPL/APS.78,81
Antibodies anti-SSA/Ro and anti-SSB/La and neonatal lupusPregnancies exposed to anti-SSA/Ro and anti-SSB/La have an increased risk of developing neonatal lupus, a passively acquired autoimmune disease mediated by maternal antibodies. Manifestations include cutaneous involvement, abnormal liver function tests and cytopenia, which usually resolve between 6 and 8 months of life. Autoimmune congenital heart block (CHB) is the most severe form of neonatal lupus, with a mortality rate of 18% and need for a pacemaker in 70% of survivors.82
Neonatal lupus is a consequence of active transfer of maternal antibodies to the fetus via the placental FcRn receptor, starting at 11 weeks of gestation.83 Among patients with anti-SSA/Ro antibodies, the risk of having a child with CHB is roughly 1–2%. However, in mothers with a prior child with neonatal lupus or CHB the risk increases to 19%.84 Higher titers of anti-Ro antibodies in mothers of CHB-affected children have been reported, as compared to those with unaffected children.85
The exact mechanism by which anti-Ro/La autoantibodies cause cardiac injury is unclear. One hypothesis is that intracellular Ro/La antigens translocate to the cardiomyocytes surface, undergoing normal physiological remodeling, allowing these antigens to be bound by circulating autoantibodies and trigger subsequent proinflammatory and fibrotic responses. Immune complex formation on phagocytic cardiocytes may impair their clearance by healthy cardiocytes, hindering a function critical to normal fetal heart development.86 HLA-related genetic alterations in the fetus have also been found.
Congenital heart block is predominantly diagnosed during pregnancy, and typically within a specific timeframe. Isolated cases have been reported as early as 16 weeks, although 75% of the cases are diagnosed between 20 and 29 weeks.83 Congenital heart block is usually preceded by lower degrees of conduction delays that can be reversed with early treatment. Close monitoring of anti-SSA/Ro and/or anti-SSB/La positive pregnant women with serial fetal echocardiography between 16 and 26 weeks of gestation is recommended.13,14,87 Detection of an early conduction defect such as a prolonged PR interval should be considered a danger signal.
Different therapeutic strategies have been evaluated for CHB. Fluorinated steroids such as dexamethasone cross the placenta and may have the potential to mitigate inflammation in autoimmune-CHB affected children, but there is conflicting data regarding its efficacy for either treatment or prophylaxis. To date, no evidence supports that dexamethasone improves mortality and morbidity or prevents heart block progression; therefore the decision to use this therapy must be weighed against the potential risk of maternal and fetal toxicity.86,88,89
Preventive therapy of anti-SSA/Ro and/or anti-La/SSB positive pregnant women is under investigation. Hydroxychloroquine administration during pregnancy has been associated with a decrease of recurrent neonatal lupus in retrospective studies.90 Recently, a multicenter open-label single-arm phase 2 clinical trial showed a >50% reduction in CHB recurrence in mothers who received HCQ 400mg/day starting before 10 weeks of gestation, confirming its role in preventing CHB in high risk patients.91
Fig. 3 summarizes an algorithm for pregnancy approach in patients with SLE.
Approach for pregnancy in women with SLE. Strategic approach before, during and after pregnancy in SLE women. Data adapted from Refs. 13, 14. APO: adverse pregnancy outcomes; aPL: antiphospholipid; APS: antiphospholipid syndrome; HCQ: hydroxychloroquine; LMWH: low molecular weight heparin; LDA: low dose aspirin.
Pregnancy and SLE are closely related as active disease is associated with increased risk of APO and pregnancy-related changes may impact on maternal disease by triggering disease flares. Pregnancy outcomes may be improved by planning conception during stable disease and while on pregnancy-compatible medications.
Besides disease activity, the presence of aPL and anti-SSA/Ro antibodies can adversely influence pregnancy, increasing the risk of maternal and fetal complications such as pregnancy loss, late gestational complications and neonatal lupus; therefore aPL and anti-SSA/Ro antibodies should ideally be identified prior to pregnancy to implement a preventive strategy and close fetal and maternal surveillance.
Hydroxychloroquine administration during pregnancy is an important strategy to reduce the risk of maternal disease flares and prevent recurrent congenital heart block. Recent research has also shown a potential beneficial effect of adding hydroxychloroquine to standard treatment in women with aPL/aPS. Ongoing clinical trials will probably shed some light in this regard. Given the higher risk of preeclampsia in SLE pregnancies, initiation of LDA before 16 weeks is recommended.
Pregnancy in SLE patients with LN represents a major challenge for both nephrologists and rheumatologists due to a higher risk of adverse perinatal outcomes and hypertension-associated complications of pregnancy. Active LN may be clinically indistinguishable from pre-eclampsia, especially after 20 weeks; however, novel tools such as the sFLT1/PlGF ratio and the mean pulsatility index of the uterine arteries are useful in making this distinction.
Maternal and fetal monitoring during pregnancy by an experienced multidisciplinary team should be the standard-of-care in pregnant women with SLE.
Carefully monitoring in SLE patients during pregnancy by a multidisciplinary team is the key to prevent maternal and fetal complications.
Potential risks to the fetus must always be weighed against the benefits of disease control when making treatment decisions in pregnant patients with SLE.
Contrary to old beliefs, in patients with inactive or stable disease, pregnancy is safer for both the mother and the baby, with good outcomes in around 80% of patients.
Additional biomarkers should be evaluated to identify high-risk patients.
The authors have no conflict of interest to disclose.