Systemic lupus erythematous (SLE) is a systemic autoimmune disease with the potential to involve any organ. The neurological manifestations are one of the main causes of morbidity and mortality related to SLE, and they can be expressed in the central or peripheral nervous system. Given their complexity, their diagnosis and treatment are a challenge for clinicians. Although there are plenty of helpful laboratory tests and diagnostic imaging tools to achieve a good diagnosis, there is no gold standard available yet. Finding biomarkers with adequate sensitivity and specificity are still being studied. A review is presented in this article on the specific antibodies that have been associated with, or that may trigger, the neurological manifestations in SLE, their pathophysiological importance, prevalence, and their association with this clinical presentation of the illness.
El lupus eritematoso sistémico (LES) es una enfermedad autoinmune multisistémica que puede comprometer cualquier órgano. El compromiso neurológico es una de las mayores causas de morbimortalidad en estos pacientes. Las manifestaciones pueden ser muy variadas y comprender compromiso tanto del sistema nervioso central como del periférico. Estas manifestaciones representan un reto para el clínico, puesto que son de difícil diagnóstico y tratamiento. Aunque existen diversas ayudas de laboratorio e imagenológicas que se han reportado como potencialmente útiles para el diagnóstico del compromiso neurológico en LES, no existe aún un estándar de oro disponible en el presente, por lo que esfuerzos para identificar biomarcadores que puedan mejorar la sensibilidad y la especificidad del diagnóstico del compromiso neurológico en LES son materia de estudio actualmente. Puesto que algunas de estas manifestaciones son mediadas o relacionadas a la presencia de anticuerpos específicos, en este artículo se revisan diferentes anticuerpos asociados al compromiso neuropsiquiátrico del LES, su posible rol fisiopatológico, su prevalencia y su asociación en esta forma de presentación clínica.
Systemic lupus erythematous (SLE) is a chronic autoimmune disease, characterized by multiple immunological abnormalities leading to multiple organ dysfunction.1 The clinical course of the disease if variable and usually relapsing and intermittent.2,3 The prevalence of SLE seems to be growing over time, possible due to the higher rates of early diagnosis and improved survival of the disease.1,4,5 However, the mortality rate is still high, approximately three-fold higher than in healthy individuals.6 Moreover, the mortality rate increases with disease-associated risk factors, including a high level of SLE activity, the presence of renal damage, the presence of neuropsychiatric, pulmonary or hematological disorders, or the association with antiphospholipid syndrome.6
Within the wide clinical spectrum of SLE, the nervous system dysfunction represents the major diagnostic and therapeutic challenge, since it comprises a broad range of neurological manifestations.7 Such manifestations can range from focal to diffuse, central, peripheral, isolated, complex, mild or severe presentations.8,9 And, they may present at the point during the disease,1 regardless of its active or inactive clinical status.9,10
In terms of epidemiology, the prevalence reported for neuropsychiatric SLE (NPSLE) ranges from 12 to 95%.7,10 Approximately 93% of these cases compromise the central nervous system (CNS) and the remaining 7% compromises the peripheral nervous system (PNS).9 Moreover, 60% are primary and 40% are secondary to the adverse effects associated with immunosuppressive medications or with infections.11 Finally, 40 to 50% of the manifestations are associated with an elevated SLE activity index (SLEDAI), as compared to patients with no NPSLE.12 Therefore this mode of presentation, together with lupus nephritis, represent the most severe forms of SLE, and are more often associated with a worse prognosis and poorer quality of life.7,13
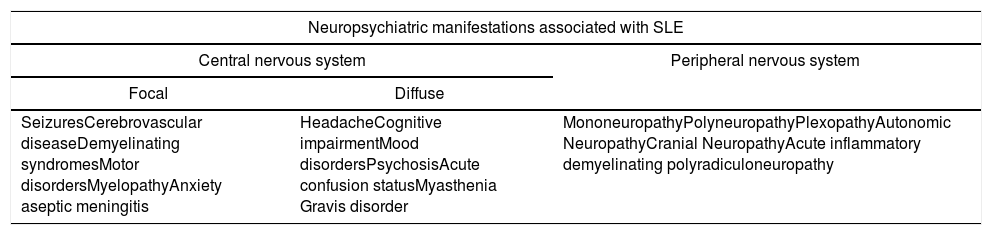
The different impacts and symptoms that may arise as a consequence of NPSLE led the American College of Rheumatology (ACR) to establish in 1999 a list of 19 neurological manifestations defining the disease (Table 1). 12 of them involve the CNS and 7 the PNS.7,8,14 Likewise, the CNS manifestations can by divided into focal and diffuse.9
Neuropsychiatric manifestations associated with SLE.
| Neuropsychiatric manifestations associated with SLE | ||
|---|---|---|
| Central nervous system | Peripheral nervous system | |
| Focal | Diffuse | |
| SeizuresCerebrovascular diseaseDemyelinating syndromesMotor disordersMyelopathyAnxiety aseptic meningitis | HeadacheCognitive impairmentMood disordersPsychosisAcute confusion statusMyasthenia Gravis disorder | MononeuropathyPolyneuropathyPlexopathyAutonomic NeuropathyCranial NeuropathyAcute inflammatory demyelinating polyradiculoneuropathy |
Notwithstanding the large number of paraclinical and imaging tools reported as potentially valuable for the diagnosis of NPSLE, there is no gold standard yet available.9 This is why efforts are currently deployed to identify biomarkers with the ability to improve the sensitivity and the specificity of the diagnosis of this pathology.
This article reviews different NPSLE-associated antibodies, their potential pathophysiological role, and the relationship to this form of clinical presentation of SLE.
MethodsLiterature search methodsA systematic search of articles was conducted until May 2018 in the following databases: Scielo, Clinical Trials, Clinics Review Article, Academic Search Ultimate, Medline, Embase and Google Scholar. The search was limited to English and Spanish literature and there were no restrictions in terms of dates. When conducting the Medline search, it was carried out through Pubmed, using the terms MeSH: Systemic lupus erythematous, Neurological involvement, Antibodies. Subsequently, a link to the Boolean connector AND was used.
Selection of articles and data miningAt the end of the search, the articles were stored in an Excel database. So any duplicate articles were excluded and the selection process was implemented from those relevant articles for this publication. The articles including the keywords in the title or in the abstract were considered. Each article was analyzed to ensure compliance with the inclusion criteria, and finally a consensus was reached among the authors to unify and check the database.
Inclusion criteria- •
Types of study: we included cohort, case, and control studies, randomized and non-randomized trials, and topic reviews.
- •
Type of population: adult patients with neurological involvement due to SLE, in whom specific antibodies were evaluated.
- •
Intervention: studies describing the function, laboratory techniques used for detection and clinical application of these antibodies.
- •
Articles for which the full text was not available.
- •
Duplicated articles and case reports.
After the initial search, 66 articles were found, most of which were available in Medline and Google Scholar. Upon excluding the articles to which full access was not possible, and the duplicated articles, a total of 56 articles were selected.
Pathophysiological mechanisms of neuropsychiatric lupusThe pathogenesis of NPSLE is particularly complex and involves a series of pathophysiological mechanisms that have not yet been fully understood.7,9 However, the current evidence suggests two probable mechanisms.
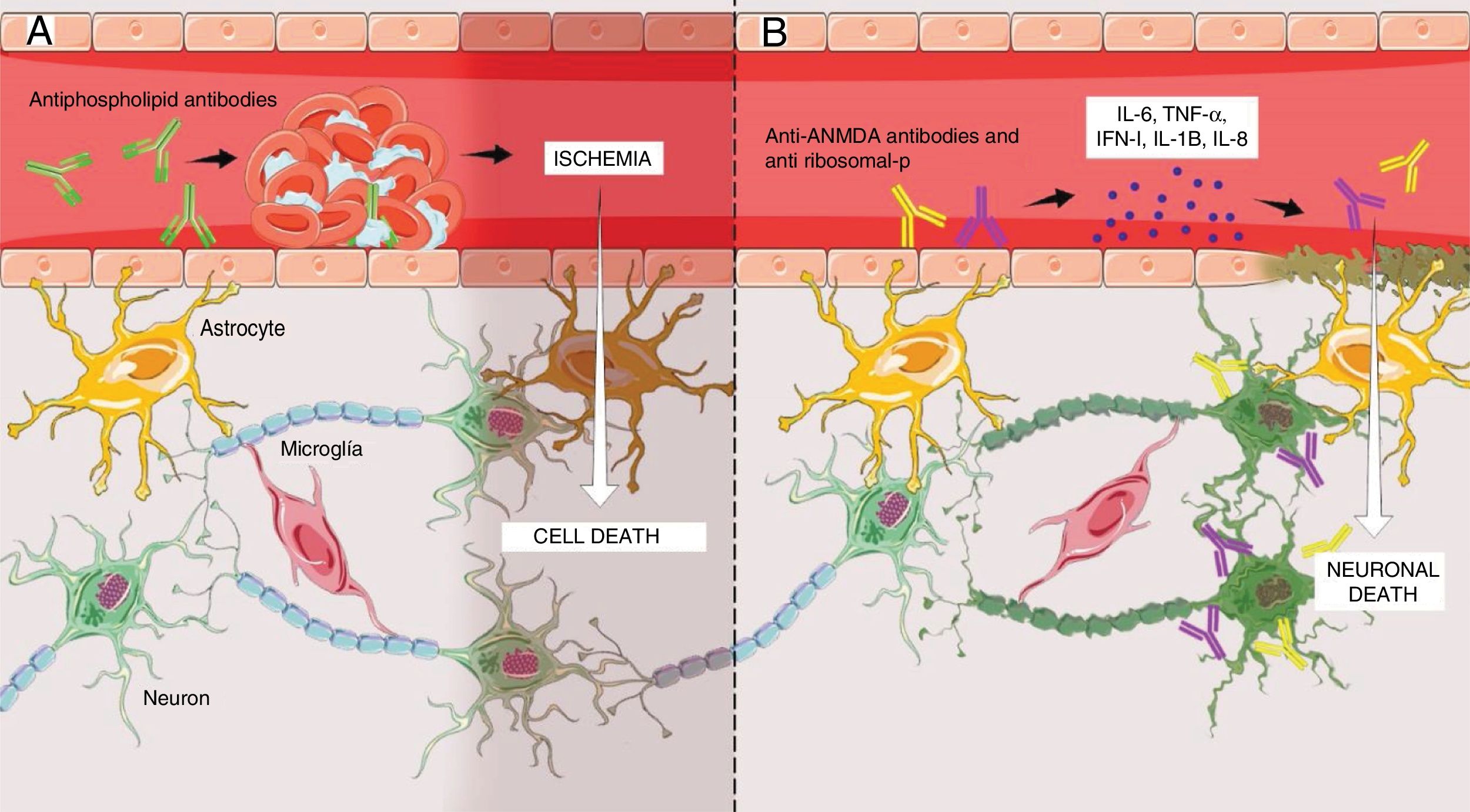
The first is a non-inflammatory vasculopathy (Fig. 1A) where the principal triggers are the antiphospholipid antibodies.7 These antibodies intervene in the coagulation cascade and bind to endothelial cells, hence giving rise to a pro-coagulating status.15 This situation results in thrombosis and ischemia of brain vessels of varying sizes, ultimately leading to the clinical presentation of NPSLE, usually of a focal nature,7,15 which in 92% of the cases are correlated with high levels of antibodies.14
(A) Antiphospholipid antibodies-mediated non-inflammatory vascular mechanism resulting in the development of micro-thrombi in the brain, with subsequent ischemia and cell death in the segment involved. This mechanism essentially explains the focal manifestations of NPSLE. (B) Inflammatory autoimmune mechanism mediated by antibodies, primarily anti-NMDA and anti-ribosomal, that promote the release of proinflammatory cytokines that could result in disruption of the endothelium of the blood-brain barrier and subsequent crossing of those antibodies into the brain parenchyma, ultimately resulting in neuronal apoptosis. This mechanism explains the diffuse manifestations of NPSLE. Figure developed by Lady J. Rios-Serna.
The second one is an autoimmune inflammatory mechanism (Fig. 1B), where the primary triggers are the anti rhibosomal-P or anti-N-methyl-d-aspartate antibodies (NMDA); however, other associations have been identified, such as the anti-endothelial and anti-neural.7 The presence of elevated levels of such antibodies in the cerebrospinal fluid (CSF) has shed some doubts among the researchers regarding whether these antibodies are formed intrathecally or peripherally.7,9 The results of the research indicate that antibodies are formed peripherally and then cross a dysfunctional blood-brain barrier (BBB)7 that has previously been made permeable by factors such as hypertension, smoking, and infections,7,15 or by SLE-dependent factors such as the deposit of immune complements in the endothelium, promoting the release of cytokines (IL-6, IL-8, IL-1β, TNF-α, INF-1) that cause cell inflammation and finally disruption of the BBB, to facilitate the crossing of antibodies.7,15–20 Once inside the brain parenchyma, these induce cell death through direct action and through the release of proinflammatory cytokines on the neurons.19 It should be highlighted that some studies have also shown that antibodies may be formed intrathecally,7,9,15 and that they have a particular tropism on the neurons located in the brain areas responsible for memory and behavior, such as the hippocampus and the amygdala.19 For this reason, this second mechanism has been associated with diffuse NPSLE.7,9
Anti ribosome P antibodiesThese are antibodies that recognize an antigenic determinant of 22 amino acids located at the C-terminal chain of 3 ribosomal proteins (P0, P1; P2).19 This antigen is also shared by protein NSPA (Neuronal Surface P Antigen) which is expressed on the cell surface of neurons distributed in the areas of the brain that are responsible for controlling emotions, cognitive functions, and memory.21–23 This antibody, via its direct action on such neurons, causes protein synthesis inhibition,22 calcium inflow into the extracellular space, and finally leads to cell apoptosis.24 High titles of anti ribosome P antibodies have been mostly associated with diffuse NPSLE,25 and psychosis is the manifestation they have been most often correlated to.19,21,26 Their first association was shown in a study in which the titles of these antibodies were elevated in 18 (90%) of 20 patients with SLE that presented psychosis; in contrast, in other diffuse manifestations of NPSLE, they were elevated in only 15%.27 Though the results are controversial, there is ongoing research to determine their association with other diffuse manifestations, such as depression and cognitive impairment.21,23
On the other hand, a meta-analysis including 1537 patients concluded that the sensitivity and specificity of the anti ribosomal P antibodies for diagnosing NPSLE are 26 and 80% and, for the diagnosis of psychosis or mood disorders, are 27 and 80%,28 respectively; further studies have shown that the titles of these antibodies rise before and during the episodes of psychosis, or during the periods of clinical activity of SLE, hence suggesting a potential predictive value in the identification of future crisis.23,27
Anti ribosomal P antibodies are detected through ELISA or immunoblot,29 and may be present both in the blood or in the CSF of patients with NPSLE.21,23 Finally, their sensitivity and specificity in patients with SLE but not with NPSLE is 37 and 96%, respectively,27 and their prevalence varies according to age, with a higher prevalence among young individuals as compared to adults (20–40% vs. 10–20%),19,27 and lower in Caucasians that in Asians (6–20% vs. 36–40%).29,30
Anti-N-methyl-d-aspartate antibodiesThese have been described as a subset of double-stranded anti-DNA antibodies that cross react against the NR2 subunit of the N-methyl-d-aspartate (NMDA) receptor.31 This receptor is widely distributed across different areas of the brain, including the amygdala, the anterior hypothalamus, and the hippocampus.32 Several studies have shown that the effect of these antibodies on said receptor is dose-dependent: the receptor is activated at low concentrations, promoting neuronal excitation, while at high concentrations promotes over-excitation and subsequent cell apoptosis.31,33 Interestingly enough, no neuronal loss has been observed when the BBB remains intact, giving rise to the hypothesis that other factors are needed to make the BBB permeable for anti-NMDA antibodies to cross and make an effect on the neurons.9,14 Elevated titles of these antibodies, both in serum as in the CSF of patients with SLE,31 have been associated with cognitive impairment, primarily short-term memory involvement.14,22 One particular article showed that the prevalence of anti-NMDA antibodies in patients with cognitive impairment and SLE was 31%,34 close to the findings of another study with 48%.35 Furthermore, they have been associated with mood disorders, such as depression.9,22 However, the relationship of these antibodies to such NPSLE manifestations is still controversial, due to the differences among the various clinical and laboratory methods used in the studies.35–37 Finally, in patients with SLE but without NPSLE, a prevalence between 25 and 50% has been reported.22,33,34
Antiphospholipid antibodiesOne of the most frequently studied antibodies with regards to NPSLE are the antiphospholipids (anti-cardiolipin, lupus anticoagulant, and anti-β2-glycoprotein).25 In patients with SLE and concomitant antiphospholipid syndrome (APS) a higher risk of thrombosis has been identified due to the presence of the anti-β2-glycoprotein that may affect any site in the body, including the CNS.38 Furthermore, another research study conducted to assess the relationship between antiphospholipid antibodies and NPSLE, found that among the patients that met the criteria for APS, the prevalence of NPSLE was extremely high, with elevated titles for both IgG and IgM anti-cardiolipins.39 Another recent study further emphasizes the importance of actively searching the antiphospholipid antibodies in patients with SLE, since around 40% of these patients have them, even without clinical manifestations of APS, and are associated with a higher risk of both focal and diffuse neurological complications.40 There are then two relevant aspects: the presence of these antibodies points to a higher risk of neurological manifestations in patients with SLE, and the prevalence of these antibodies in these patients is extremely high; hence the extreme relevance of determining their positivity.41
Other antibodies associated with neuropsychiatric systemic lupus erythematous- -
Anti-neuronal antibodies. It has been said that these antibodies are able to react against a broad range of neuronal antigens of 22–130kDa; however, their clear identification has escaped us.42 Numerous research studies have shown elevated titles of these antibodies in serum and in the CSF of patients with diffuse NPSLE.43–46 One study found high titles in 20 (74%) of 27 patients with NPSLE, as compared with only 2 (11%) of 18 that were positive, without NPSLE.44 Another study found elevated titles in 15/50%) of 31 individuals with NPSLE, in contrast to only 4 (29%) of 14 without NPSLE.44 One more study found elevated titles in 13 of 15 patients with NPSLE,45 while one last study reported elevated levels in 39 of 41 NPSLE patients.46 Higher concentrations of these antibodies have been observed in patients with psychosis as compared against the non-psychotic individuals; furthermore, the levels drop when the acute episode improves.46 The above findings show an important association of these antibodies with NPSLE; however, studies with a larger number of patients are needed to determine their diagnostic sensitivity and specificity.
- -
Anti-endothelial antibodies. These are also a heterogeneous group of antibodies reacting against various antigens in the membrane of endothelial cells.22,47 However, research is currently underway to identify one antigen in particular.47 Anti-endothelial antibodies have been associated with the endothelial disruption of the BBB in patients with NPSLE, a mechanism that as mentioned above, is extremely important in the pathogenesis of the disease.47 One study showed elevated titles of these antibodies in 5 of 14 subjects with NPSLE,48 mainly in those with psychosis and depression.49
- -
Anti-aquaporin 4 antibodies. Aquaporins (AQP) are transmembrane channels responsible for water transport through the formation of pores on the cell membrane. AQPs can be found in different sites of the human body and play various physiological roles. In humans, the AQPs able to transport water are AQP0, AQP1, AQP2, AQP4, AQP5, AQP6, and AQP7. In this case, emphasis is placed on AQP4,50 which is found in the astrocytes and glial cells as the only AQP present in these cells.51 In 2004, an anti-IgG1 antibody was identified, with a specificity of 91% and a sensitivity of 73%, which binds to AQP4 expressed in astrocytes and was associated with neuromyelitis optica (NMO), which was considered until then a variant of multiple sclerosis.52 The IgG-NMO or anti-AQP4 are IgG1 autoantibodies that bind to AQP4 and activate the complement, hence initiating the deposit of immune complexes that attack the cell membrane. An important leukocyte infiltrate is produced, that triggers a CNS injury with subsequent myelin loss,53 finally leading to NMO or Devic's disease – a demyelinating inflammatory autoimmune CNS disease whose diagnosis is based on three criteria, including the presence of an IgG-AQP4 positive serology.50 In a study with 89 patients with SLE, 2.2% of them had anti-AQP4 antibodies for 11 years, without any NMP manifestations.54 However, 1–2% of the patients with SLE develop myelitis, of which between 21 and 48% present in the context of neuromyelitis optica spectrum disorder (NMOSD). Moreover, this study showed a low incidence of NMOSD with positive AQP4 in 0.54%54 of the cases. Hence, it may be argued that whilst anti-AQP4 may be present in patients with SLE, it is a rare occurrence. Therefore, although they may be responsible for some neurological manifestations, these are not the antibodies with the major impact on NPSLE.
Other tools, in addition to serum antibodies, have been described in the literature to support the diagnosis of this type of complications. One of them is the administration of neuropsychiatric tests, in which the most frequent diagnosis in patients with confirmed NPSLE is attention deficit disorder.20 Another option is the analysis of the CSF, though in some cases it may be normal20; nevertheless, proteinomics studies have been introduced to differentiate markers specifically expressed in NPSLE.55 The use of the quantitative encephalogram has been considered because of its 80% sensitivity to identify the CNS involvement and for its contribution of objective measures regarding the severity of said compromise.56 Moreover, nuclear magnetic resonance excels as the most helpful imaging technique to rule out differential diagnoses and to identify abnormalities in individuals with focal symptoms.20
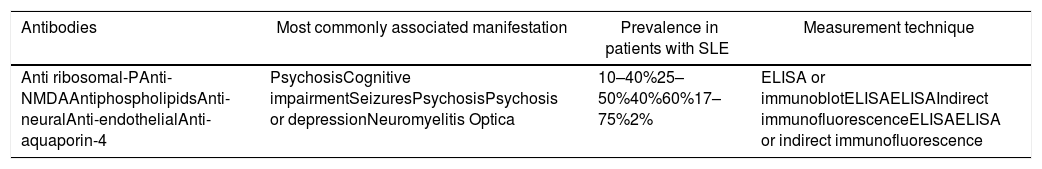
ConclusionNotwithstanding the fact that NPSLE is a condition for which no diagnostic gold standard is yet available, the number of studies focusing on an understanding of its pathogenesis should be highlighted. These studies have guided the clinicians towards the search for biomarkers associated with the manifestations of the disease. Some of those strongly linked to the condition are the antiphospholipid antibodies, the anti ribosomal-P, and the anti-NMDA. Consequently, it is important to do further research directed at showing their value in patients with NPSLE with a view to using them in our future clinical practice (Table 2).
Principal antibodies associated with manifestations of neuropsychiatric lupus in patients with SLE.
| Antibodies | Most commonly associated manifestation | Prevalence in patients with SLE | Measurement technique |
|---|---|---|---|
| Anti ribosomal-PAnti-NMDAAntiphospholipidsAnti-neuralAnti-endothelialAnti-aquaporin-4 | PsychosisCognitive impairmentSeizuresPsychosisPsychosis or depressionNeuromyelitis Optica | 10–40%25–50%40%60%17–75%2% | ELISA or immunoblotELISAELISAIndirect immunofluorescenceELISAELISA or indirect immunofluorescence |
The authors have no conflict of interests to disclose.
Please cite this article as: Marín J-D, Posso-Osorio I, Vargas S, Nieto-Aristizábal I, Ríos-Serna LJ, Tobón GJ. Anticuerpos asociados al lupus neuropsiquiátrico: rol fisiopatológico, prevalencia y utilidad diagnóstica. Rev Colomb Reumatol. 2019;26:111–117.