Pannus refers to synovial tissue proliferation, and has been considered a late, inactive and irreversible manifestation of rheumatoid arthritis (RA), contrary to historical findings. A literature search was performed on terminology about pannus and its historical role in the pathophysiology of RA. Light microscopy studies have shown the destructive impact of pannus tissue with very specific abnormalities, corroborated a year later with electronic microscopy. Some of these findings are the isolation of the immunological cells inside the tissue, especially one cell line with particular capacities, called synoviocytes similar to fibroblasts. This cellular component is the source a large quantity of cytokines and proteinases that perpetuate and cause bone and cartilage damage. Inflammation has been seen in many image techniques, such as magnetic resonance and ultrasound. These show the role of tissue widening and hyper-vascularization in tissue damage, and some reversibility after treatment of RA. With the evidence presented it is possible to conclude that pannus refers to a histological (more than clinical) term for synovial hypertrophy, and includes a large component of cell activity that generates and perpetuates inflammation and thus the disease.
La proliferación del tejido sinovial, que es llamada pannus, se ha considerado como una manifestación tardía, inactiva e irreversible de la artritis reumatoide (AR), contrario a lo que históricamente se ha estudiado. Se realizó una búsqueda de la literatura para realizar una revisión narrativa e histórica respecto al surgimiento del término pannus y su papel en la artritis reumatoide. Estudios de microscopia de luz han mostrado el carácter destructivo de este tejido con hallazgos característicos de la AR, corroborados con microscopia electrónica años más tarde. Estos hallazgos llevaron a caracterizar el componente celular del pannus con gran número de células inmunológicas y de líneas celulares específicas con propiedades especiales como los sinoviocitos similares a fibroblastos. Este componente celular es el origen de una gran cantidad de citoquinas y proteinasas que perpetúan y causan el daño óseo y del cartílago. Este componente inflamatorio ha sido evidente también con el desarrollo de técnicas de imágenes, como la resonancia magnética y la ultrasonografía, que muestran un papel activo del tejido sinovial engrosado, junto a la hipervascularización en el daño articular y la reversibilidad de estos cambios tras el tratamiento. Las evidencias contempladas permiten concluir que el pannus como evidencia histológica (más que clínica) se refiere a la proliferación del tejido sinovial e incluye un gran componente celular activo que genera y perpetúa la inflamación y, por tanto, la enfermedad.
Since the initial clinical description of rheumatoid arthritis (RA), pannus has played a key role in the development of the disease and its understanding has improved alongside with the development of histological, molecular biology, and imaging techniques.
In the clinic, pannus has been considered a late, inactive, and irreversible manifestation of the disease, although the term is purely histological. The confusion becomes increasingly apparent when dealing with the clinical monitoring of RA and the use of terms such as pannus, synovitis, or chronic synovitis, with a blurred idea about the activity of the disease.
A search of the literature in Pubmed and Embase was conducted for this review, using the terms pannus, rheumatoid arthritis, ultrasonography, magnetic resonance, from 1900 to 2015. Non-indexed texts and books on the topic were searched, in order to make a narrative and historical description about pannus, its development, and its pathological role in RA.
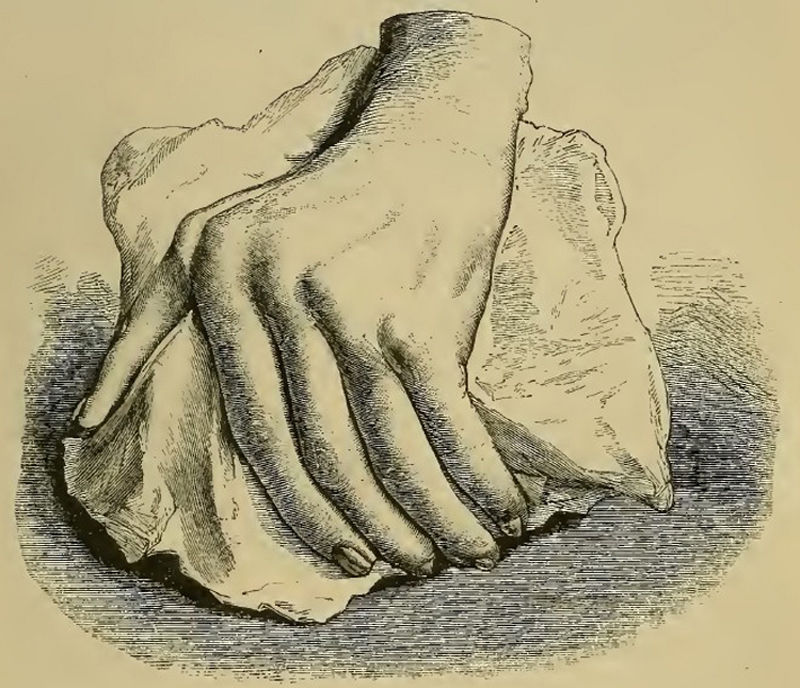
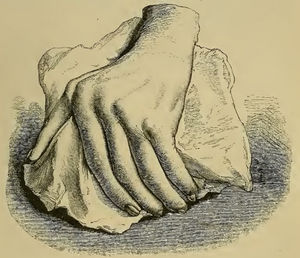
The initial clinical and histological description of pannus in rheumatoid arthritisAlthough similar terms to RA had been used prior to 1859, it was Garrod (Fig. 1) who in Chapter XV of his book “The nature and treatment of gout and rheumaticgout”1,2 accurately described the disease as we know it today, differentiating it from gout and rheumatic fever. He offered detailed illustrations (Fig. 2) of the typical deformities and underscored the serious nature of the disease as compared to other conditions, due to the difficulty in controlling the disease and to its disabling clinical evolution (Fig. 3).
Photograph of Sir. Alfred Baring Garrod, 1819–1907.3
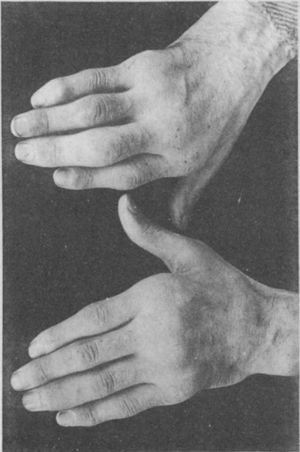
Illustration from Garrod's book depicting a deformed hand with ulnar deviation resulting from rheumatoid arthritis.1,2
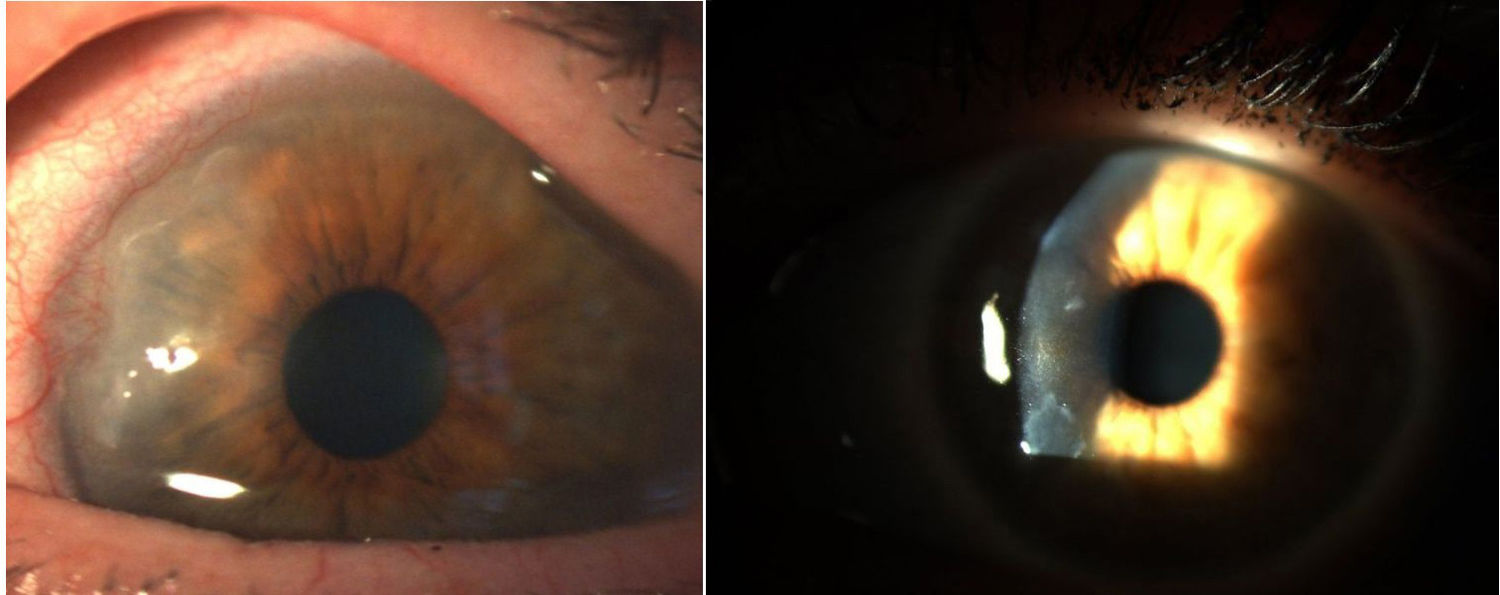
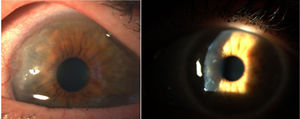
Corneal pannus.8
Despite a very thorough clinical description of RA, Garrod failed to mention the joint involvement from the histopathological point of view. Decades later, William Ord, a physician at the St. Thomas's Hospital in London in his manuscript published in the British Medical Journal in 1880, tried to clarify some doubts about the term RA which as he pointed out, was frequently used in a non-specific manner to classify different diseases. Ord suggested that the term RA should only be used for the type of inflammatory joint involvement with effusion different from gout, rheumatic fever (rheumatism), or scrofula, which is characterized by bone and cartilage atrophy, in addition to synovial hypertrophy. This statement pointed to a relationship with the pathophysiogenesis of RA.4
In 1905 and 1909, Nichols and Richardson in their work published in the Journal of Medical Research and in the Boston Medical and Surgical Journal5 respectively, submitted one of the best clinical-pathological descriptions of rheumatic diseases, and differentiated 2 groups of patients: one depicting the clinical characteristics similar to RA and the other the characteristics of osteoarthritis.6 Notwithstanding the fact that both articles by Nichols and Richardson refer to cases of non-scrofulous deforming arthritis, the thorough representation of the clinical manifestations, accompanied by pictures and radiographic images, clearly depicts cases of RA. These authors also compiled a number of histology specimens from 75 clinical cases, arriving at an important conclusion: there are 2 histological patterns of joint involvement, one of deterioration of the joint cartilage, and the other of thickening of the synovial membrane. The latter is closely associated with the invasion of the bone tissue and the joint cartilage, causing destruction and occasionally disorganized bone neoformation. This same publication by Nichols and Richardson includes a description of proliferative arthritis with very vascularized granulation tissue formation, with a mesenchymal origin cell component, primarily of the synovial membrane and of the so called pannus-like tissue. This is the first time that the term articular pannus was used.5 It should be highlighted that the term pannus had already been used at that time in ophthalmology to describe some type of corneal lesions, and was defined by the American Academy of Ophthalmology as fibrous and vascular tissue growth between the epithelium and Bowman's membrane, often present in cases of chronic corneal edema, following the inflammation of the cornea7 (Fig. 7). In both cases, synovial pannus and corneal pannus, the lesions described are similar to a pannus, the Latin word for “cloth”.
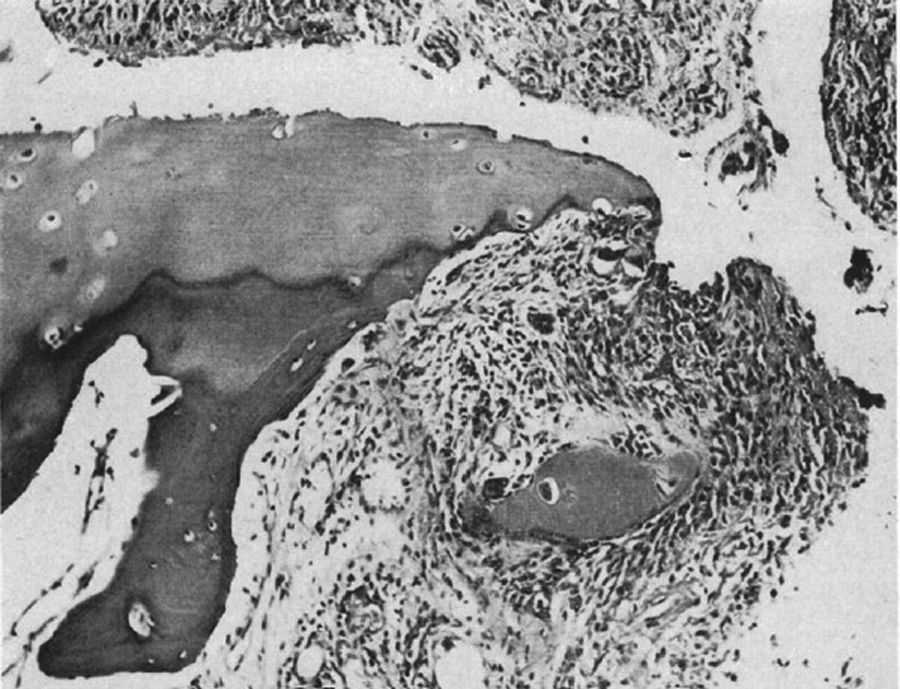
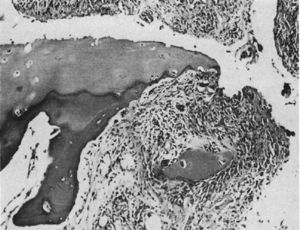
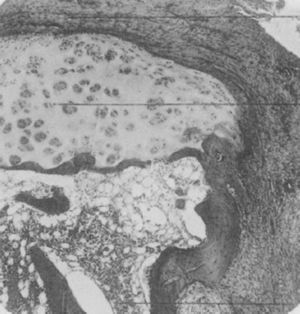
Light microscopy histology. Destructive lesion at the interphalangeal joint margin. The synovial tissue or pannus can be appreciated next to the granulation tissue – the so called clasp movement isolating a fragment of the cartilage marked “C”.9
Some of the images and descriptions in the study by Nichols and Richardson in 19095 are depicted in Figs. 4–6.
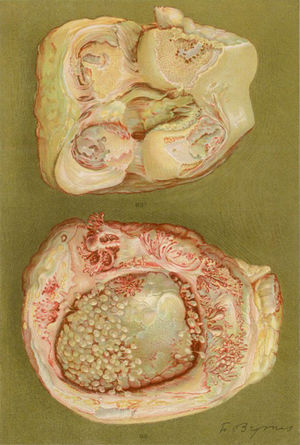
Light microscopy photograph of the distal phalanx of a patient suffering from the disease then called extreme type proliferative arthritis. It shows the formation of thick granulation tissue invading and destroying the articular cartilage.5,6
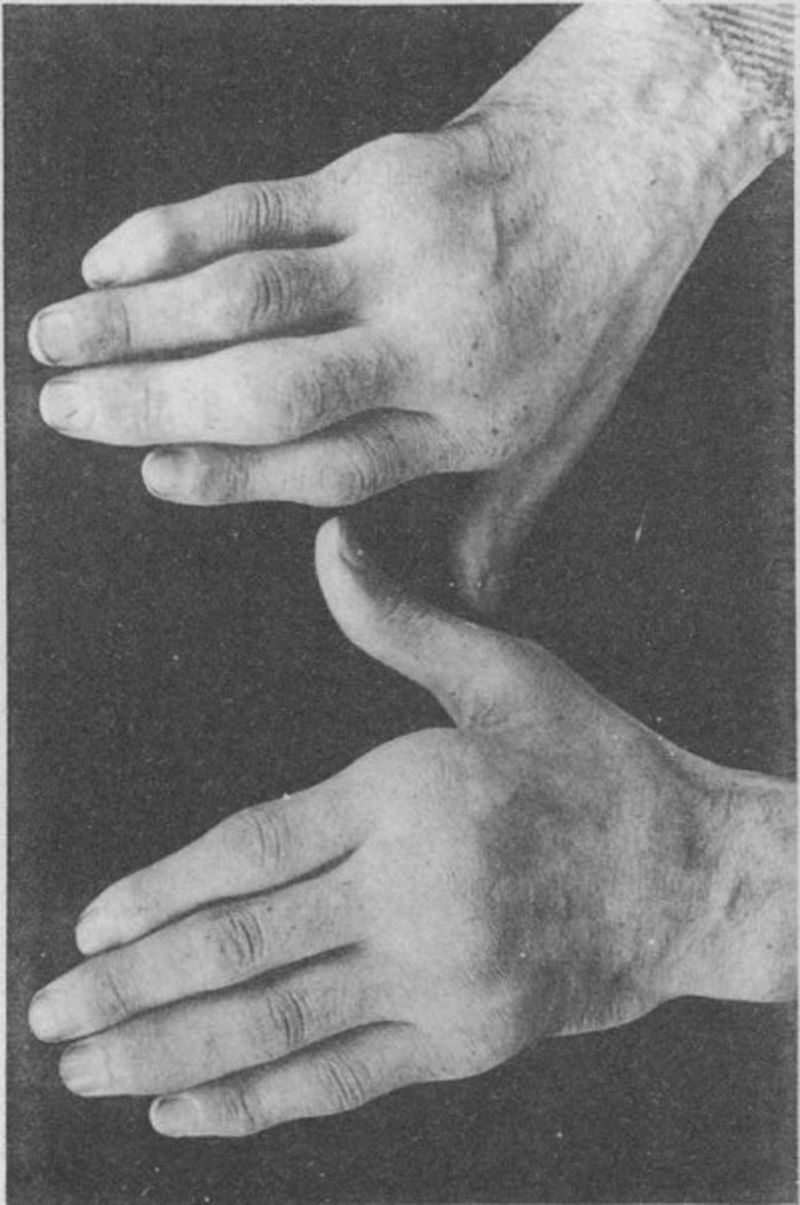
Photograph of the hands of a 35-year old patient with a history of gonococcal urethritis, who years later progressively developed polyarthritis of the small and large joints. Classified as proliferative arthritis.5,6
Drawing of the necropsy of a 40-year old woman with a clinic of polyarthritis of the large and small joints leading to significant disability and ankyloses. There is evidence of loss of the joint cartilage and bone formation zones giving rise to knee ankyloses.5,6
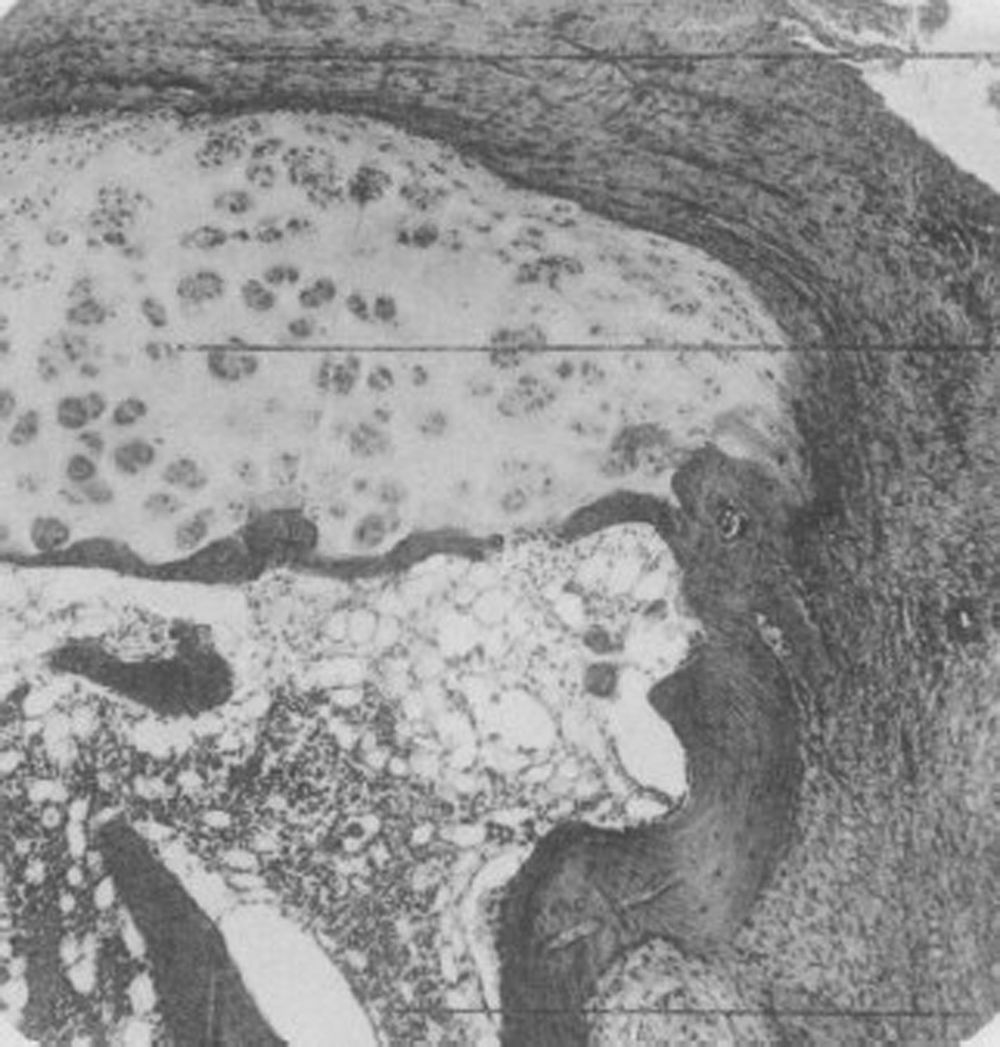
In 1959 Kulka published a review of the pathogenesis of RA,9 using the work by Nichols and Richardson5 as a reference, which classified the histological lesions in RA into 3 types: The first type was classified as inflammatory changes of the synovial membrane, the second as connective tissue degeneration and necrosis, and the third as vascular inflammatory infiltration of the arterioles and venules. The first type of lesions corresponds to the pannus described in 1909 by Nichols and Richardson, consisting of a synovial thickening that causes cartilage resorption. Kulka found using light microscopy that on the one hand, the destructive lesion of the cartilage was characterized by the chronically inflamed synovial tissue growth – or pannus – and on the other, that the granulation tissue that proliferated from the subchondral bone marrow spaces and extended through the subchondral plate to attach to the pannus and surrounded it with a clamp motion, may progress to isolate fragments of the marginal cartilage (Fig. 7).9
Kulka in his paper defined the histological criteria to diagnose RA, if at least 3 of the 5 distinctive characteristics were met, in the absence of any other probable diagnosis:
- 1.
Villous proliferation
- 2.
Proliferation of the superficial synovial cells, usually palisading
- 3.
Marked infiltration of inflammatory cells (lymphocytes or plasma cells) with a tendency to develop lymphoid nodules
- 4.
Compact fibrin deposit
Although emphasis was placed on the fact that none of these were specific, the combination of 3 or more characteristics in the absence of other pathologies was of considerable diagnostic value for RA (Fig. 8).
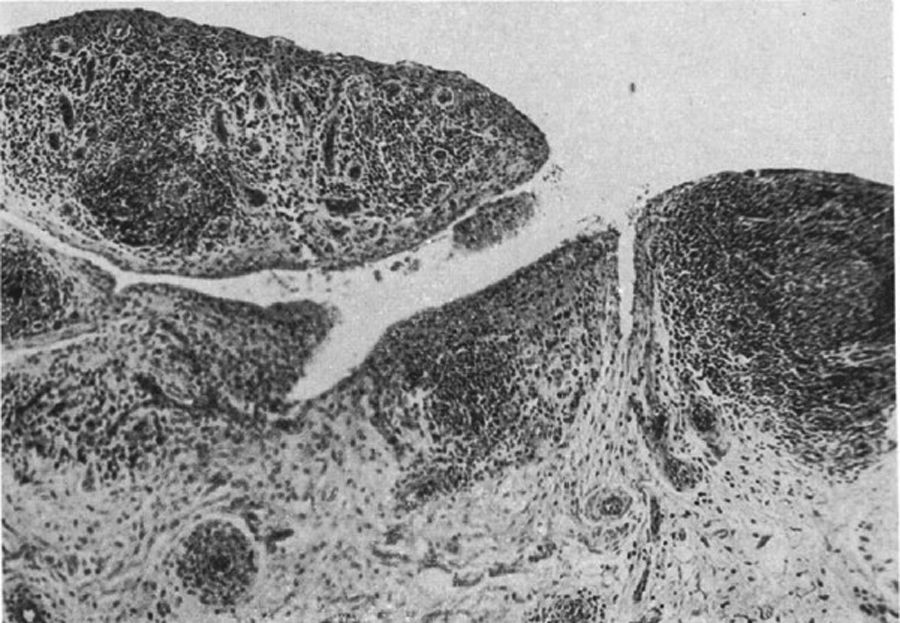
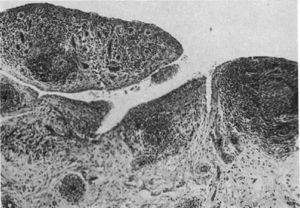
Light microscopy histology. Classical knee synovitis in RA depicting villous hypertrophy, vascular infiltration and lymphoid nodules with a germinal center in the right corner.9
In 1975, Kobayachi and Ziff10 conducted electron microscopy studies of pannus and its attachment to the cartilage, in order to contribute to the understanding of joint damage. Although back in 196011 Ziff had already described alterations of the synovial membrane, both authors described that lysosomal proteases could develop inside the pannus, which degrade the mucoproteins in the cartilage and could be secreted into specific sites, such as the articular margins where the initial pannus production takes place; in other words, at the interface of the synovial membrane and the articular cartilage.12
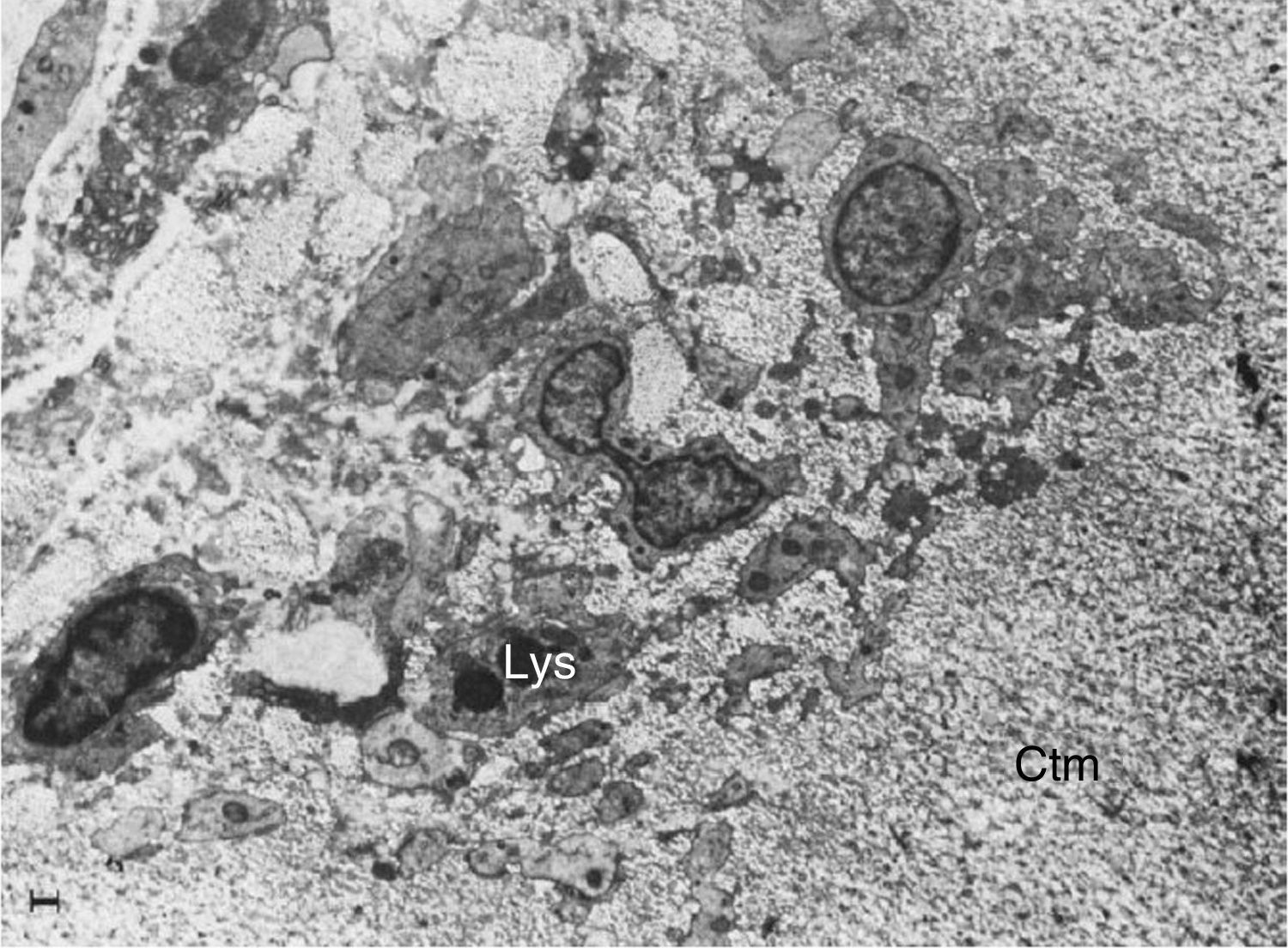
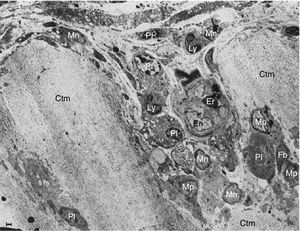
These researchers analyzed samples of the synovial-cartilage interface in 8 patients with a diagnosis of RA, and identified 3 types of lesions: the first with a mononuclear cell infiltration of the vessels encroaching into the cartilage, occasionally forming cell nests or aggregates inside the cartilage matrix (in addition to plasma cells, lymphocytes and granulocytes). Moreover, dissolved circulating collagen was also found inside these nests, which led to the theory of a proteolytic enzyme secretion (Fig. 9). The second type of lesions correspond to a direct invasion of the cartilage of monocytic cells and poorly differentiated fibroblasts that can manifest as phagocytic or fibroblastic, with an amorphous cytoplasmic granular content with cell prolongations that degrade the cartilaginous collagen matrix and can be seen inside the cellular extensions (Fig. 10). The third type of lesion presents as a thick layer of fibrous and vascularized tissue covering the cartilage and interfering with the normal vascularization of the articular cartilage.
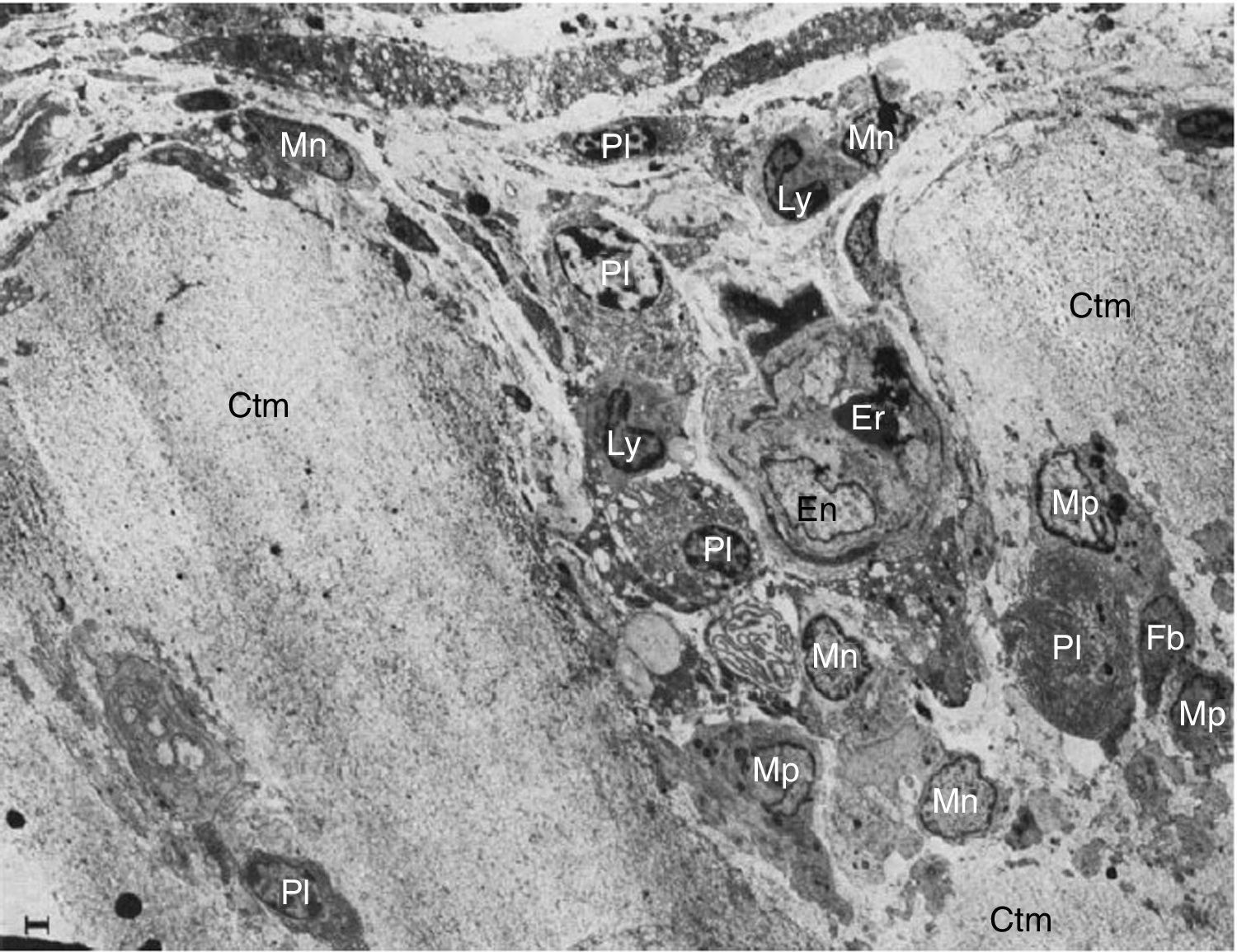
Electron microscope images of pannus. Illustration of a vessel invasion (En: endothelium; Er: erythrocyte) at the pannus/cartilage interface (Ctm) by lymphocytes (Ly), plasma cells (Pl), monocytes (Mn), macrophages (Mp) and fibroblasts (Fb).10
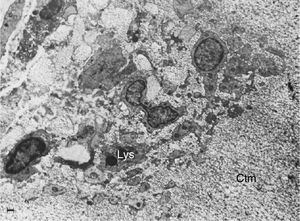
Electron microscope image showing fibroblasts with immersed lysosomes (Lys) and multiple cytoplasmic extensions invading the cartilage matrix (Ctm).10
Over the next 2 decades, the leading role of pannus in the pathophysiology of joint disease in RA was not only limited to describing the destruction of the cartilage. Edward Harris in 199013 published one of the most comprehensive reviews on the pathophysiology of RA, classifying it into 5 types, in accordance with the immunological characteristics of the joint damage. He described that in the initial stages (stage 2) the non-thickened synovia and cytokine-mediated neovascularization of the macrophages14 with accumulation of the perivascular lymphocytes played a role.15 As previously demonstrated by Kobayashi10 using electron microscopy, lymphocytes – mostly T CD4+16 with scarce T regulators17 – activate the local B lymphocytes maturing to antibody-producing plasma cells in the synovial tissue,18 compromising this tissue as part of the onset and progression of the disease. Harris also claimed that in more advanced stages of the disease (stage 4) a profuse proliferation of the synovia with up to 100 fold its normal weight, which was interpreted as a behavior similar to that of a tumor with increased cell activity of the synoviocytes, as was confirmed in enriched culture medium where the cells grew isolated and autonomously,19 leading to consider for the first time the oncogenes model, as is the case in cancer.
Pannus cell componentsThough several researchers continued to study the thickened tissue or pannus component, no agreement was reached with regards to its pathogenesis. In 1983, Burmester et al.20 studied pannus non-lymphocytic cells via monoclonal antibodies targeted against macrophage and fibroblast antigens. They identified 3 types of synovia line cells; the first were mostly (40% of the total) monocytic cells or macrophages with phagocytic ability, with Fc receptors and HLA DR expression or type II. The second cell type was similar to monocytes, but non phagocytic, with HLA-DR expression and no other monocytic antigens found. The last cell line found was positive for fibroblast markers, but with no HLA-DR expression or other monocytic antigens (between 20% and 40% of the total), which was confirmed in 1987 with cloned cells and cell cultures.21 The significance of these synovial cells was among other factors, the activation of the proto-oncogene c-jun in response to the stimulus of the IL-a,22 which is similar to the neoplastic cells that increase the collagenase expression with the ability to destroy, together with stromelysin and other metalloproteinases, the cartilage and bone matrix,23,24 a finding previously attributed to Ziff back in 1960.11
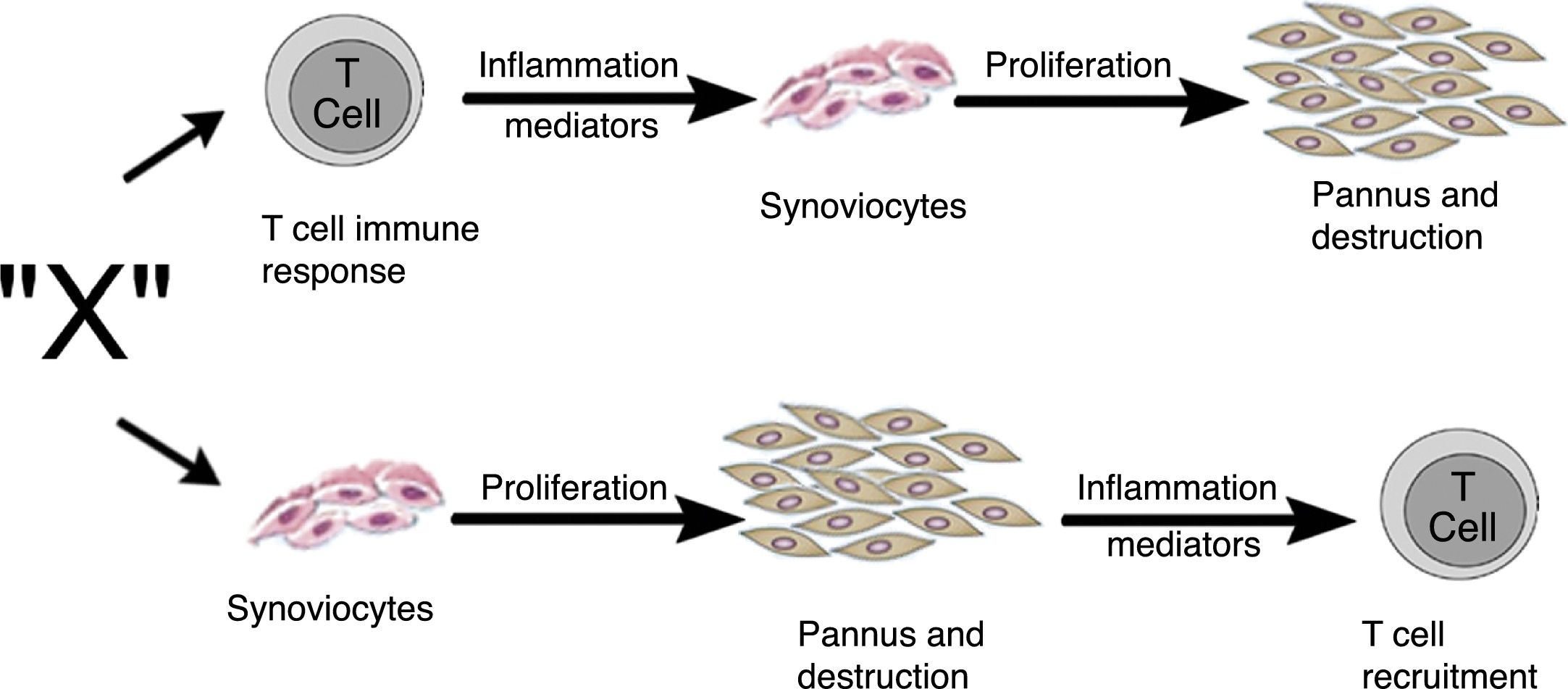
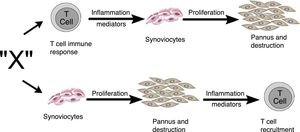
Zvaifler and Firestein25 in 1994 considered that the origin of pannus was unclear and that the evidence was divided into a chronically inflamed synovial tissue, and a specialized, highly vascularized connective tissue that started at the synovia-bone interphase and historically had been defined as the site of onset of the destructive process.5,10,26,28 Two possibilities about the origin of these synoviocytes were considered in this review, which are similar to fibroblasts and were given the name of pannocytes.20 The first hypothesis was that the immature mesenchymal cells were the first to invade the vessels that penetrate the cartilage and that could be replaced o could evolve to fibroblasts,27 in a process mediated by unknown inflammatory factors (factor X), independent from immune T-cell derived factors, as has been also shown in animal models.29,30 The second less supported hypothesis, and based on studies conducted early in 90s,31-33 was dependent on the cytokines of lymphocytes and described the reduced genome expression of lymphocytes and lymphocytic products in the tissues and in the synovial fluid (Fig. 11).
Alternate pathways through which the unknown factor “X” in RA could induce the formation of pannus. It may start through the T-cells dependent pathway or through the synoviocytes pathway.25
The review by Zvaifler and Firestein25 attempts to understand, not just the origin, but the perpetuation of pannus, based on 2 possibilities: one – as mentioned back in 1989 – considers a tumor model in which the synovial cells are transformed by a signal triggering proliferation and inducing the expression of oncogenes and the production of enzymes that degrade the matrix or adhesion proteins.34,35 These signals may stem from soluble molecules such as cytokines or from unspecified etiological agents. From this perspective, tissues such as cartilage or bone, are not part of their development, but are affected by pannus. The second possibility is based on the idea that compounds generated by chondrocytes or immune complexes immersed in the cartilage are the ones responsible for activating the synoviocytes to invade the tissue and abandon their natural environment.
However, the process whereby the fibroblast-like synoviocytes, lacking Fc receptors, are affected by these immune complexes36 has not been established, but it is likely that the monocytic-macrophage cells are the intermediaries. A likely explanation of this event is the binding of complement proteins and immune complexes to vitronectin and its receptors, which affects the differentiation, migration, and proliferation of fibroblasts and which is subsequently amplified by the contact between these cells and collagen, fibronectin, and other metalloproteinases,37,38 via the expression of receptors such as VCAM-1, which until then was the only known adhesion molecule overexpressed in the fibroblast-like synoviocytes.39
According to this second scenario, the cartilage will not only be the pannus affected tissue, but the inducer and perpetuator of this response. Notwithstanding the above, it is still clear that the conservation of the pannus depends on the cytokines that stimulate cell growth and of the production of enzymes synthetized by the monocyte-derived synoviocytes40 and of the fibroblast-like synoviocytes. It was shown in vitro that the enzymes were synthetized41 via the regulation and intermediation of T-lymphocytes, monocytes, and their byproducts which account for most of the cells in the pannus.42
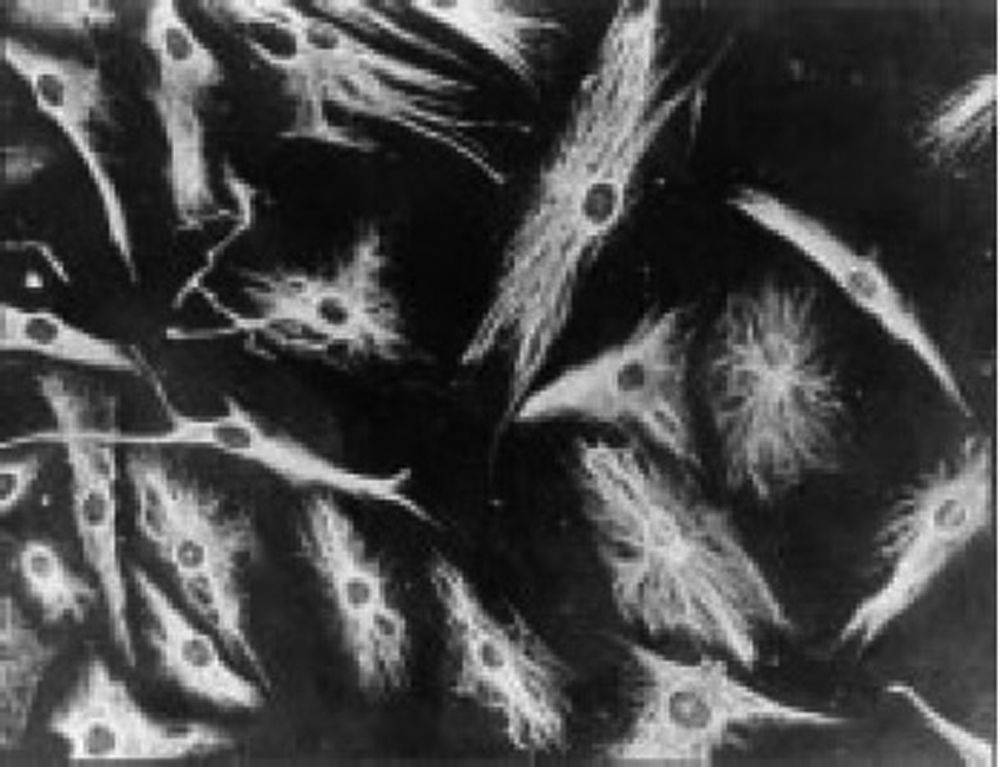
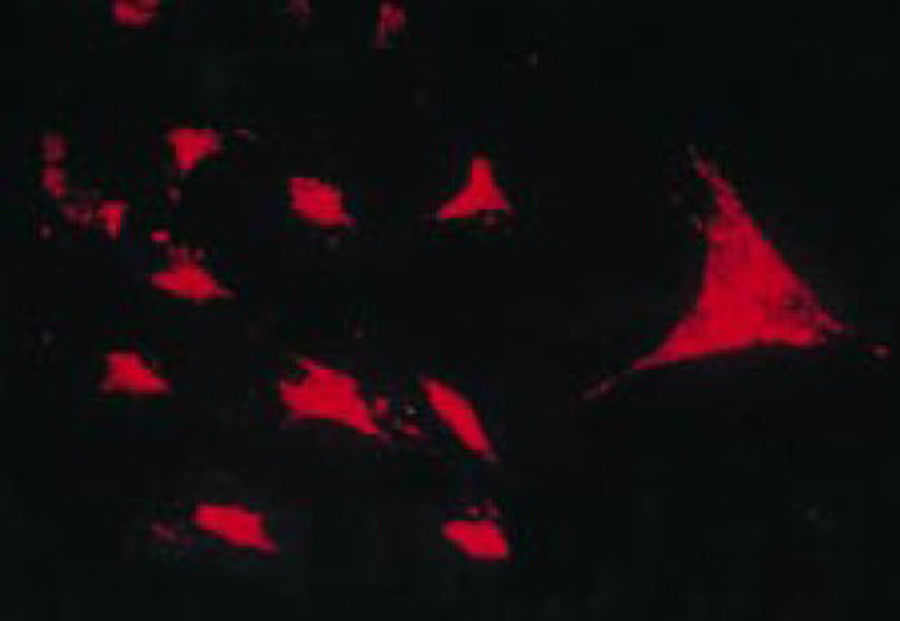
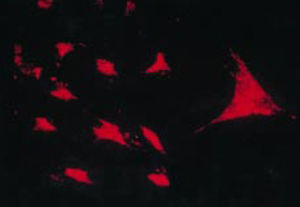
The work by Burmester in 198320 was fundamental for the continuity of the study of the cellular component of the pannus. Xue's group in 1997 studied arthroplasty specimens containing pannus of patients with RA and observed PSC cells that were phenotypically studied via microscopy and immunohistochemistry, with a specific protein and genomic expression.43 These cells were found to be positive for vimentin filaments, immunofluorescence markers (Fig. 12) and were morphologically similar to fibroblasts under the microscope. However, the safranin O marker that reacts with proteoglycans (Fig. 13), as well as the mRNA expression of type II collagen which is used to identify chondrocyte-like cells, were also positive; hence the conclusion that these cells are a combination of fibroblasts and chondrocytes.
Immunofluorescence micrograph showing the positivity of the PSC cells to anti-vimentin antibodies labeled with fluorescein isothiocyanate. This is similar to the findings in fibroblasts.43
In situ reverse transcriptase assay to show the expression of type II collagen RNA in a PSC culture, which conveys chondrocyte-like characteristics.43
The genome expression study also evidenced the high activity of proto-oncogenes for c-myc, c-jun and c-fos that enables the high production of enzymes such as cathepsin B and L and some collagenase.
In later years further evidence was disclosed about the characteristics of the tissue in early joint disease and the increased expression of the p53 gene,44 mutations which were more often seen in advanced stages.45 In 2003 Smeets et al. observed differences in the cellular characteristics, cytokine expression, angiogenic factors and metalloproteinases of the pannus, obtained from specimens of patients with late disease that were undergoing joint replacement with no signs of clinical activity of arthritis, as compared against patients undergoing arthroscopy and considered to be in the early stages of the disease because they still had clinical activity of arthritis.46 The differences between both groups were the larger number of macrophages and T-lymphocytes derived from the arthroscopy specimens or of early disease, and the higher expression of TNF-a, IL-6, metalloproteinases (MMP-1, MMP-3, MMP-13) and of the angiogenesis factor (VEGF).
Pannus is often considered a fibrous tissue without major biological activity and a remnant of the joint damage that seems to be irreversible. However, pannus behaves as a medium producing large amounts of proteolytic enzymes, with a central pathological role, such as the MMP 1, 3, 13 and 14.47 MMP 3 or stromelyn (mentioned above)23,24,48 in particular, is intensively produced as shown in 200549 through mRNA detection at the synovium-cartilage junction, and is IL-1b dependent for synthesis stimulation. MMP 3 differs from MMP 1 or collagenase type 1, because it is less abundant and is stimulated by TNF-a.
Pannus and Kappa B nuclear factorThe kappa B nuclear transcription factor (NF-kB) is one of the most important regulators of the genomic transcription of cytokines, chemokines, and adhesion molecules; it plays a role in cartilage and articular bone degradation,50 is increased both in the synovial tissue of both patients and animal models with RA,51–53 mainly at the pannus-cartilage junction53 where there is a larger number of factor-producing cells, as compared to other synovial sites outside of this junction. The relevance of the NFkB factor in terms of joint damage is not only reflected by the increased number of cytokines, but by the osteoclastogenesis – a key process in bone erosion,54,55 which depends on the myeloid line and the RANK-RANKL complex,56–58 enabling the activation, migration, and survival of the osteoclast.59,60 RANKL derives from T-lymphocytes, fibroblast-like synoviocytes, and cells outside the pannus-bone interface.50,61–63 Pettit et al., in 2006,64 studied the RANKL expression pattern, NF-kB and osteoprotegerin (natural inhibitor of RANKL that blocks its bond to the receptor and therefore prevents osteogenesis) in pannus specimens of patients with RA, specifically of the pannus-bone interface where it had never been studied. The result was that both RANKL and osteoprotegerin are expressed in microenvironments where the pannus interfaces with the bone or the cartilage and there is erosion or tissue damage, always in proportions that favor the maturation of the osteoclast and its activation. This molecular imbalance was ratified in 2008.65
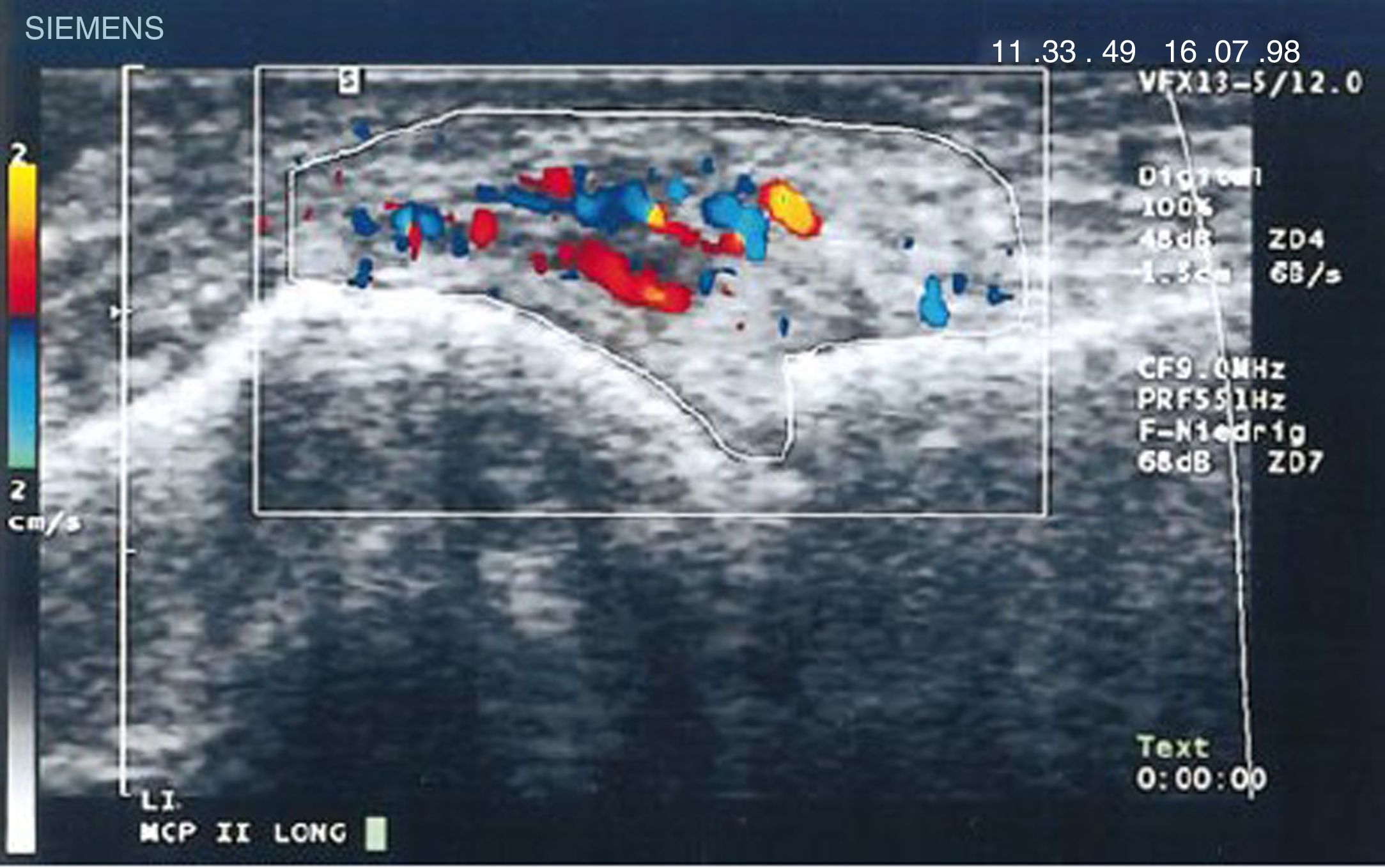
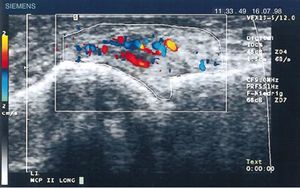
Diagnostic images and pannusIn 1978 Cooperberg was the first to show synovitis with ultrasound, observing a thickening of the synovial membrane of the knee in a greyscale image.66 Further details were observed in the work by De Flaviis in 1988, implementing an ultrasound protocol in the hand of patients with RA.67 6 years went by until Newman used the Doppler method on tendons and bursae,68 and 2 years later he used it for synovial thickening in the knees of patients with RA.69 Natias Hau in 1999, with high resolution ultrasound, used the Doppler mode for the first time on the pannus of hands of patients with RA, showing hypervascularization70 of the knee joints, hence confirming the increased vascularization already described in studies conducted one century before (Fig. 14).5,12,14,15
Longitudinal ultrasound of the metacarpophalangeal joint showing the first Doppler images with increased pannus vascularization in a patient with RA.70
Having shown changes in the synovial tissue using ultrasound, the next step was to establish whether this pannus, as seen clinically, also presents involution. The first one to do this type of ultrasound study was Newman in 1996, when he used Doppler in RA69 noticing changes in the amount of vascularization following intra-articular steroid therapy. These same results were seen in the work by Stone in 2001.71 He compared the images of metacarpophalangeal joints, before and after intravenous methylprednisolone or oral prednisolone therapy. Using TNF-a inhibitors, Hau et al. published a paper in 2002, showing the changes in the vascularization of the pannus72 in etanercept-treated patients, and also in subsequent studies.73–76 Taylor showed the change of hypervascularization with infliximab77 and Naredo did the same in 2008.78
The use of magnetic resonance that started in 1988 with Gilkenson's work showed that this was a better method to detect hand erosions in patients with RA79; Kusunoglu in 1990 showed it was useful to differentiate synovial thickening from joint effusion.80 The visualization of the pannus using this method began in 1994, when for the first time Ostergaard assessed the synovial growth and its relationship to the activity of the disease,81 confirmed by a more extensive work by Sugimoto in 1998,82 and finally in 2003 the OMERACT group defined synovitis in nuclear magnetic resonance as an increase in the synovial thickness following contrast injection; this is definitely the evidence of pannus.83
ConclusionsHistory has allowed for an understanding of the critical role of pannus in joint destruction, which is sometimes similar to a tumor lesion because of its rapid and abundant growth, favoring the production of antibodies that perpetuate the autoimmune process. This contravenes the idea that pannus is a barely active granulation tissue, limited to being merely a residual scar tissue. These characteristics interfere with the processes of development, progression, and destruction of the joint bone and cartilage that have been evidenced for over 100 years, and proven with microscopy and molecular biology techniques. Therefore, the idea that pannus is a definitive or irreversible condition is incorrect, since it is not an active part of the disease, and on the contrary, it can be reversed in response to treatment. The incorrect use of the term should be avoided to make the clinical evaluation of the disease less confusing. These changes have been historically attributed to synovitis, which is simply the inflammation of the synovial tissue clinically evaluated. The term synovitis cannot be differentiated from pannus, since it refers to a histological finding and hence pannus is not comprised in the initial clinical evaluation or in the follow-up of patients with RA by the various scientific societies.
Further studies on pannus as an indispensable component of the pathogenesis of RA will contribute to a better understanding of the disease from its very onset, and open new pathways for its clinical or imaging evaluation, in addition to identifying more targeted therapies that inhibit the destruction of the joints.
Conflict of interestsThe authors have no conflict of interests to disclose.
Please cite this article as: Cajas LJ, Casallas A, Medina YF, Quintana G, Rondón F. Pannus y artritis reumatoide: evolución histórica y fisiopatológica. Rev Colomb Reumatol. 2019;26:118–128.