Adherence to treatment is one of the pillars to achieve control of any disease and this also applies to rheumatoid arthritis. There are different ways to assess the level of adherence, and measurement scales are one of the methods most used due to their easy application and low cost. The aim of this study was to translate and validate the Compliance Questionnaire on Rheumatology (CQR) to Spanish and calibrate the scores according to the activity of the disease measured by the DAS-28.
Materials and methodsThe translation process was carried out in the first phase of the study using guidelines for translation and cultural adaptation of measures focused on patients. This was followed by cognitive clarification process. Finally, a study of diagnostic tests was carried out to determine the best cut-off point for the score on the CQR scale for identifying the level of adherence to rheumatic therapy in patients with rheumatoid arthritis using the DAS-28 as subrogated adherence.
ResultsThe study included 233 patients, with a mean age of 46.3 (±13.9) years and mean time with the disease of 11.2 (±9.6) years. The CQR cut-off to establish adherence to treatment was 80.7. This achieved a sensitivity of 80.2% (95% CI: 71.9–86.9%) and specificity of 72.3% (95% CI: 63.1–80.4%). With this cut-off point, it was established that there was 43.8% (n=102) patient adherence to oral rheumatic therapy.
ConclusionCQR Spanish version of the CQR was developed and calibrated obtaining a practical tool, with acceptable sensitivity and specificity.
La adherencia al tratamiento es uno de los pilares para lograr el control de cualquier enfermedad, y esto aplica también en la artritis reumatoide. Existen diferentes formas de evaluar el grado de adherencia, siendo las escalas de medición uno de los métodos más usados por su facilidad en aplicación y bajo costo. El objetivo de este estudio fue traducir y validar el Compliance Questionnaire on Rheumatology (CQR) al español y calibrar los puntajes de acuerdo con la actividad de la enfermedad medida mediante DAS-28.
Materiales y métodosEn la primera fase del estudio se llevó a cabo el proceso de traducción empleando las guías para la traducción y adaptación cultural de medidas centradas en pacientes. Posteriormente se realizó un proceso de aclaración cognoscitiva. Finalmente se llevó a cabo un estudio de pruebas diagnósticas para determinar el mejor punto de corte para el puntaje de la escala CQR en la identificación del nivel de adherencia al tratamiento antirreumático en pacientes con artritis reumatoide, empleando el DAS 28 como subrogado de adherencia.
ResultadosUn total de 233 pacientes con edad promedio de 46,3 (±13,9) años y tiempo promedio de evolución de enfermedad de 11,2 (±9,6) años. El punto de corte del CQR para establecer adherencia al tratamiento fue de 80,7, con lo cual se obtuvo una sensibilidad del 80,2% (IC 95%: 71,9-86,9%) y una especificidad del 72,3% (IC 95%: 63,1-80,4%). Con este punto de corte se estableció que el 43,8% (n=102) de los pacientes eran adherentes a la terapia antirreumática oral.
ConclusiónSe desarrolló una versión en español del CQR y se calibraron los puntos de corte obteniendo una herramienta práctica y de rápida aplicación clínica, con aceptables sensibilidad y especificidad.
Rheumatoid arthritis (RA) is a chronic autoimmune disease, characterized by inflammation and joint deformity, for which the introduction of therapy using synthetic disease modifying rheumatoid arthritis drugs (DMARDs) is intended to halt the clinical and radiological progression of the disease. One of the major limitations to treatment effectiveness is non-compliance.1 It has been reported that patients with high levels of compliance show better clinical effects in controlling the disease.2,3 Compliance has been defined as 80% or more of medication use4,5; however, the best method to measure such compliance has not yet been established, though the literature reports several methods such as patient's self-reporting, the judgment of the clinician, measuring metabolites, tablet counting, electronic monitors, drug dispensation audits, or measurement scales.6–9 The use of measurement scales to determine the level of compliance offers significant advantages, including simplicity and cost, as well as producing relevant information to make daily life changes to optimize patient compliance. The most popular questionnaire to measure medication compliance in RA is the Compliance Questionnaire on Rheumatology (CQR), with a sensitivity of 98% and a specificity of 67%, allowing for an approximate estimate of the patient's compliance to anti-rheumatic therapy.6
According to the CQR score, zero means a total lack of compliance and 100 means perfect compliance. One of the studies to validate this questionnaire used as the “gold standard” an electronic mechanism to monitor opening and defined a satisfactory level of compliance when there was evidence that the patient took the medication more than 80% of the time. The final analysis of this study concluded that the CQR was an appropriate strategy to measure adherence to treatment with DMARDs.4 The aim of this study was to translate and validate the CQR into Spanish, and to calibrate the scores in accordance with the activity of the disease measured with DAS-28.10,11
The patient should express the his/her level of agreement with each statement, with the following options and scores marked on a Likert scale: strongly disagree (one point), disagree (two points), agree (three points), strongly agree (four points). Five items are expressed in a negative form (items 4, 8, 11, 12 and 19) and these are recoded in an inverse fashion to those expressed positively: strongly disagree (four points), disagree (three points), agree (two points), strongly agree (one point). The total CQR score is estimated by adding the total number of points for all the items, subtracting 19 and dividing into 0.57. the CQR score ranges from 0 (complete non-compliance) to 100 (perfect compliance).
Materials and methodsParticipantsTwo hundred and thirty-three patients with a diagnosis of RA based on the ACR-EULAR 201012 classification, who were all managed with oral anti-rheumatic therapy, participated in the study. The patients were collected from the rheumatology outpatient clinic in a specialized care center between October 2015 and October 2016.
CQR translationIn order to complete the translation process, the guidelines of the ISPOR Task Force for translation and cultural adaptation of patient-centered measures were used.13 During the first phase of the study, the translation of the original CQR was made into Spanish. The translation was done by two official translators (Spanish native tongue) and the resulting document was translated into English by two official translators (native English tongue), and finally the two documents in English were contrasted against the original, choosing the one with the strongest similarity to the original English questionnaire. A cognitive clarification process was then conducted in a sample of 10 patients with RA, in order to assess the level of understanding of the questions, as well as the responsiveness and completion of the instrument. There was no need to make any changes to the items or to the answer options. The final result of this process with the Spanish version of the CQR is shown in Table 1.
Spanish version of the Compliance Questionnaire on Rheumatology (CQR).
| 1. If the rheumatologist tells me to take the arthritis medicines, I do so |
| 2. I take my anti-rheumatic medicines because I then have fewer problems (joint pain, joint stiffness, joint inflammation) |
| 3. I definitively don’t dare to miss my anti-rheumatic medications |
| 4. If I can help myself with alternative therapies, I prefer that to what my rheumatologist prescribes |
| 5. My medicines are always stored in the same place and that's why I do not forget to take them |
| 6. I take my medicines because I hace complete confidence in my rheumatologist |
| 7. The most important reason to take my anti-rheumatic medicines is that I can still do what I want to do |
| 8. I don’t like to take medicine. If I can do without them, I will |
| 9. When I am on vacation, it sometimes happens that I don’t take my medicines |
| 10. I take my anti-rheumatic drugs, for otherwise what's the point of consulting a rheumatologist? |
| 11. I don’t expect miracles from my anti-rheumatic medicines |
| 12. If you can’t stand the medicines you might say: “Throw it away, no matter what” |
| 13. If I don’t take my medicines regularly, the inflammation returns |
| 14. If I don’t take my anti-rheumatic medicines, my body warns me (I feel bad) |
| 15. My health is my number one priority, and if I have to take medicines to keep well, I will |
| 16. I use a pill box for my medicines |
| 17. I do what my doctor tells me to do |
| 18. If I don’t take my anti-rheumatic medicines, I have more symptoms |
| 19. It happens every now and then, I go out for the weekend and then I don’t take my medicines |
A diagnostic test study was designed to establish the best cut-off point for the CQR scale score, using the formula:
∑i=119Xi−190.57 in the identification of the level of compliance to anti-rheumatic treatment in patients with RA in the outpatient clinic. Socio-demographic information was collected, in addition to compliance self-report (in a scale from 0 to 100%), the presence of medication-associated adverse events, and the level of disease activity measured using DAS-28.10,11 The disease activity was considered the best indicator of compliance, based on the concept of treatment goals, since the primary objective of anti-rheumatic therapy is to achieve remission or low activity of the disease, and hence to reduce joint inflammation, control pain, and halt the clinical and radiological progression of the disease. The assumption to conduct this study was that moderate to high levels of disease activity in a patient involve some level of poor adherence to treatment.
DAS-28 was classified into: clinical remission, low, moderate and high activity, and the CQR score was calculated in accordance with the guidelines from the DeKlerk et al. study.4 The DAS-28 result was re-classified into 2 subgroups: remission – low activity, and moderate-high activity.
Statistical analysisOperating characteristics of the receiver curves were developed, identifying the best cut-off point in the CQR score for patient classification, using DAS-28 as an approximation to the level of adherence. The intent was to maximize the sensitivity with an acceptable level of specificity. All of the analyses were conducted using the STATA version 14.1 solution.14
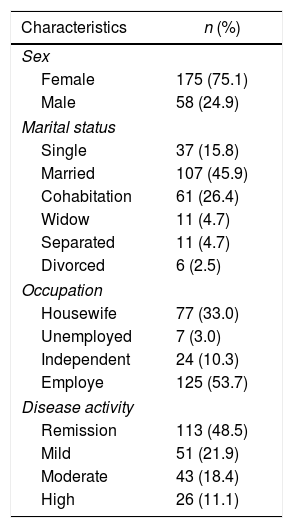
ResultsThe characteristics of the 233 patients participating in the study are illustrated on Table 2. The mean age was 46.3 (±13.9) years. The average duration of RA was 11.2 (±9.6) years.
Baseline characteristics of the participants in the calibration study.
| Characteristics | n (%) |
|---|---|
| Sex | |
| Female | 175 (75.1) |
| Male | 58 (24.9) |
| Marital status | |
| Single | 37 (15.8) |
| Married | 107 (45.9) |
| Cohabitation | 61 (26.4) |
| Widow | 11 (4.7) |
| Separated | 11 (4.7) |
| Divorced | 6 (2.5) |
| Occupation | |
| Housewife | 77 (33.0) |
| Unemployed | 7 (3.0) |
| Independent | 24 (10.3) |
| Employe | 125 (53.7) |
| Disease activity | |
| Remission | 113 (48.5) |
| Mild | 51 (21.9) |
| Moderate | 43 (18.4) |
| High | 26 (11.1) |
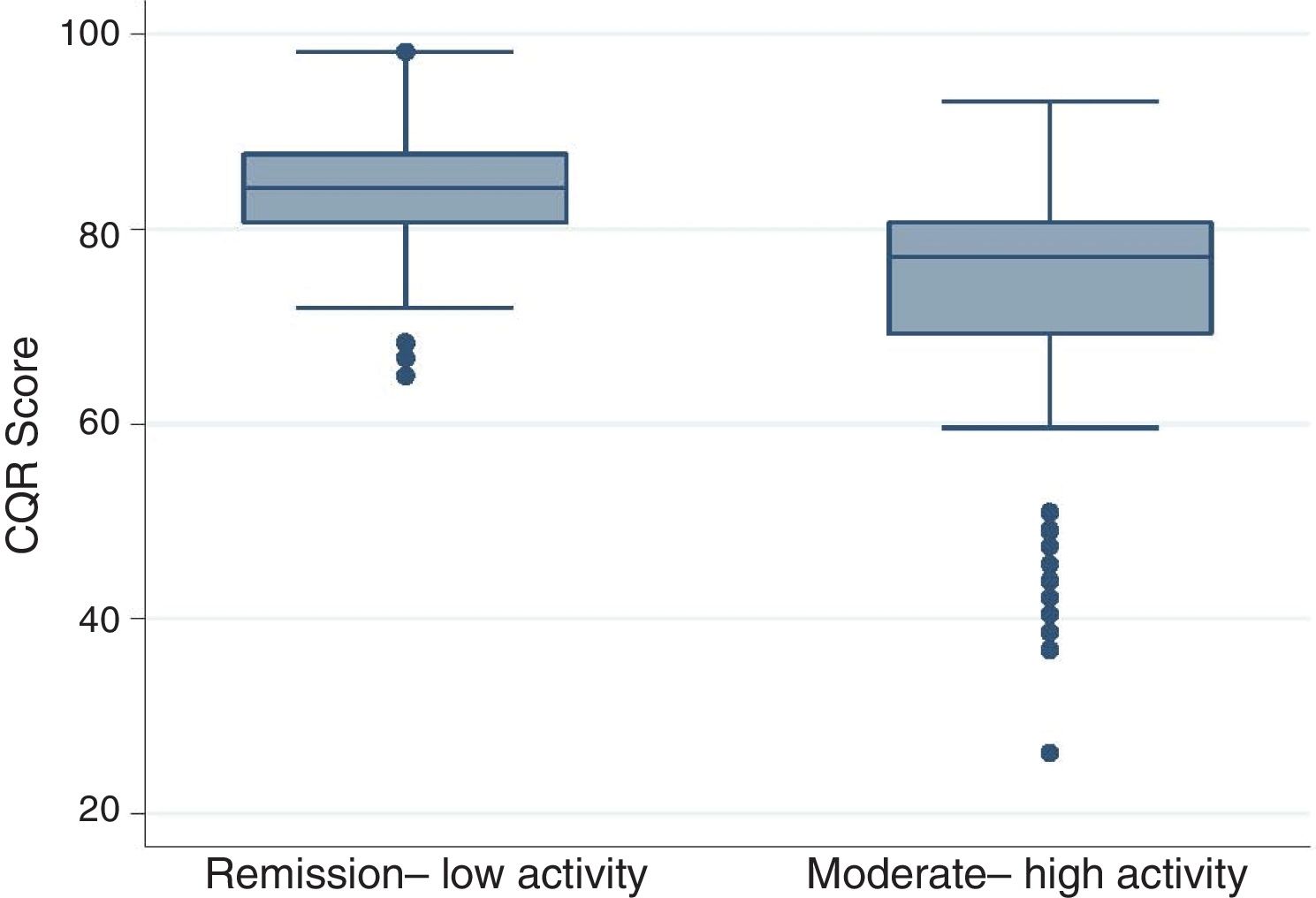
Considering that the primary objective of anti-rheumatic therapy is to take the patient into remission or to achieve a low activity of the disease (in our case, measured with DAS-28), the group was reclassified into two subgroups: patients in clinical remission and low activity (48.4%) and patients with moderate to high activity of the disease (51.6%). As seen in Fig. 1, the subjects in remission had higher CQR scores (median 84.2; RIQ 7) than those with some level of activity (median 77.2; RIZ 11.4).
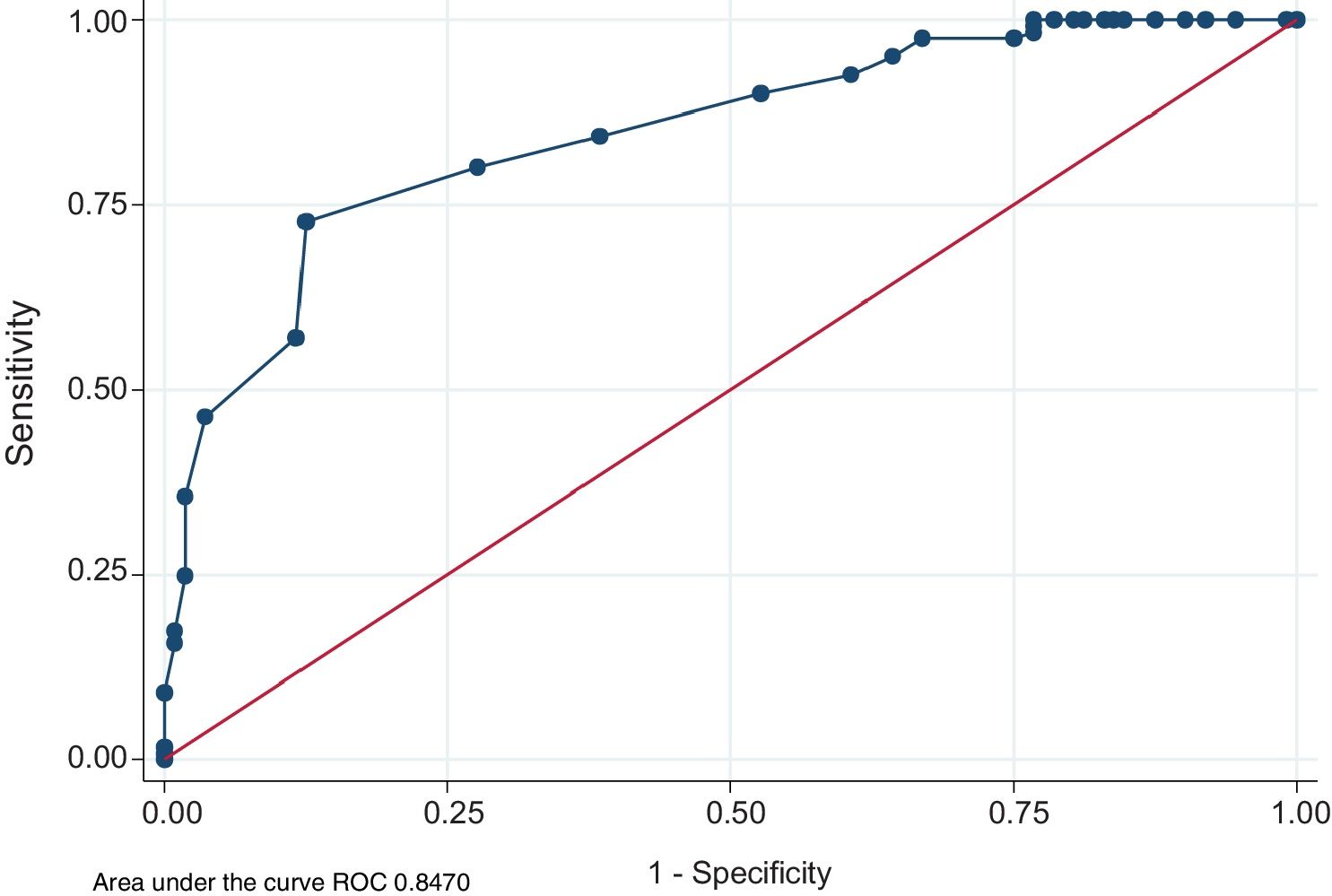
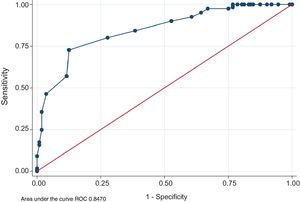
Fig. 2 illustrates the curve of operating characteristics of the receiver (ROC) for the CQR score. The area under the curve (0.84; 95% CI: 0.79–0.89) indicates that the CQR score could be used for the classification of adherence measured with DAS-28 as an approximation. For the identification of the best cut-off point, trying to maximize the sensitivity with an acceptable specificity, 80.7 was selected as the CQR cut-off point. This means that someone with a score below this number shall be considered non-adherent. This cut-off point has an 80.2% sensitivity (95% CI: 71.9–86.9%) and a specificity of 72.3% (95% CI: 63.1–80.4%). With the aim of enhancing the validity of this cut-off point, the treatment compliant and the non-compliant groups were compared, based on the CQR score, using the self-reported treatment adherence values. It could be seen that the subjects classified as compliant reported in average 10 more points of adherence than those classified as non-compliant (95% CI: 7–12).
Using a cut-off point of 80.7 to establish adherence, 43.8% (n=102) in our sample were found to be adherent to oral anti-rheumatic therapy, whilst 56.2% (n=131) were found to be non-adherent. No relationship was found between the level of adherence and the presence of adverse effects reported by patients, their age, occupation, or marital status.
DiscussionThis study developed a Spanish version of the CQR and calibrated the cut-off points in accordance with the activity of the disease measured using DAS-28, identifying a cut-off point of 80.7 that favors sensitivity without sacrificing specificity for patients with RA. The activity of the disease as an approximation to adherence has been assessed by the multicenter veterans cohort (VARA) and its results indicate that the average DAS-28 was lower in the high adherence group as compared to the low adherence group.15 However, this is a far from perfect method because of the multiple influences affecting the RA activity, beyond taking the medicine. The results of our study show that patients in remission had higher CQR scores as compared to those with moderate or high activity. Using the cut-point of 80.7, the percentage of compliant patients is low (43.8%), as compared to the findings reported by Bart et al., who found a adherence profile ranging between 60 and 68%.16 The results did not show any relationship between adherence measured with CQR ad variables such as age, occupation, marital status, or adverse events reported. The psychometric characteristics of the CQR show that the adherence results when using this instrument are valid and reliable.4,17,18 The administration of the questionnaire takes around 5min in the rheumatology outpatient clinic, and provides relevant information to discuss with the patient the decisions regarding adherence to treatment and the effects thereof.
The Spanish version of the CQR is a tool that may be used in the Colombian population with RA, in order to assess oral anti-rheumatic therapy adherence, with a cut-off point of 80.7. Considering the structure of the survey, it may be administered to assess treatment compliance in other rheumatic disease, but further studies are needed to assess the operating characteristics of the instrument in other clinical scenarios.
ConclusionA Spanish version of the CQR was developed and the cutoff points were calibrated to obtain a practical and rapid to administer tool in the clinic, with acceptable sensitivity ad specificity.
FundingThis study was financed with the researchers’ own resources, with no external funding.
Conflict of interestThe authors have no conflict of interests to disclose with regards to this research paper.
Please cite this article as: Fernández-Avila DG, Accini M, Tobón M, Moreno S, Rodríguez V, Gutiérrez JM. Validación y calibración al español del cuestionario CQR (Compliance Questionnaire on Rheumatology) para la medición de adherencia a la terapia antirreumática en un grupo de pacientes colombianos con artritis reumatoide. Rev Colomb Reumatol. 2019;26:105–110.