A renal biopsy is the ‘gold standard’ for diagnosis and classification of lupus nephritis (LN). The role of repeat renal biopsy in lupus nephritis (LN) to guide treatment or predict prognosis has been controversial. A systematic literature review was conducted based on retrospective and prospective studies. The studies were identified using English electronic scientific databases, including MEDLINE PUBMED, published between January 1990 and August 2020. The eligibility criteria were studies including adult LN patients with at least one follow-up renal biopsy with appropriate longitudinal information. Case reports, studies with incomplete information or including duplicate patients were excluded. Based on the inclusion and exclusion criteria, a total of 73 publications were identified. This study included a total of 1167 repeat biopsies in LN patients from 15 studies. The primary indication for a repeat biopsy was relapse in 44-78% of the cases, and lack of response in 13-51%. Additionally, several repeat biopsies were done according to the protocol, after induction and maintenance therapy. In terms of histopathological class switches, there was a higher frequency of changes from nonproliferative to proliferative lesions. Only two studies provide a definition of histological response. There were often changes in the therapeutic approach after a repeat biopsy. Repeat kidney biopsies are helpful in patients with LN flare/relapse, and in patients with poor treatment response. Histological transformation was a common finding. The histologic and clinical responses are discordant. A repeat biopsy could be of prognostic value for therapeutic decision-making.
La biopsia renal es el «estándar de oro» para el diagnóstico y la clasificación de la nefritis lúpica (NL). El papel de la biopsia renal repetida en nefritis lúpica para orientar el tratamiento o predecir el pronóstico ha sido controversial. Se llevó a cabo una revisión sistemática de la literatura basada en estudios retrospectivos y prospectivos. Los estudios se identificaron a través de bases de datos científicas electrónicas en inglés, incluyendo Medline PubMed, de publicaciones entre enero de 1990 y agosto del 2020. Los criterios de elegibilidad fueron estudios que incluyeran a pacientes adultos con NL, quienes tuvieran al menos una biopsia renal de seguimiento, con información longitudinal apropiada. Se excluyeron informes de casos, estudios con información incompleta o con pacientes duplicados. Basándose en los criterios de inclusión y exclusión, se identificaron 73 publicaciones. En la presente revisión se analizaron un total de 1.167 biopsias repetidas en pacientes con NL en 15 estudios. Las principales indicaciones para la biopsia repetida fueron: recidiva en 44-78% de los casos, y falta de respuesta en 13-51%. Adicionalmente, varias biopsias repetidas se hicieron conforme al protocolo, luego de la terapia de inducción y de mantenimiento. Con respecto a los cambios de clase histopatológica, hubo una mayor frecuencia de cambios de lesiones no proliferativas a lesiones proliferativas. Solamente dos estudios ofrecen una definición de respuesta histológica. Con frecuencia hubo cambios en el abordaje terapéutico después de realizar la biopsia repetida. Las biopsias renales repetidas son útiles en pacientes con exacerbación/recidiva y en pacientes con falta de respuesta a tratamiento. La transformación histológica fue un hallazgo frecuente; las respuestas histológicas y clínicas son discordantes. Una biopsia repetida puede ser de valor pronóstico para la toma de decisiones terapéuticas.
Treatment response in patients with lupus nephritis (LN) is primarily assessed according to specific clinical metrics.1 Renal biopsy is highly recommended for all subjects with suspected LN, since the biopsy allows the clinician to recognize and classify the type of renal involvement, assess its activity, and thus guide the intensity of treatment.2,3
Currently, the most common indication for a repeat biopsy has been to assess response one or more years after the initial diagnostic biopsy, and usually due to adverse outcomes, such as deteriorating kidney function, worsening of proteinuria, suspicion of a renal flare or suspicion of an LN class change. Some reports suggest that routinely repeating a kidney biopsy after induction therapy could be helpful to decide the next steps in LN treatment. Other authors have observed that after induction therapy, histologic and clinical responses are discordant.1
The usefulness of repeat kidney biopsy is still controversial; there are questions about how it impacts patient management and the risk of potential complications, mainly related to bleeding. Considering the risk-benefit ratio, some authors are reluctant to do repeat biopsies since there is no clear evidence regarding which patients undergoing a second biopsy will have clear therapeutic consequences that justify the risk.2
Moreover, arguments have been raised that repeat biopsies should be a standard procedure to define response after immunosuppressive treatment, thereby identifying patients who may need prolonged or intensified therapy, but also to avoid overtreatment.4 However, the value of repeat kidney biopsy as a tool for monitoring LN is still controversial. Although renal flares and conversion from one class of LN to another are common, the need for a repeat biopsy is not yet clearly established.
The purpose of this article was to assess adult LN patients, who had at least one follow-up renal biopsy and compare it against the reference kidney biopsy, identifying the indications of repeat kidney biopsy, the histopathological transitions observed, the definition of histologic remission, and any therapeutic decisions after a repeat biopsy to establish its clinical value.
MethodsInclusion Criteria- -
Population: Patients 18 years and older with Systemic Lupus Erythematosus (SLE).
- -
Intervention: A reference biopsy and at least one repeat kidney biopsy. At least 2 of 4 findings: indication for repeat kidney biopsy, histopathological transition, definition of histologic remission, and therapeutic decision after repeat biopsy.
- -
Language: English.
- -
Publication dates: From January 1990 to August 2020.
- -
Study design: observational, descriptive and clinical trials.
- -
Study design: case reports.
- -
Articles without access to full text.
- -
Duplicate patients.
The study was designed based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines.5 A bibliographic research was carried out from 1990 to august 2020, to find studies that met the inclusion criteria for MEDLINE PUBMED.
The Medline research was conducted via PubMed using the MeSH terms: repeat biopsy, lupus nephritis, reference biopsy, immunosuppressive treatment, histopathological response. Only articles published in English language were included.
Study selectionAll studies were independently assessed by three reviewers (GAGB, SMSC and MDQ) who followed the inclusion and exclusion criteria before their consensus meeting. The information was organized using Microsoft Excel (Version 16.45).
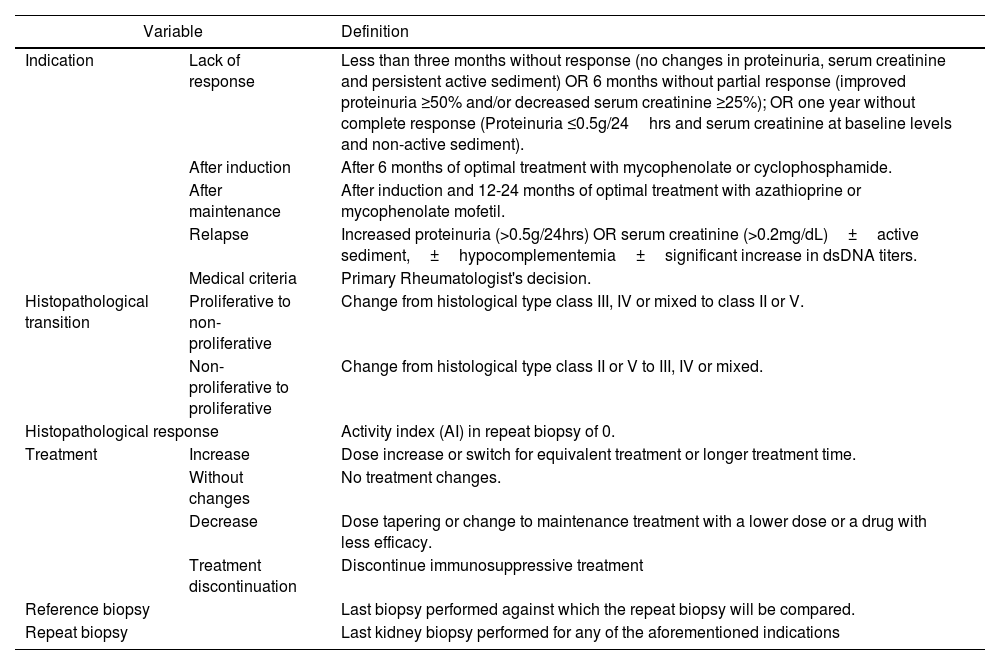
The information collected from the selected studies included: study characteristics (authors, study design), number of patients with lupus nephritis, and renal histology categorized according to the most recent classification of the International Society of Nephrology/ Renal Pathology Society (ISN/RPS). Table 1 summarizes the variables considered in this study.
Definition of Study variables.
| Variable | Definition | |
|---|---|---|
| Indication | Lack of response | Less than three months without response (no changes in proteinuria, serum creatinine and persistent active sediment) OR 6 months without partial response (improved proteinuria ≥50% and/or decreased serum creatinine ≥25%); OR one year without complete response (Proteinuria ≤0.5g/24hrs and serum creatinine at baseline levels and non-active sediment). |
| After induction | After 6 months of optimal treatment with mycophenolate or cyclophosphamide. | |
| After maintenance | After induction and 12-24 months of optimal treatment with azathioprine or mycophenolate mofetil. | |
| Relapse | Increased proteinuria (>0.5g/24hrs) OR serum creatinine (>0.2mg/dL)±active sediment,±hypocomplementemia±significant increase in dsDNA titers. | |
| Medical criteria | Primary Rheumatologist's decision. | |
| Histopathological transition | Proliferative to non-proliferative | Change from histological type class III, IV or mixed to class II or V. |
| Non-proliferative to proliferative | Change from histological type class II or V to III, IV or mixed. | |
| Histopathological response | Activity index (AI) in repeat biopsy of 0. | |
| Treatment | Increase | Dose increase or switch for equivalent treatment or longer treatment time. |
| Without changes | No treatment changes. | |
| Decrease | Dose tapering or change to maintenance treatment with a lower dose or a drug with less efficacy. | |
| Treatment discontinuation | Discontinue immunosuppressive treatment | |
| Reference biopsy | Last biopsy performed against which the repeat biopsy will be compared. | |
| Repeat biopsy | Last kidney biopsy performed for any of the aforementioned indications | |
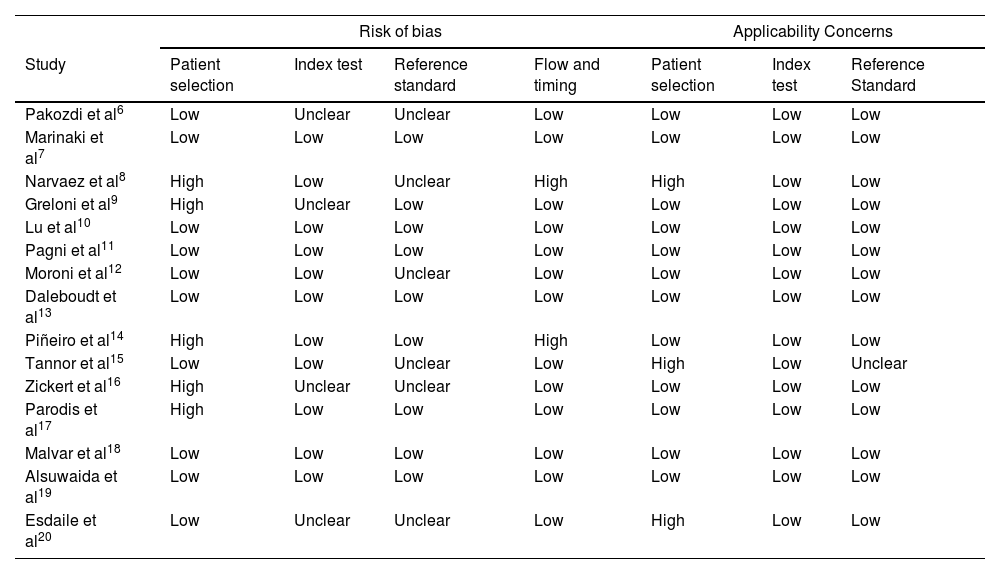
The QUADAS-2 tool was used to assesses the risk of bias of all the articles included. The assessment was conducted by two authors (GAGB and MDQ), who established the risk of bias by mutual agreement (Table 2).
Risk of bias based on QUADAS-2.
| Risk of bias | Applicability Concerns | ||||||
|---|---|---|---|---|---|---|---|
| Study | Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference Standard |
| Pakozdi et al6 | Low | Unclear | Unclear | Low | Low | Low | Low |
| Marinaki et al7 | Low | Low | Low | Low | Low | Low | Low |
| Narvaez et al8 | High | Low | Unclear | High | High | Low | Low |
| Greloni et al9 | High | Unclear | Low | Low | Low | Low | Low |
| Lu et al10 | Low | Low | Low | Low | Low | Low | Low |
| Pagni et al11 | Low | Low | Low | Low | Low | Low | Low |
| Moroni et al12 | Low | Low | Unclear | Low | Low | Low | Low |
| Daleboudt et al13 | Low | Low | Low | Low | Low | Low | Low |
| Piñeiro et al14 | High | Low | Low | High | Low | Low | Low |
| Tannor et al15 | Low | Low | Unclear | Low | High | Low | Unclear |
| Zickert et al16 | High | Unclear | Unclear | Low | Low | Low | Low |
| Parodis et al17 | High | Low | Low | Low | Low | Low | Low |
| Malvar et al18 | Low | Low | Low | Low | Low | Low | Low |
| Alsuwaida et al19 | Low | Low | Low | Low | Low | Low | Low |
| Esdaile et al20 | Low | Unclear | Unclear | Low | High | Low | Low |
Consideration was given to developing a meta-analysis; however, due to the homogeneity of the outcomes and the limited amount of evidence available from the studies included in this systematic review, this option was ruled out.
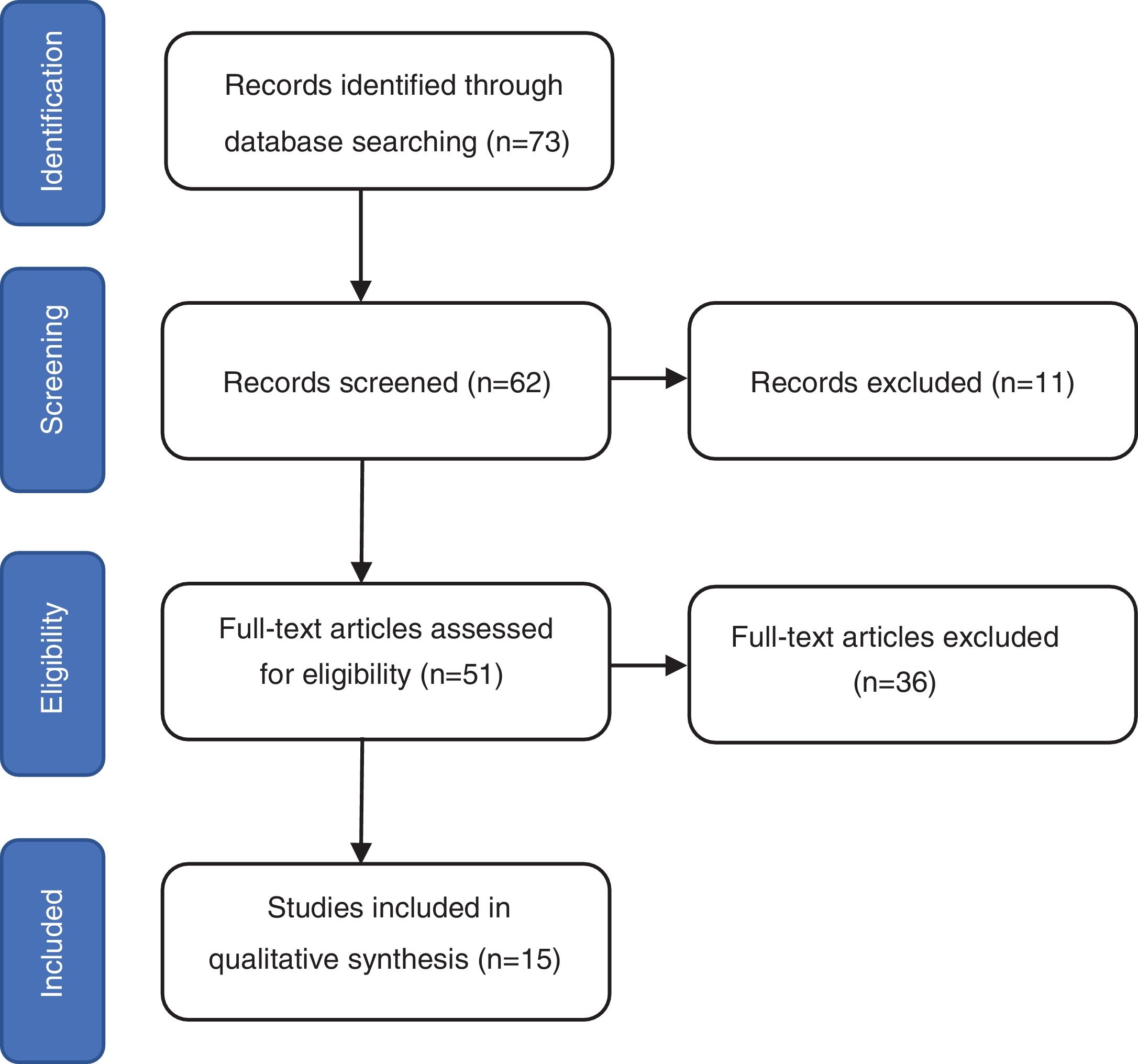
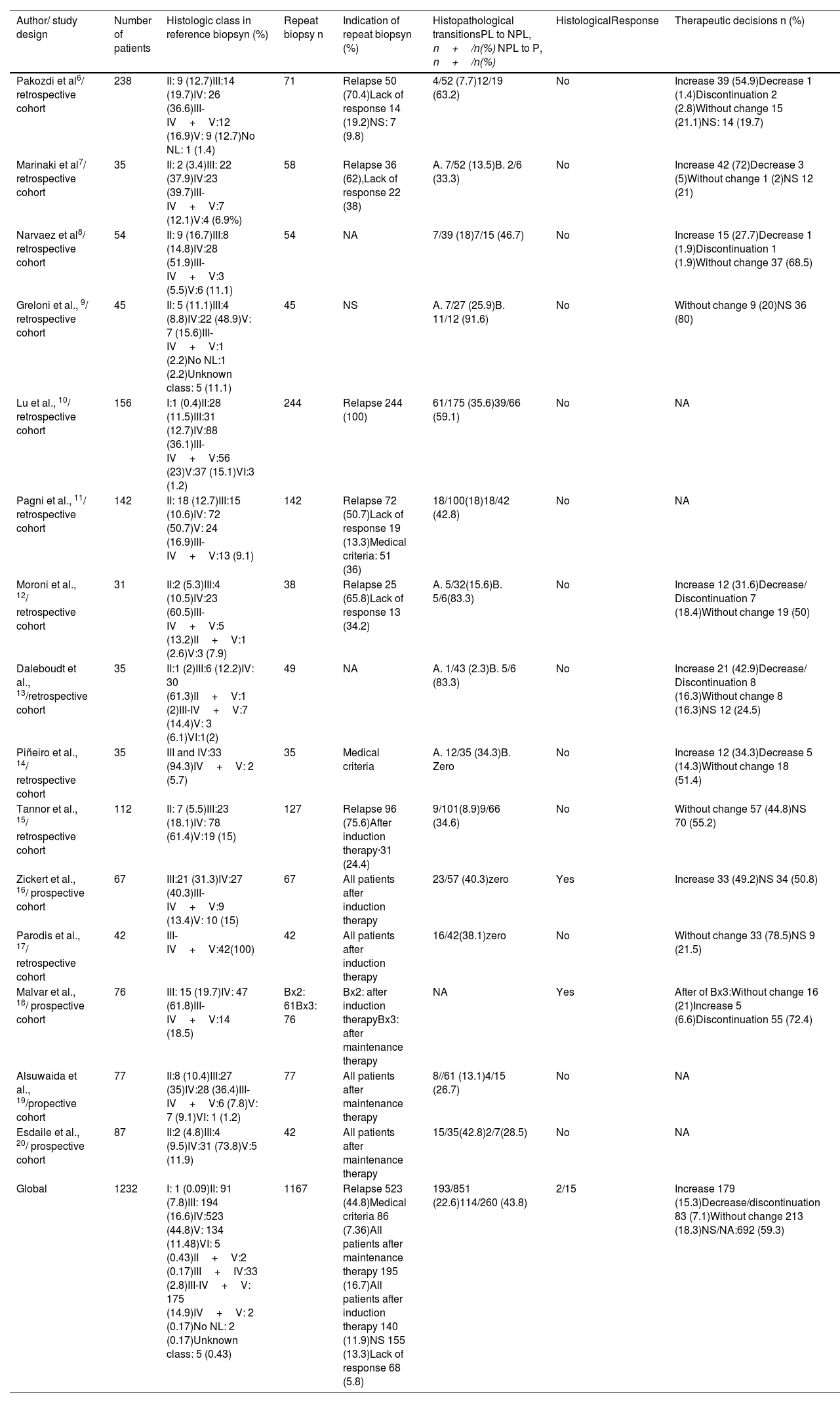
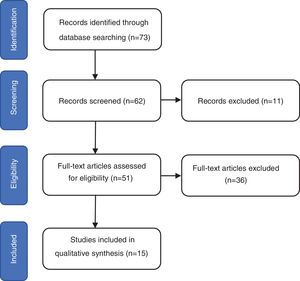
ResultsThe search identified 73 studies in MEDLINE PUBMED. 62 studies were selected after applying the filters based on the above-mentioned inclusion and exclusion criteria, and then a selection according to title and abstract led to 51 articles. Finally, 15 studies were included in the review (figure 1) and a total of 1167 repeat biopsies. Table 3 summarizes the characteristics of the studies selected from literature. This search was conducted by three authors (SMSC, MGSF and JRD).
The findings the repeat biopsy in lupus nephritis.
| Author/ study design | Number of patients | Histologic class in reference biopsyn (%) | Repeat biopsy n | Indication of repeat biopsyn (%) | Histopathological transitionsPL to NPL, n+/n(%) NPL to P, n+/n(%) | HistologicalResponse | Therapeutic decisions n (%) |
|---|---|---|---|---|---|---|---|
| Pakozdi et al6/ retrospective cohort | 238 | II: 9 (12.7)III:14 (19.7)IV: 26 (36.6)III-IV+V:12 (16.9)V: 9 (12.7)No NL: 1 (1.4) | 71 | Relapse 50 (70.4)Lack of response 14 (19.2)NS: 7 (9.8) | 4/52 (7.7)12/19 (63.2) | No | Increase 39 (54.9)Decrease 1 (1.4)Discontinuation 2 (2.8)Without change 15 (21.1)NS: 14 (19.7) |
| Marinaki et al7/ retrospective cohort | 35 | II: 2 (3.4)III: 22 (37.9)IV:23 (39.7)III-IV+V:7 (12.1)V:4 (6.9%) | 58 | Relapse 36 (62),Lack of response 22 (38) | A. 7/52 (13.5)B. 2/6 (33.3) | No | Increase 42 (72)Decrease 3 (5)Without change 1 (2)NS 12 (21) |
| Narvaez et al8/ retrospective cohort | 54 | II: 9 (16.7)III:8 (14.8)IV:28 (51.9)III-IV+V:3 (5.5)V:6 (11.1) | 54 | NA | 7/39 (18)7/15 (46.7) | No | Increase 15 (27.7)Decrease 1 (1.9)Discontinuation 1 (1.9)Without change 37 (68.5) |
| Greloni et al., 9/ retrospective cohort | 45 | II: 5 (11.1)III:4 (8.8)IV:22 (48.9)V: 7 (15.6)III-IV+V:1 (2.2)No NL:1 (2.2)Unknown class: 5 (11.1) | 45 | NS | A. 7/27 (25.9)B. 11/12 (91.6) | No | Without change 9 (20)NS 36 (80) |
| Lu et al., 10/ retrospective cohort | 156 | I:1 (0.4)II:28 (11.5)III:31 (12.7)IV:88 (36.1)III-IV+V:56 (23)V:37 (15.1)VI:3 (1.2) | 244 | Relapse 244 (100) | 61/175 (35.6)39/66 (59.1) | No | NA |
| Pagni et al., 11/ retrospective cohort | 142 | II: 18 (12.7)III:15 (10.6)IV: 72 (50.7)V: 24 (16.9)III-IV+V:13 (9.1) | 142 | Relapse 72 (50.7)Lack of response 19 (13.3)Medical criteria: 51 (36) | 18/100(18)18/42 (42.8) | No | NA |
| Moroni et al., 12/ retrospective cohort | 31 | II:2 (5.3)III:4 (10.5)IV:23 (60.5)III-IV+V:5 (13.2)II+V:1 (2.6)V:3 (7.9) | 38 | Relapse 25 (65.8)Lack of response 13 (34.2) | A. 5/32(15.6)B. 5/6(83.3) | No | Increase 12 (31.6)Decrease/ Discontinuation 7 (18.4)Without change 19 (50) |
| Daleboudt et al., 13/retrospective cohort | 35 | II:1 (2)III:6 (12.2)IV: 30 (61.3)II+V:1 (2)III-IV+V:7 (14.4)V: 3 (6.1)VI:1(2) | 49 | NA | A. 1/43 (2.3)B. 5/6 (83.3) | No | Increase 21 (42.9)Decrease/ Discontinuation 8 (16.3)Without change 8 (16.3)NS 12 (24.5) |
| Piñeiro et al., 14/ retrospective cohort | 35 | III and IV:33 (94.3)IV+V: 2 (5.7) | 35 | Medical criteria | A. 12/35 (34.3)B. Zero | No | Increase 12 (34.3)Decrease 5 (14.3)Without change 18 (51.4) |
| Tannor et al., 15/ retrospective cohort | 112 | II: 7 (5.5)III:23 (18.1)IV: 78 (61.4)V:19 (15) | 127 | Relapse 96 (75.6)After induction therapy·31 (24.4) | 9/101(8.9)9/66 (34.6) | No | Without change 57 (44.8)NS 70 (55.2) |
| Zickert et al., 16/ prospective cohort | 67 | III:21 (31.3)IV:27 (40.3)III-IV+V:9 (13.4)V: 10 (15) | 67 | All patients after induction therapy | 23/57 (40.3)zero | Yes | Increase 33 (49.2)NS 34 (50.8) |
| Parodis et al., 17/ retrospective cohort | 42 | III-IV+V:42(100) | 42 | All patients after induction therapy | 16/42(38.1)zero | No | Without change 33 (78.5)NS 9 (21.5) |
| Malvar et al., 18/ prospective cohort | 76 | III: 15 (19.7)IV: 47 (61.8)III-IV+V:14 (18.5) | Bx2: 61Bx3: 76 | Bx2: after induction therapyBx3: after maintenance therapy | NA | Yes | After of Bx3:Without change 16 (21)Increase 5 (6.6)Discontinuation 55 (72.4) |
| Alsuwaida et al., 19/propective cohort | 77 | II:8 (10.4)III:27 (35)IV:28 (36.4)III-IV+V:6 (7.8)V: 7 (9.1)VI: 1 (1.2) | 77 | All patients after maintenance therapy | 8//61 (13.1)4/15 (26.7) | No | NA |
| Esdaile et al., 20/ prospective cohort | 87 | II:2 (4.8)III:4 (9.5)IV:31 (73.8)V:5 (11.9) | 42 | All patients after maintenance therapy | 15/35(42.8)2/7(28.5) | No | NA |
| Global | 1232 | I: 1 (0.09)II: 91 (7.8)III: 194 (16.6)IV:523 (44.8)V: 134 (11.48)VI: 5 (0.43)II+V:2 (0.17)III+IV:33 (2.8)III-IV+V: 175 (14.9)IV+V: 2 (0.17)No NL: 2 (0.17)Unknown class: 5 (0.43) | 1167 | Relapse 523 (44.8)Medical criteria 86 (7.36)All patients after maintenance therapy 195 (16.7)All patients after induction therapy 140 (11.9)NS 155 (13.3)Lack of response 68 (5.8) | 193/851 (22.6)114/260 (43.8) | 2/15 | Increase 179 (15.3)Decrease/discontinuation 83 (7.1)Without change 213 (18.3)NS/NA:692 (59.3) |
Abbreviations: Bx2: second biopsy. Bx3: third biopsy. NPL: non- proliferative lesions. PL: proliferative lesions. NA=Data not available. NL: nephritis lupus. NS=data not specified.
Narvaez et al reference biopsy was the second biopsy.
Greloni et al reference biopsy was the first biopsy and repeat biopsy were 71.
Piñeiro et al, Malvar et al and Parodis et al only include proliferative class or mixed.
Zickert et al didn’t include class II.
Parodis et al included 8 from Euro-Lupus and 25 from MAINTAIN.
Percutaneous kidney biopsy, introduced in the 1940s and adopted into clinical practice since the 1950s, remains the gold standard for diagnosis of LN, and is highly recommended for the identification and classification of renal involvement, to assess disease activity and thus guide intensity of treatment and also to predict prognosis.21 Protocol repeat biopsies are sometimes controversial, but emerging data from observational cohort studies suggest that such biopsies may assist in making treatment decisions and help predict long-term renal outcomes. This systematic review discusses the indications for repeat kidney biopsy, histopathological transitions, definition of histologic remission and therapeutic decisions after repeat biopsy.
The 2019 updated European League Against Rheumatism–European Renal Association–European Dialysis and Transplant Association (EULAR/ ERA-EDTA) recommendations for the management of LN states that repeat kidney biopsy should be considered in selected cases, such as worsening of kidney variables, non-responsiveness to immunosuppressive or biologic treatment or relapse, to demonstrate possible histologic class transition or changes in chronicity and activity indices; to provide prognostic information; and detect other pathologies.22
The primary indications for repeat biopsy were identified as relapse (44-78%) and lack of response (13-51%). Pagni et al 11also mentioned as an indication of repeat biopsy the potential reduction of immunosuppressive therapy. In some studies, repeat biopsy was performed per protocol after induction and maintenance therapy.15–20 Protocol biopsies have taught us some important lessons. Although very few studies have been conducted with repeat biopsy immediately after induction treatment, findings from such biopsies suggest that repeat biopsies are more predictive of long-term kidney and patient outcomes than reference biopsies. Furthermore, such biopsies showed that aggressive immunosuppression and rapid control of clinical disease activity did not necessarily prevent chronic damage in LN. Thus, the clinical findings after induction therapy may not reflect what is happening in the kidney regarding inflammation and chronic damage.
One study of patients with proliferative LN, where renal biopsies after induction treatment were done per protocol, showed that one-third of patients with complete clinical response still had high histologic activity on the second biopsy, and that 62% of patients with complete histologic remission on re-biopsy still showed persistent clinical activity. The results show that after induction therapy, histologic and clinical responses are discordant.23
Repeat biopsy after maintenance treatment has also shown continuing histologic activity in a significant number of patients. The discontinuation of maintenance immunosuppression in such patients may put them at risk of renal flare. In a study, 44% of the patients were found to have persistent histologic activity.24 These data demonstrate that despite extensive and long-term immunosuppression, patients with LN who go into complete clinical renal remission still have a high relapse rate following withdrawal of maintenance immunosuppression. Relapse-prone patients cannot be identified a priori by clinical or demographic variables. However, examination of kidney histology during treatment and after clinical remission provides useful information to predict who is likely to relapse and who is likely to remain in remission after discontinuation of immunosuppression24,25.
It is well known that LN class may change to a different grade during flares. Nevertheless, a repeat biopsy during LN flare remains controversial as some research has shown that proliferative LN on the first/reference biopsy does not commonly change to non-proliferative LN during flare. Therefore, treatment adjustments may initially be made based on clinical and laboratory signs. It has been shown that clinically relevant class switches are more frequently observed in patients with nonproliferative lesions, ranging from 26 to 91%; however, as expected, patients who initially had proliferative lesions rarely switched to nonproliferative nephritis, ranging from 3 to 40%. Based on these data, a repeat biopsy would allow for the identification of patients with proliferative changes who transitioned to a non-proliferative class or vice versa. A second biopsy showing chronicity or inactive disease may also help in guiding immunosuppressive reduction.
Very few studies 16,18provide a definition of histological response; these studies have shown discordance between clinical and histologic findings in patients with LN who have undergone protocol kidney biopsies during induction or maintenance therapy. Most studies show the mean AI both in reference biopsies and in repeat biopsies; however, they fail to specify how many still have AI >0.
The therapeutic decision after repeat biopsy shows that in many studies treatment changes are introduced. However, as mentioned above, patients with complete histological recovery may have persistently abnormal clinical findings, suggesting that repeat biopsies may be a valuable tool to guide the decision to withdraw immunosuppressive therapy. Furthermore, discontinuing immunosuppression in patients with ongoing marked renal inflammation may put them at risk of renal relapse, while continuing treatment in patients with signs of clinical activity (proteinuria) but with no histological activity, may expose patients to increased morbidity from unnecessary immunosuppression.26
There are some limitations to this systematic review including the retrospective nature of the studies which focused on indications of repeat kidney biopsy, histopathological transitions, the definition of histologic remission, and the therapeutic decision after repeat biopsy. Morevoer, the study fails to assess the association among the clinical, laboratory and histopathological characteristics; few studies show the relationship between clinical and histopathological response, and long-term outcomes. Furthermore, most studies fail to provide treatment details before repeat kidney biopsy.
ConclusionsThe current data suggest that a repeat biopsy should be considered in patients with LN flare/relapse, especially when the reference biopsy was ISN/RPS class I/II or V, as histology changes are likely to impact treatment options. A repeat biopsy should also be considered in patients with poor treatment response as a tool to guide treatment choices and eventually discontinuing or tapering immunosuppression. Histological transformations are common. Treatment changes are introduced following a repeat biopsy. The repeat biopsy could have prognostic value for therapeutic decision-making.
FundingThis research was not funded by any public, commercial, or not-for-profit institutions.
Conflict of interestThe authors have no conflict of interest to disclose.