Low mannose-binding lectin (MBL) concentrations in serum are due mainly to the presence of three punctual mutations in the coding region of the MBL2 gene. SLE patients, who are homozygous for MBL allele variants, have a significantly greater risk of developing infections. With the purpose of examining the association of MBL locus haplotypes with disease activity and past history of infection in SLE, we studied a group of patients treated in the Rheumatology Outpatient Clinic of the UANL University Hospital.
ObjectiveDetermine the prevalence of MBL2 locus haplotypes and the causal associations between MBL2 locus haplotypes and SLE determining the Hardy–Weinberg law for specific genotypes in both groups of study.
Materials and methodsAn observational, cross-sectional, retrospective study was performed. Hardy–Weinberg equilibrium for genotypic frequencies was proven with the X2 test. The risk of lupus associated with MBL2 genotypes as a genetic factor and the strength of the association of the genotypes with the frequency of clinical characteristics was estimated by calculation of odds ratio with a 95% confidence interval. Statistical significance was taken as a value of P<.05.
ResultsThe findings suggest potential genetic associations between allelic systems and the risk of SLE. A relationship was found regarding the MEX-SLEDAI index, as well as the number of infections among patients with differences in structural gene polymorphisms and promoter gene polymorphisms.
ConclusionsThere are significant differences in the polymorphisms of the promoter region regarding the risk for developing SLE.
Las bajas concentraciones de lectina de unión a manosa (MBL) en suero se deben principalmente a la presencia de tres mutaciones puntuales en la región codificante del gen MBL2. Los pacientes con lupus eritematoso sistémico (LES), que son homocigotos para las variantes del alelo MBL, tienen un riesgo significativamente mayor de desarrollar infecciones. Con el propósito de examinar la asociación de los haplotipos del locus MBL con la actividad de la enfermedad y los antecedentes de infección en el LES, estudiamos a un grupo de pacientes atendidos en la Consulta Externa de Reumatología del Hospital Universitario de la Universidad Autónoma de Nuevo León (UANL).
ObjetivoDeterminar la prevalencia de los haplotipos del locus MBL2 y las asociaciones causales entre los haplotipos del locus MBL2 y el LES, determinando la ley de Hardy-Weinberg para genotipos específicos en ambos grupos de estudio.
Materiales y métodosSe realizó un estudio observacional, transversal y retrospectivo. El equilibrio de Hardy-Weinberg para las frecuencias genotípicas se comprobó con la prueba de X2. El riesgo de LES asociado a los alelos de MBL2 como factor genético y la fuerza de la asociación de los genotipos con la frecuencia de las características clínicas se estimó mediante el cálculo de la odds ratio, con un intervalo de confianza del 95%. Se consideró significación estadística un valor de p<0,05.
ResultadosLos hallazgos sugieren potenciales asociaciones genéticas entre los sistemas alélicos y el riesgo de LES. Se encontró relación respecto al índice MEX-SLEDAI, así como al número de infecciones entre los pacientes con diferencias en los polimorfismos del gen estructural y del gen promotor.
ConclusionesExisten diferencias significativas en los polimorfismos de la región promotora respecto al riesgo de desarrollar LES.
Mannose-binding lectin (MBL) is a calcium-dependent serum protein that is secreted by the liver as an acute-phase protein, whose gene is found on the long arm of chromosome 10.1–3 The protein is a multimeric molecule of up to six functional subunits, each one formed by three polypeptidic chains with a structure that is analogue to the C1q protein. MBL plays an important role in the innate immunity defense system, opsonizing microorganisms rich in mannose and N-acetylglucosamine and activating macrophages through the C1q4 receptor. MBL activates the third pathway of the complement system and the lectin pathway through two associated serine proteases (MASP1 and MASP2).5,6
Deficient and low MBL concentrations in serum are due mainly to the presence of three punctual mutations in the structural (coding) region of the MBL2 gene in codons 54, 57, and 52, which affect protein production and are called, in general, O alleles (in contrast to the normal A allele) and their particular denominations are B, C, and D. Additionally, three mutations have been reported in the 5′ untranslated region of the same gene (promoter region), which have a quantitative effect on the production and serum concentration of the protein.7,8 These mutations are located at positions −550 and −221 and constitute the polymorphic systems H/L and X/Y, respectively.
As a consequence of unbalanced binding phenomena, six haplotype systems composed of alleles of the promoter and structural regions of the MBL2 gene: HYA, LYA, LXA, HYD, LYC and LYB, have been observed in humans; these haplotypes are combined in different genotypes.9 The HYA haplotype is associated with high levels of MBL while the LYA haplotype is associated with moderate levels, and the LXA haplotype with low production of MBL.10
Allelic and genotypic frequencies have been defined in different populations11; distribution of these haplotypes have been observed in Africa (Kenya and Mozambique) 50% for LYA, 24% for LYC, 18% for LXA, and 7% for HAY12; in Europe (Denmark) 30% for HYA, 24% for LXA, 21% for LYA, 12% for LYB and 7% for HYD13; in Asia (Japan) 44% for HYA, 32% for LYB, 11% for LXA, 7% for LYA14; in Australia, 75% for HYA and 21% for LYA15; in Greenland, 81% for HYA, and 12% for LYB12; and in South America (Argentina) 48% for HYA, and 43% for LYB.13
MBL deficiency is associated with a high risk of infection in children and adults,16 and there is controversy about the pathogenic role of these polymorphisms in systemic lupus erythematosus (SLE) and rheumatoid arthritis.17
Infections cause 25–50% of morbidity in SLE patients, and severe infections are an important cause of hospital admissions.18 Common bacteria are responsible for most infections in SLE patients. The most frequently recorded bacteria are gram-negative rods and gram-positive cocci.19
In addition, infections are the main cause of death among SLE patients in developing countries. In a cohort of Chinese patients followed from 1992 to 1996, 66% of deaths were caused by infections.20,21 In series of autopsies performed in SLE patients in Brazil, infections were responsible for 58% of the deaths; 34% were attributed to SLE activity. In developed countries, infection is also one of the main causes of mortality among SLE patients and it is considered the first or second most frequent cause of mortality in various studies.20,21 These high mortality rates caused by infections are probably the result of more aggressive use of steroids, immunosuppressants, and support therapy (including dialysis and admission to a intensive care unit) in the search to control activity and complications of SLE.
Opportunistic infections are emerging as an important cause of mortality. These are frequently associated with increased use of high-dose steroids and immunosuppressors, and are also frequently diagnosed only post-mortem.21
Variant alleles in the promoter segment of the MBL2 gene have been associated with lower levels of MBL, which plays an important role in the phagocytosis of microorganisms and has a function similar to C1q. SLE patients, who are homozygous for MBL allele variants also have a significantly greater risk of developing infections such as pneumococcal pneumonia. It has been shown that the annual incidence of infections requiring admission to a hospital is four times greater in these patients than in those who are heterozygous for variant alleles or homozygous for the normal allele.20,21
With the purpose of examining the proposed role of MBL deficiency in SLE, we studied a group of patients treated in the Rheumatology Outpatient Clinic of the UANL University Hospital. The association of MBL locus haplotypes with disease activity and past history of infection was studied in these patients. These patients reside mainly in the states of Nuevo León, Coahuila, Tamaulipas, San Luis Potosi, Zacatecas, and Veracruz, and only those who signed the informed consent form approved by the UANL-UH ethics committee participated.
ObjectivesTo determine the prevalence of MBL2 locus haplotypes and the possible (causal) associations between MBL2 locus haplotypes and SLE determining genetic equilibrium for specific genotypes in both groups of study.
Material and methodsPopulationPatients were recruited during the period from January to July 2007. Both groups were selected consecutively by convenience, controls were purposively searched for.
The patient population consisted of people seen at the UANL-UH Rheumatology Outpatient Clinic and at a private rheumatology practice. Inclusion criteria were both sexes, older than 16 years, diagnosis of SLE was established as four or more of the criteria proposed in 1982 and revised in 1997 by the American College of Rheumatology (ACR)22 for the classification of SLE. Patients with other concomitant autoimmune diseases or undifferentiated connective tissue disease, and those who did not agree to sign the informed consent form were excluded.
Inclusion criteria for controls included both sexes, older than 16 years, clinically healthy who agreed to sign the informed consent. These were hospital staff, medical students and healthy women in normal puerperium from the obstetrics ward; all accepted to voluntarily collaborate in the study.
Demographic and clinical data were obtained retrospectively form clinical records, and criteria for data recollection was standard for all patients.
MethodologyRheumatology specialists from the outpatient clinic of the University Hospital were in charge of examining the patients and performed the Mexican SLE Disease Activity Index (MEX-SLEDAI).23 From the applied questionnaires, the number of infections in the last 6 months was collected.
Specialists in genetics and molecular biology from the Department of Biochemistry of the UANL School of Medicine performed determinations of the MBL2 gene haplotype in patients and controls. Allele and haplotype determinations were performed from genomic DNA isolated from 200μl of peripheral blood anticoagulated with EDTA. DNA was isolated using phenol–chloroform extraction according to the protocol of the Department of Biochemistry.24,25 After isolation, DNA samples were stored at −20°C until used in the genotypification assays.26
Allele typification in the promoter and structural regions of the MBL2 gene was performed by PCR; PCR-amplified DNA products with specific probes of the polymorphism were cleaved with restriction endonucleases. Afterwards these were verified by restriction fragment length polymorphism (RFLP); probes were sent to Invitrogen (Carlsbad, California) for synthesis.24,25 MBL-E1-FOR and MBL-E2-REV probes were used to amplify the polymorphic sites in exon 1, while MBL-PRO-FOR and MBL-PRO-REV were used for the polymorphic sites in the promoter region of the gene.27 For visualizing the digested fragments of promoter exon I, gel electrophoresis with 2% agarose for structural and 3% for the promoter were used; ethidium bromide was used for marking and photographs with exposure to UV light were taken. Additionally, the ELISA kit for MBL 030 (Antibody Shop, Gentofte, Denmark) was used for quantification of mannose-binding lectin levels in serum.
With the results obtained, the following genotypes were defined: Group A/A consisting of two wild-type alleles of the structural fragment with high-expression promoter region alleles (YA/YA), two wild-type alleles in the structural region with a low-expression promoter region allele (XA/YA), two wild-type alleles in the structural region with two low-expression promoter region alleles (XA/XA); a wild-type allele and a mutated allele O for the structural region (genotype A/O) were combined with either high-expression promoter region alleles (YA/YO), or with low-expression promoter region alleles (XA/XO); and finally, the O/O genotype for defective alleles in the structural region with whichever allele in the promoter region (XOXO, YO/YO, XO/YO).
ThJe protocol was approved by the UANL-HU Institutional Committee with number RE007-009.
Statistical analysisAn observational, cross-sectional, retrospective, exploratory study was performed. Hardy–Weinberg equilibrium for genotypic frequencies was proven with the X2 test. The risk of lupus associated with MBL2 alleles as a genetic factor and the strength of the association of the genotypes with the frequency of clinical characteristics was estimated by calculation of odds ratio with a 95% confidence interval. All tests were two-tailed. Statistical significance was taken as a value of P<.05.
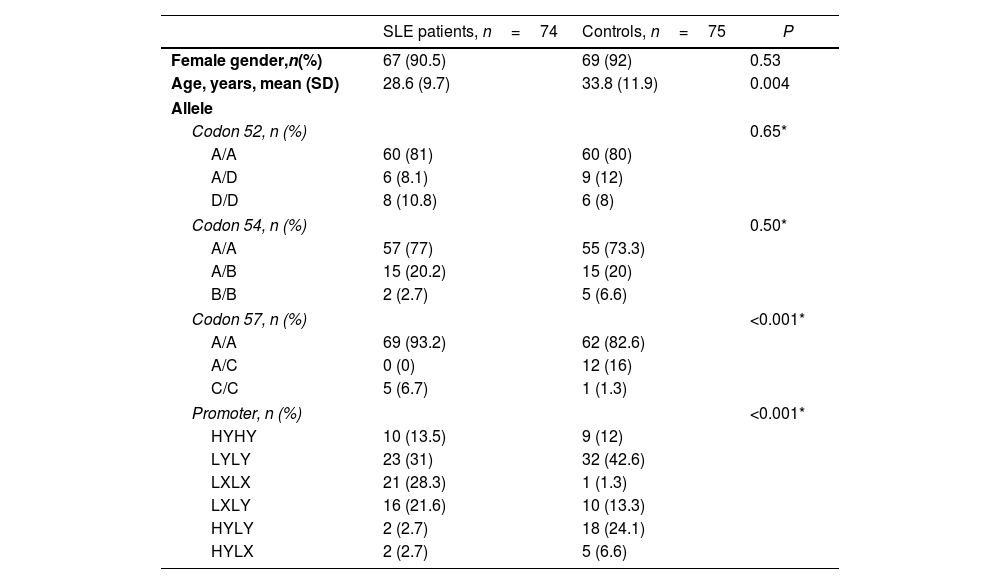
ResultsThe study population consisted of 74 patients with SLE and 75 healthy controls. Of the SLE patients, 67 were female (91%). The mean age was 33 years, with an age range of 16–62 years. In the control group, 69 were female (92%) with a mean age of 27 years and a range of 17–77 years (P=0.05). The demographic characteristics of both groups are shown in Table 1.
Demographics and frequencies of MBL structural and promoter genotypes.
| SLE patients, n=74 | Controls, n=75 | P | |
|---|---|---|---|
| Female gender,n(%) | 67 (90.5) | 69 (92) | 0.53 |
| Age, years, mean (SD) | 28.6 (9.7) | 33.8 (11.9) | 0.004 |
| Allele | |||
| Codon 52, n (%) | 0.65* | ||
| A/A | 60 (81) | 60 (80) | |
| A/D | 6 (8.1) | 9 (12) | |
| D/D | 8 (10.8) | 6 (8) | |
| Codon 54, n (%) | 0.50* | ||
| A/A | 57 (77) | 55 (73.3) | |
| A/B | 15 (20.2) | 15 (20) | |
| B/B | 2 (2.7) | 5 (6.6) | |
| Codon 57, n (%) | <0.001* | ||
| A/A | 69 (93.2) | 62 (82.6) | |
| A/C | 0 (0) | 12 (16) | |
| C/C | 5 (6.7) | 1 (1.3) | |
| Promoter, n (%) | <0.001* | ||
| HYHY | 10 (13.5) | 9 (12) | |
| LYLY | 23 (31) | 32 (42.6) | |
| LXLX | 21 (28.3) | 1 (1.3) | |
| LXLY | 16 (21.6) | 10 (13.3) | |
| HYLY | 2 (2.7) | 18 (24.1) | |
| HYLX | 2 (2.7) | 5 (6.6) | |
SLE: systemic lupus erythematosus; IQR: interquartile range.
In the allelic system of the structural region A/C for codon 57, i.e., the C allele, A/A homozygotes were present in 69 SLE patients (63%) and in 62 controls (82%). No heterozygous A/C patients were found in the SLE patient group, whereas 12 subjects in the control group (16%) had it. In addition, C/C homozygosity was identified in 5 patients with SLE (7%) and in 1 subject in the control group (1.3%) (P<0.001) (Table 1).
In the H/L, X/Y promoter region allelic system, the LX/LX polymorphism was detected in 21 patients with SLE (28%) and in 1 subject in the control group (1.3%) (P<0.001) (Table 1). The HY/LY polymorphism was identified in 2 patients with SLE (2.7%) and in 18 subjects in the control group (24%).
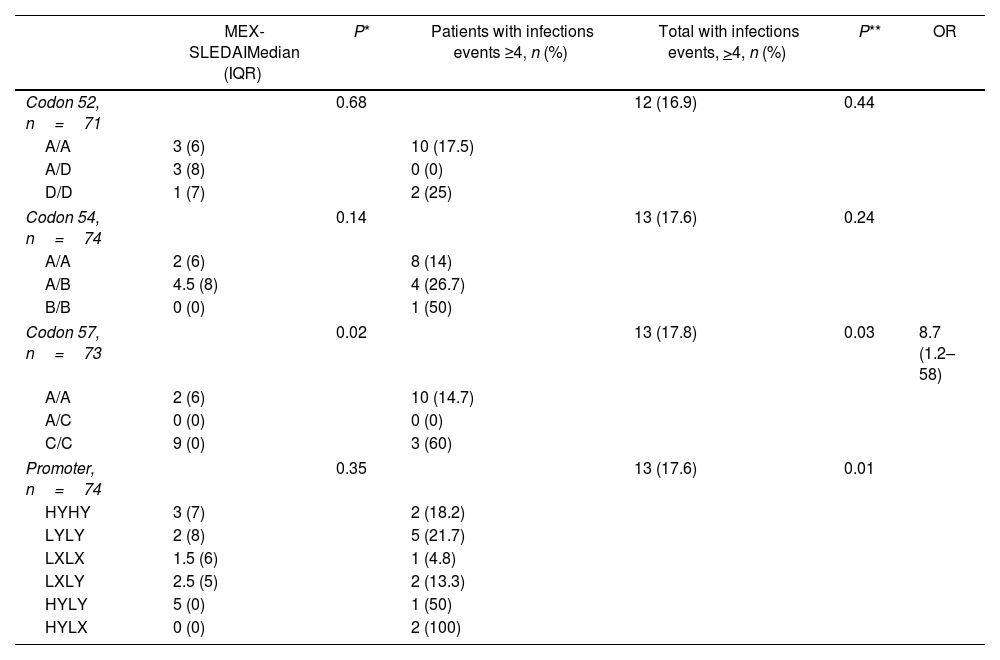
Homozygous C/C polymorphisms of codon 57 of the structural region presented a median of 9 (RIQ 0) in the MEX-SLEDAI and 3 patients (60%) had >4 infections. While the 2 (100%) HY/LX heterozygotes of the promoter region were associated with increased risk of infections (P=0.01) (Table 2).
Association between MEX-SLEDAI and infections with MBL structural and promoter genotypes.
| MEX-SLEDAIMedian (IQR) | P* | Patients with infections events ≥4, n (%) | Total with infections events, >4, n (%) | P** | OR | |
|---|---|---|---|---|---|---|
| Codon 52, n=71 | 0.68 | 12 (16.9) | 0.44 | |||
| A/A | 3 (6) | 10 (17.5) | ||||
| A/D | 3 (8) | 0 (0) | ||||
| D/D | 1 (7) | 2 (25) | ||||
| Codon 54, n=74 | 0.14 | 13 (17.6) | 0.24 | |||
| A/A | 2 (6) | 8 (14) | ||||
| A/B | 4.5 (8) | 4 (26.7) | ||||
| B/B | 0 (0) | 1 (50) | ||||
| Codon 57, n=73 | 0.02 | 13 (17.8) | 0.03 | 8.7 (1.2–58) | ||
| A/A | 2 (6) | 10 (14.7) | ||||
| A/C | 0 (0) | 0 (0) | ||||
| C/C | 9 (0) | 3 (60) | ||||
| Promoter, n=74 | 0.35 | 13 (17.6) | 0.01 | |||
| HYHY | 3 (7) | 2 (18.2) | ||||
| LYLY | 2 (8) | 5 (21.7) | ||||
| LXLX | 1.5 (6) | 1 (4.8) | ||||
| LXLY | 2.5 (5) | 2 (13.3) | ||||
| HYLY | 5 (0) | 1 (50) | ||||
| HYLX | 0 (0) | 2 (100) | ||||
MEX-SLEDAI: Mexican Systemic Lupus Erythematosus Disease Activity Index; IQR: interquartile range; OR: odds ratio.
SLE is a multifactorial disease influenced by a triad of genetic, immune, and environmental factors.28 The role of genetic factors continues to be investigated, suggesting alterations in candidate genes involved in this matter. The degree of influence on disease manifestations, including complications observed when alterations in these genes affect multiple functions simultaneously, as well as the immune response against infectious agents and autoimmune effects due to inadequate management of self-tolerance, varies in different populations and families.29
Based on previous studies, serum levels of MBL are found to be lower than those in healthy controls.30,29 According to Thakur et al., there is no relationship between MBL levels and disease activity measured by SLEDAI, as well as the presence of infections or hematological manifestations in Indian patients.29 In our study, quantitative measurements of MBL were not performed; however, the absence of the A/C genotype in the patient group was identified, along with an association with increased activity and genetic variations present at codon 57. In contrast to this information, it was demonstrated that in the Egyptian population, carrying the variant B allele at codon 54 of MBL2 could be a risk factor for developing SLE,30 whereas in our patients, variants at the same codon showed no significant differences between both groups.
Findings described by Saldanha et al. correlate hospital admissions due to infection with the C allele and HY and LY haplotypes in patients from Brazil,31 similar to the patients in our study.
While the role of the MBL2 gene in SLE has been previously studied, there are other complement genes that may be associated, such as the ITGAM gene, which has also been linked to rheumatoid arthritis.32,33 When there are suspected mutations or polymorphisms, they are not always reproducible among researchers from different populations due to their different penetrance and interaction with environmental factors, and reproduction in animal models is not always feasible.
We acknowledge that our study has some limitations. Being an exploratory study, the results cannot be generalized to the entire population. Furthermore, besides the MBL2 gene polymorphisms, other genetic polymorphisms, and even factors such as the patient's current treatment, could influence the risk of infection.
Our study confirmed findings from other studies regarding the heterogeneity of haplotype frequency. This suggests the need for further research to corroborate the utility of MBL2 polymorphisms in healthy individuals to determine the risk of developing SLE prospectively. Other studies can be conducted to correlate the clinical characteristics of SLE with different MBL2 polymorphisms.
ConclusionsThe findings of our study confirm the presence of MBL gene polymorphisms in patients with SLE and their association with a higher risk of infections.
Ethical approvalThe protocol was approved by the institutional ethics committee under number RE07-009.
FundingThe authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interestThe authors declare that they have no conflict of interest.