Systemic lupus erythematosus (SLE) is the most representative disorder within systemic autoimmune diseases. The treat-to-target strategy in SLE was established half a decade ago and, since then, remarkable advances have been made. An international consensus has defined and unified the term remission and also low disease activity has been proposed as an alternative and, perhaps, more realistic target. Both of them have proven to be meaningful in terms of improving several outcomes, and have opened the path for future research in clinical trials.
El lupus eritematoso sistémico (LES) es el trastorno más representativo dentro de las enfermedades autoinmunes sistémicas. La estrategia de tratamiento por objetivos en el LES se estableció hace media década y desde entonces se han producido notables avances. Un consenso internacional ha definido y unificado el término remisión y también se ha propuesto la baja actividad de la enfermedad como un objetivo alternativo y quizás más realista. Ambos han demostrado ser significativos en cuanto a la mejora de varios resultados y han abierto el camino para futuras investigaciones en ensayos clínicos.
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by a chronic, relapsing-remitting course. It is notable for its clinical heterogeneity that may involve different organ/systems in various combinations1 with potentially fatal consequences. Despite being the most paradigmatic systemic autoimmune disease, it is considered a rare disease due to its low incidence.2 However, if SLE has any advantage over other more prevalent entities, such as hypertension, diabetes or dyslipidaemia, it is precisely the opportunity to take advantage of and extrapolate those same strategies that have been more successful in the management of these patients. This is the case of the treat-to-target (T2T) strategy, which has been implemented for decades in hypertension, dyslipidaemia or diabetes.3–5
In essence, the treat-to-target strategy is based on the principle that treating patients towards a specific goal, and adjusting the treatment if this goal is not met, achieves better outcome results. Thus, in hypertensive patients, evidence from clinical trials demonstrate that lowering blood pressure to a certain threshold (i.e. 140/90mmHg vs 160/100mmHg) is capable of reducing the occurrence of cardiovascular events, and that the lower the threshold, the better the outcome.6 Likewise, with dyslipidaemia and cardiovascular risk, treatment with statins is associated with a decrease in mortality and myocardial infarction, and intensive treatment with different goals of LDL-cholesterol are recommended varying with the baseline individual cardiovascular risk.4 However, when defining the target, benefits expected need to be weighed against potential risks. Thereby, while it is true that an intensive glycaemic control reduces the risk of developing microvascular diabetes complications, it also may increase the risk of hypoglycaemia.7,8
Nevertheless, extrapolating this T2T strategy from other diseases to SLE is not that simple. First, SLE is not characterized by one main manifestation. Instead, several organs with variable prognostic importance can be affected as a direct consequence of the disease, which implies that many factors have to be taken into account. Second, it is yet to be clarified which is the best method of quantifying all these factors, how to optimally evaluate the activity of the disease. This aspect still creates controversy, since not all scales are able to adequately capture every degree of activity, nor measure every organ/system, and not all have the same feasibility in the daily practice. In other words, is a global approach better than an individual organ/system approach? Third, it has to be defined what are the most suitable targets to pursue, because SLE is not a single-target disease. Besides diminishing mortality, many other aspects, such as controlling disease activity, preventing damage accrual, minimizing treatment-related toxicities or improving the quality of life of the patients, among others, are desirable goals to achieve. Fourth, appropriate and universal definitions of this targets, agreed preferably by consensus, have to be reached. And, finally and more important, these universally defined and agreed targets have to prove a significant impact on relevant outcomes.
In this review, we aim to summarize the overarching principles of the T2T strategy in SLE and the major obstacles in the application of the T2T principle in SLE patients.
Defining the target in systemic lupus erythematosusIn order to start developing the T2T strategy is SLE, an international and multidisciplinary task force with expertise in the field of SLE, both in research and clinical management, gathered in various meetings that were held through 2012 and 2013, to make an initial proposal of what would be the definitions and the key pivotal lines of the treat-to-target (T2T) in SLE as we know it today.9 The main recommendations are summarized in Table 1.
T2T overarching principles and key messages. Adapted from Ref. 9.
| General principles |
| A. The management of systemic lupus erythematosus (SLE) should be based on shared decisions between the informed patient and her/his physician(s). |
| B. Treatment of SLE should aim at ensuring long-term survival, preventing organ damage, and optimising health-related quality-of-life, by controlling disease activity and minimising comorbidities and drug toxicity. |
| C. The management of SLE requires an understanding of its many aspects and manifestations, which may have to be targeted in a multidisciplinary manner. |
| D. Patients with SLE need regular long-term monitoring and review and/or adjustment of therapy. |
| Particular recommendations |
| 1. The treatment target of SLE should be remission of systemic symptoms and organ manifestations or, where remission cannot be reached, the lowest possible disease activity, measured by a validated lupus activity index and/or by organ-specific markers. |
| 2. Prevention of flares (especially severe flares) is a realistic target in SLE and should be a therapeutic goal. |
| 3. It is not recommended that the treatment in clinically asymptomatic patients be escalated based solely on stable or persistent serological activity. |
| 4. Since damage predicts subsequent damage and death, prevention of damage accrual should be a major therapeutic goal in SLE. |
| 5. Factors negatively influencing health-related quality of life (HRQOL), such as fatigue, pain and depression should be addressed, in addition to control of disease activity and prevention of damage. |
| 6. Early recognition and treatment of renal involvement in lupus patients is strongly recommended. |
| 7. For lupus nephritis, following induction therapy, at least 3 years of immunosuppressive maintenance treatment is recommended to optimise outcomes. |
| 8. Lupus maintenance treatment should aim for the lowest glucocorticoid dosage needed to control disease, and if possible, glucocorticoids should be withdrawn completely. |
| 9. Prevention and treatment of antiphospholipid syndrome (APS)-related morbidity should be a therapeutic goal in SLE; therapeutic recommendations do not differ from those in primary APS. |
| 10. Irrespective of the use of other treatments, serious consideration should be given to the use of antimalarials. |
| 11. Relevant therapies adjunctive to any immunomodulation should be considered to control comorbidity in SLE patients. |
At that moment, it was convened that a long-term meaningful goal should be “ensuring long-term survival, preventing organ damage, and optimizing health-related quality-of-life”, based on shared decisions between an informed patient and a multidisciplinary team. To achieve that, it was required “controlling disease activity and minimizing comorbidities and drug toxicities”,9 thus defining short-term meaningful goals.
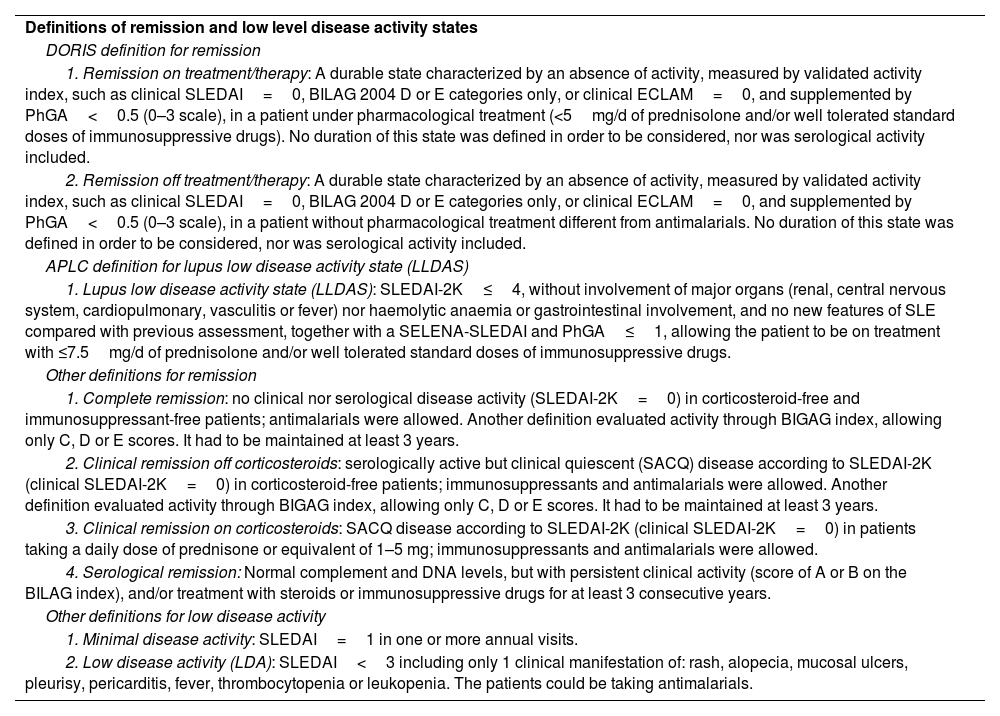
This is based on the fact that several previous epidemiological studies have shown that high levels of disease activity, assessed at baseline or at any timepoint of the disease and measured by distinct validated activity indices, are associated not only with increased mortality, but also with damage accrual, as well as other poor patient reported outcomes, such as higher pain or fatigue scores, and lower quality of life, assessed by a health-related quality of life (HRQoL) questionnaire.10–15 Therefore, achieving remission or, if this is not feasible, then aiming for the lowest activity possible, became two main targets. However, one of the major drawbacks at that time was the absence of a valid, accepted and widespread definition for any of both targets. During the last years, several authors had formulated various definitions of remission with certain degree of overlap.16–21 More recently, an international consensus task force - known as Definition Of Remission In SLE (DORIS) group - proposed the basic principles that a definition of remission should contain.22 The main differences among these definitions included variations in the disease activity index used, in the inclusion or not of immunological parameters, in the allowance or not of immunosuppressants together with corticosteroids, or in the requirement of a minimum timeframe to be considered as remission. The main definitions are summarized in Table 2.
Definitions of remission and low lupus disease activity states. Adapted from Refs. 16–25.
| Definitions of remission and low level disease activity states |
| DORIS definition for remission |
| 1. Remission on treatment/therapy: A durable state characterized by an absence of activity, measured by validated activity index, such as clinical SLEDAI=0, BILAG 2004 D or E categories only, or clinical ECLAM=0, and supplemented by PhGA<0.5 (0–3 scale), in a patient under pharmacological treatment (<5mg/d of prednisolone and/or well tolerated standard doses of immunosuppressive drugs). No duration of this state was defined in order to be considered, nor was serological activity included. |
| 2. Remission off treatment/therapy: A durable state characterized by an absence of activity, measured by validated activity index, such as clinical SLEDAI=0, BILAG 2004 D or E categories only, or clinical ECLAM=0, and supplemented by PhGA<0.5 (0–3 scale), in a patient without pharmacological treatment different from antimalarials. No duration of this state was defined in order to be considered, nor was serological activity included. |
| APLC definition for lupus low disease activity state (LLDAS) |
| 1. Lupus low disease activity state (LLDAS): SLEDAI-2K≤4, without involvement of major organs (renal, central nervous system, cardiopulmonary, vasculitis or fever) nor haemolytic anaemia or gastrointestinal involvement, and no new features of SLE compared with previous assessment, together with a SELENA-SLEDAI and PhGA≤1, allowing the patient to be on treatment with ≤7.5mg/d of prednisolone and/or well tolerated standard doses of immunosuppressive drugs. |
| Other definitions for remission |
| 1. Complete remission: no clinical nor serological disease activity (SLEDAI-2K=0) in corticosteroid-free and immunosuppressant-free patients; antimalarials were allowed. Another definition evaluated activity through BIGAG index, allowing only C, D or E scores. It had to be maintained at least 3 years. |
| 2. Clinical remission off corticosteroids: serologically active but clinical quiescent (SACQ) disease according to SLEDAI-2K (clinical SLEDAI-2K=0) in corticosteroid-free patients; immunosuppressants and antimalarials were allowed. Another definition evaluated activity through BIGAG index, allowing only C, D or E scores. It had to be maintained at least 3 years. |
| 3. Clinical remission on corticosteroids: SACQ disease according to SLEDAI-2K (clinical SLEDAI-2K=0) in patients taking a daily dose of prednisone or equivalent of 1–5 mg; immunosuppressants and antimalarials were allowed. |
| 4. Serological remission: Normal complement and DNA levels, but with persistent clinical activity (score of A or B on the BILAG index), and/or treatment with steroids or immunosuppressive drugs for at least 3 consecutive years. |
| Other definitions for low disease activity |
| 1. Minimal disease activity: SLEDAI=1 in one or more annual visits. |
| 2. Low disease activity (LDA): SLEDAI<3 including only 1 clinical manifestation of: rash, alopecia, mucosal ulcers, pleurisy, pericarditis, fever, thrombocytopenia or leukopenia. The patients could be taking antimalarials. |
Similarly to remission, various definitions of low disease activity have been proposed,23,24 particularly if among this group were considered patients clinically inactive but serologically active, which may, for some authors, overlap with the term remission. Like the DORIS consensus, the Asia-Pacific Lupus Collaboration group proposed and validated a definition of a low disease activity state in SLE, named Lupus Low Disease Activity State (LLDAS), based on the principle of a “tolerated” or “acceptable” level of disease activity on a patient with a stable treatment and low dose of corticoisteroids with a low likelihood of adverse outcome.25 These most recent definitions are summarized in Table 2.
It is important to note that both LLDAS and DORIS remission definitions were determined a priori, based on consensus processes following a thorough systematic literature search, involving multinational expert panels convened specifically for this purpose, and not fabricated ad hoc for a specific study, conversely to other definitions, which confers them a solid face and construct validity.
Remission and LLDAS: validating the targetA critical step in the generation of a target is to prove that reaching such target will have a significant impact on a given outcome, and that this results are reproducible in various cohorts of patients. Thus, reaching both remission and low disease activity have shown consistently to have clinically meaningful impact on several outcomes.
Achieving both remission and low disease activity is associated with less damage accrual.18,26–30 The Padua cohort showed in one study18 that of 224 patients followed during 5 years, 7.1% achieved prolonged complete remission, 14.7% prolonged clinical remission off corticosteroids (immunosuppressants and antimalarials were allowed) and 15.6% clinical remission on corticosteroids, while the rest did not. Damage accrual occurred less frequently and with a lesser degree in patients on clinical remission than in unremitted patients. Moreover, in patients on remission, damage accrual was more frequently observed in patients with clinical remission on corticosteroids than in those with clinical remission off corticosteroids or complete remission, which suggested that steroids withdrawal could be a potential target also. Another study of the same cohort showed that achieving LLDAS more at least 2 years prevented from damage accrual, and the higher the duration of LLDAS, the lower the increase in SDI.28
In the Hopkins Lupus Cohort, which included 1356 SLE patients, those who achieved clinical remission on treatment even less than 25% of their follow-up had 50% reduction in organ damage than those who never achieved remission, whereas patients who achieved LLDAS at least 50% of their follow-up had substantially lower rates of damage than those who do not.26
Another Italian cohort of 115 patients followed 5 years showed that 6% of the patients achieved prolonged remission off treatment the whole follow-up and 29.5% of the patients maintained prolonged remission on treatment, whereas 36.5% maintained prolonged LLDAS. In this cohort, damage accrual also occurred less frequently when these targets were achieved.
The Amsterdam cohort, comprised of 183 patients, showed that 32.5% of them achieved remission during a median follow-up of 5 years, whereas 64.5% achieved LLDAS more than 50% of the time of follow-up. Both states were associated with a reduced risk of damage accrual, more markedly with remission.
Finally, a recent systematic literature review on the impact of remission on damage accrual comprising more than 6000 patients concluded that achieving remission, even with less stringent definitions, prevented damage accrual.31
These targets have been tested not only on clinical outcomes, but also on patient reported outcomes. Importantly, a recent systematic review of the literature involving more than 3000 patients and exploring the impact of remission and low disease activity showed that even less stringent remission or low disease activity definitions predicted/were associated with a better HRQoL. Physical rather than mental domains were more associated with remission or low disease activity.32 The impact of remission on other outcomes has been less explored to date. However, both targets have shown to reducing the number of flares >50% and allowing for corticosteroid withdrawal in 20% of patients,33 or also reduced direct healthcare costs.34
While it is true that, according to T2T recommendations, remission should be preferable over any level of activity, it is more frequent to reach less stringent definitions, such as LLDAS, than remission,25–28 in a proportion that varies according with the definition of remission employed. However, there is indirect information regarding that probably remission would lead to more improvement, with evidence pointing to lower damage accrual, and higher corticosteroid reduction with remission than with LLDAS.26,30-33,35,36
In summary, both the DORIS definition of remission and the LLDAS have proven to be attainable targets, with a clear impact on SLE clinical and patient reported outcomes, being remission the ideal target, but LLDAS maybe a more realistic one.
Obstacles and pitfalls implementing the T2T strategy in SLEHeterogeneous nature of the diseaseOne of the first problems implementing the T2T strategy is the pleomorphic nature of the disease. Unlike other entities, the spectrum of clinical manifestations of SLE varies from articular involvement to neuropsychiatric symptoms, from different cytopenias to fatigue or thrombotic events. Moreover, the course is often unpredictable and the potential severity of the disease require management by an experienced clinician integrated in a multidisciplinary team. The management not only implies having to control the disease itself, but often side effects and complications derived from therapy. Therefore, there is no single factor, but a combination of them that play a role in the natural course and have to be taken into account to implement the strategy.
Problems measuring variablesIn diabetes mellitus or dyslipidaemia there are reliable markers, such as levels of HbA1c or levels of LDL-cholesterol, that are directly correlated with the severity and the prognosis of the disease, and also serve to monitor the response to the treatment. Also, in hypertension, there are reliable devices to quantify and register very precisely the blood pressure measurements. However, in SLE these biomarkers or instruments are lacking. To quantify the activity of the disease, there are multiple validated scales, which is the proof that none of them is perfect. Regarding global activity scales, for instance, SLEDAI-2K captures the disease in a dichotomous way, either present or absent. It qualifies the same 2 tender joints with little morning stiffness and barely limitation on daily activity than 20 extremely inflamed joints with serious impairment. On the other hand, BILAG index reflects much better different organ involvement, and is capable of capture improvement or worsening, but requires a trained clinician and lot of time, which makes it unfeasible for routine clinical practice. Regarding organ specific scales, CLASI is a scale that deeply explores cutaneous involvement, but obviously does not take into account other involvements. For all these reasons and limitations, currently, no single SLE activity index is preferred in terms of convenience of use and reflection of change in disease activity in different organs/systems. These activity indices are used mainly in investigation to capture and gauge the effect rather than guide the clinical decision of treatment.
Lack of consensus regarding definitions of targetsMany of the potential targets of the disease do not have a validated definition or scale to be measured, and if they do, they are not universally accepted. The DORIS definition of remission and the LLDAS are definitions created by an international panel of experts following a rigorous process similar to that of the new classification criteria of several rheumatic diseases. Other definitions exist since they were created previously, but that terminology is still being used, which complicates extrapolation and comparison of data.
Paucity of validated data on definitionsAlthough much advances have been made in the last years testing the different definitions of several targets in observational studies, there are still a paucity of data regarding this same targets and its performance in clinical trials.
Concluding remarksThe T2T strategy is still expanding and developing in SLE but it is important to note that much progress has been made in recent times.37 Coining terms such as remission or low disease activity is of the utmost importance for setting more objective goals in future clinical trials.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interestThe authors declare that they have no conflict of interest.