Anti-DFS70 antibodies (Dense Fine Speckled, 70kDa molecular weight) are a sub-group of anti-nuclear antibodies (ANA) that show a fine dense speckled pattern (DFS) by indirect immunofluorescence. This pattern is also recognized by irregularly distributed granular fluorescence at the interface of nuclei and chromatin. This antibody was initially described in patients with interstitial cystitis, atopic dermatitis, and malignancy, such as prostate cancer. These antigens have been found to act directly against co-activators of nuclear transcription (LEDGF/p75) encoded by the PSP1 gene and located in the C-terminal region between the a.a. 349 and 435. Additionally, associations with some physiological functions have been described, such as protection against stress-induced apoptosis, the survival of lens epithelial cells, and acting as a cofactor of HIV replication through its interaction with viral integrase. As regards autoimmunity, recent evidence has also shown the importance of this antibody as a useful biological marker in the discrimination of individuals with positive ANA that do not progress to systemic autoimmune disease (SID). This is based on the observation that these antibodies are more common in healthy individuals than in patients with SID, and that healthy individuals with positive anti-DFS70 did not develop SIDs after a clinical follow-up of four years or more. This article reviews the description of anti-DFS70 and its usefulness in clinical practice.
Los anticuerpos anti-DFS70 (Dense Fine Speckle, peso molecular de 70kd) son un subgrupo de anticuerpos antinucleares (ANA) de tipo natural, los cuales se manifiestan por un patrón moteado denso fino (DFS) por inmunofluorescencia indirecta. Este se caracteriza por la fluorescencia granular distribuida irregularmente en la interface de los núcleos y de la cromatina. Inicialmente, este anticuerpo fue descrito en pacientes con cistitis intersticial, dermatitis atópica y algunas neoplasias como el cáncer de próstata. Se ha encontrado que sus antígenos actúan directamente contra co-activadores de la transcripción nuclear (LEDGF/p75), codificado por el gen PSP1 y localizado en la región C terminal entre los a.a. 349 y 435. Adicionalmente, se documenta asociación con algunas funciones fisiológicas como la protección contra la apoptosis inducida por estrés, promover la supervivencia de las células epiteliales del cristalino y actuar como cofactor de replicación del virus VIH a través de su interacción con la integrasa viral. En el campo de la autoinmunidad se ha evidenciado recientemente la importancia de este anticuerpo como marcador biológico útil en la discriminación de personas con ANA positivos que no evolucionan a enfermedad autoinmune sistémica (EAI). Lo anterior se ha basado en la observación de que estos anticuerpos son más frecuentes en individuos sanos que en los pacientes con EAI y que los individuos sanos con anti-DFS70 positivo no desarrollaron EAI después de un seguimiento clínico por 4 años o más. Este artículo revisa la descripción de los anti-DFS70 y su utilidad en la práctica clínica.
One of the characteristics of a significant percentage of rheumatic systemic autoimmune diseases (SID) is the production of autoantibodies, which have high relevance in contributing to the diagnosis, prognosis and, in some cases, to the clinical follow-up and response to the treatment of these diseases. Among the main autoantibodies that are determined routinely in the clinical practice there are the antinuclear antibodies (ANA), which correspond to immunoglobulins that react against different nuclear and cytoplasmic autologous components.1,2 The study of these antibodies began with the identification of LE cells in patients with systemic lupus erythematosus (SLE) described by Hargraves in 1948, and for a long time this was the main confirmatory test for SLE. However, some years later was demonstrated its low specificity because these LE cells could be found in other pathologies such as rheumatoid arthritis (RA), Sjögren's syndrome (SS) and active chronic hepatitis, among others.3 But it was not until 1959 when the presence of antibodies that recognize nuclear antigens as the cause of the formation of LE cells began to be described,4 and subsequently, techniques for the detection of these antibodies such as radial immunodiffusion, hemagglutination, and a technique of microscopy using antibodies conjugated with fluorescent molecules, called indirect immunofluorescence (IIF) were developed.3 Currently, the most widely used technique for the detection of ANA is the IIF, which was developed in 1950 by Coons and Kaplan5 and was later modified by Tan and Kunkel in 1966.6 This test used as substrate mouse liver or kidney sections. The results of the standardization for the detection of ANA in patients with SID were published ten years later.7 The detection of ANA is done currently using the Hep-2 and HeLa cell lines as substrate, the first being the most used, since it has a higher concentration of autoantigens because it is an epithelial cell line (pharyngeal carcinoma), in addition to having a greater amount of mitochondria, more than 2 nucleoli and more than 46 chromosomes, making the observation of nuclear and cytoplasmic patterns an easier task.3,8 In this article we describe the usefulness of anti-DFS70 antibodies (Dense Fine Speckle, molecular weight of 70kDa) in the clinical setting, based on a quantitative systematic literature review.
MethodsMethods for literature searchA systematic search of articles was carried out until July 2017. The databases consulted include: Scielo, Clinical Trials, Clinics review Article, Academic Search Ultimate, Medline, Embase and Google Scholar. There were no restrictions of date or language during the search. The search in Medline was carried out through Pubmed using the MeSH terms: anti-DFS70; anticuerpos; inmunofluorescencia indirecta; antígeno; autoinmunidad (anti-DFS70; antibodies; indirect immunofluorescence; antigens; autoimmunity). Subsequently they were linked with the Boolean connector AND.
Selection of articles and information extractionAt the end of the search, the articles were stored in a database created in Excel. In this way the duplicate articles were excluded and the process of selection of those which were relevant for this publication was started. The articles that included the keywords in the title or in the abstract were taken into account. It was reviewed that each article met the inclusion criteria, and finally it was made a consensus among all the authors to unify and review the database.
Inclusion criteria- •
Types of studies: we included cohort studies, cases and controls, randomized and non-randomized studies, case reports and topic reviews.
- •
Population type: adult patients with anti-DFS70 antibodies.
- •
Intervention: studies describing the function, laboratory techniques used for the detection and clinical application of this subtype of ANA.
- •
Articles without access to full text.
- •
Duplicate articles.
After the initial search, 72 articles were found, most of them in Medline and Google Scholar. After excluding the articles to which there was no full access and the duplicate articles, a total of 52 articles were selected. It is worth highlighting that there are very few articles related with anti-DFS70 antibodies, especially in healthy population; the majority of reviews or studies have focused more in other types of antibodies and laboratory techniques than in their clinical applicability.
DiscussionDefinition and classification of antinuclear antibodiesThe ANA constitute a broad group of autoantibodies composed of immunoglobulins capable of interacting specifically not only with macromolecules integrated in structures of the cell nuclei, but also against some cytoplasmic components.8 They can be classified into 3 subtypes present in the systemic circulation at different titers. In the general population, natural ANA are found in low titers (produced mainly by B1 lymphocytes), without knowing so far the specific antigenic stimulus that originates their synthesis, but recognizing some of their most relevant characteristics, such as their low avidity, the polyreactivity and the lack of direct association with clinical manifestations of SID. There is a second group of antibodies, which are related to infectious processes. These, on the contrary, show high avidity, their titers decrease when the external antigenic stimulus disappears and they are not related either with clinical manifestations of autoimmunity.9 Finally, there is the group of ANA present in autoimmune pathologies, which have a multifactorial origin and can be linked to loss of immune tolerance, genetic predisposition, hormonal changes, interaction with the environment and epigenetic changes, among other factors. The latter are characterized by high avidity to the antigen used as substrate, titers that fluctuate throughout the course of the disease and may be associated with clinical manifestations of autoimmunity.3
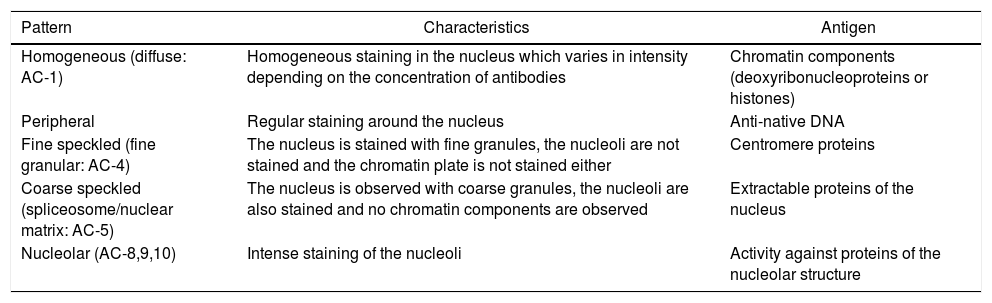
IIF is the most widely used technique for the detection of the ANA, since it has a high sensitivity, is inexpensive and easy to carry out, but is not very specific.1,10 The nuclear patterns most commonly detected in IIF using as substrate Hep-2 cells in sera of patients with autoimmune diseases are described in Table 1.1,3,10–13
Characteristics and specific antigens of the different ANA patterns most frequently found by indirect immunofluorescence.
| Pattern | Characteristics | Antigen |
|---|---|---|
| Homogeneous (diffuse: AC-1) | Homogeneous staining in the nucleus which varies in intensity depending on the concentration of antibodies | Chromatin components (deoxyribonucleoproteins or histones) |
| Peripheral | Regular staining around the nucleus | Anti-native DNA |
| Fine speckled (fine granular: AC-4) | The nucleus is stained with fine granules, the nucleoli are not stained and the chromatin plate is not stained either | Centromere proteins |
| Coarse speckled (spliceosome/nuclear matrix: AC-5) | The nucleus is observed with coarse granules, the nucleoli are also stained and no chromatin components are observed | Extractable proteins of the nucleus |
| Nucleolar (AC-8,9,10) | Intense staining of the nucleoli | Activity against proteins of the nucleolar structure |
In this table are evidenced the different ANA patterns with their respective characteristics in IIF, their current nomenclature and specific antigens.
The nuclear patterns described in Table 1 represent pure patterns; however, more than 90% of the ANA detected in the IIF present at least two different patterns: nuclear and cytoplasmic.3
The detection of ANA by IIF using Hep-2 cells has been the standard test for the serological diagnosis of the SIDs, which include SLE, SS, RA and systemic sclerosis, among others.2,12 Although there are currently available multiple tests for the determination of the ANA, the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014–2015 argues that the use of the Hep-2 cells remains the gold standard for their analysis,13 supported by the high sensitivity that this test has for the diagnosis of SID and the detection of ANA, and it is part of the diagnostic criteria for some autoimmune diseases, such as SLE and autoimmune hepatitis.13,14 However, the limited specificity of the ANA in the context of the SIDs limits accurate diagnoses in individuals with positive ANA and non-specific symptoms of SID, which represents a major limitation, even more if it is taken into account that some diseases such as SLE can start with non-specific symptoms, which in scenarios of positive ANA titers could lead to overdiagnosis of this disease.15 A relatively recent international consensus has attempted to standardize the reading of the ANA in different patterns.2
The ANA is considered a screening test in the study of patients with suspected autoimmune conditions. After their detection, additional study with anti-ENA and anti-double-stranded DNA, among others, is suggested according to the clinical context.
The extractable nuclear antigens [ENA]) are non-histone proteins associated with the RNA; of them, there are more than 100 known antigens, being anti-Ro/SS-A, anti-La/SS-B, anti-U1RNP, anti-Sm, anti-Scl70 and anti-Jo1 the most studied at present. These antibodies are of great clinical relevance, since they guide the clinician when discriminating between the different SIDs.1,8,16 The anti-Sm antibody is an immunoglobulin directed against small nuclear ribonucleoproteins (snRNP) that are part of the spliceosome, and it has been documented that it is the most specific antibody of SLE, with values close to 98%.17
Anti-DNA antibodies are a heterogeneous group of immunoglobulins directed against DNA, either the native or double-stranded (anti-dsDNA) or the denatured or single-stranded DNA. The importance of the anti-dsDNA lies on the fact that it is one of the most specific antibodies for the diagnosis of SLE, reaching almost 100% of specificity, especially at high titers.10,16
However, and despite the increasingly wide availability of autoantibody detection, some healthy individuals have high titers of ANA, without ENA specificity, and require additional assessments to determine the potential for progression into a SID. The detection of anti-DFS70 antibodies has gained importance in this clinical scenario.
Anti-DFS70 antibodiesIn recent years, multiple studies have demonstrated the presence of a new type of ANA called anti-DFS70 antibody, which is detected mainly in apparently healthy individuals or with clinical conditions other than SID.18 Thanks to this finding, this antibody has been proposed as a helpful tool in the interpretation of a positive ANA result with nonspecific symptomatology.19
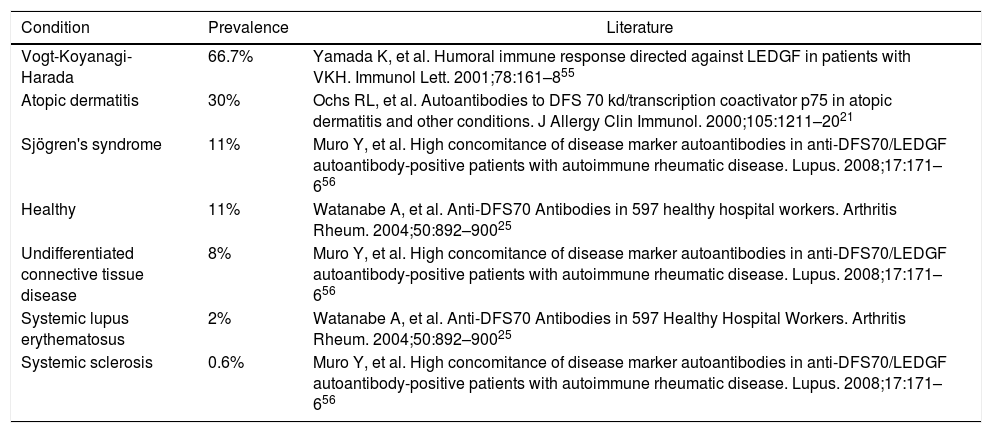
Definition and historyThe dense fine speckled pattern (DFS) or AC-2, according to the current nomenclature, differs from other nuclear patterns by the finely granular, irregularly distributed fluorescence of the interphase nuclei and the metaphase chromatin.20 This pattern is frequently observed in routine diagnoses, but in 1994 it was described as a separate pattern associated with an antibody related to interstitial cystitis. It has been associated later with other diseases, among which are included some chronic inflammatory diseases, such as asthma, psoriasis, SS, atopic dermatitis, Behçet's disease, sarcoidosis, other non-inflammatory conditions such as the chronic fatigue syndrome, and some neoplasms, such as prostate and breast cancer.21–23 Several studies have documented a prevalence of up to 66.7% in patients with Vogt-Koyanagi-Harada syndrome24 and of up 30% in atopic dermatitis,21 which contrasts with a prevalence of around 11% in healthy individuals,25 while in patients with SID is relatively low, of 2–3%.26 Despite the arguments about the lower prevalence of this antibody in patients with SID compared with healthy individuals are not conclusive so far,27 it has been described a possible relationship with genetic, racial, environmental, therapeutic or demographic factors.22 The prevalence of anti-DFS70 antibodies in different conditions is summarized in Table 2; it should be noted that it is relatively low when healthy individuals and patients with SLE are compared. Thus, it is posed in the world literature that the presence of anti-DFS70 antibodies in healthy individuals with positive ANA is related to lower progression to SID within the period of 4 years.28
Prevalence of anti-DFS70 antibodies in sera of patients with different autoimmune pathologies and in healthy controls.
| Condition | Prevalence | Literature |
|---|---|---|
| Vogt-Koyanagi-Harada | 66.7% | Yamada K, et al. Humoral immune response directed against LEDGF in patients with VKH. Immunol Lett. 2001;78:161–855 |
| Atopic dermatitis | 30% | Ochs RL, et al. Autoantibodies to DFS 70 kd/transcription coactivator p75 in atopic dermatitis and other conditions. J Allergy Clin Immunol. 2000;105:1211–2021 |
| Sjögren's syndrome | 11% | Muro Y, et al. High concomitance of disease marker autoantibodies in anti-DFS70/LEDGF autoantibody-positive patients with autoimmune rheumatic disease. Lupus. 2008;17:171–656 |
| Healthy | 11% | Watanabe A, et al. Anti-DFS70 Antibodies in 597 healthy hospital workers. Arthritis Rheum. 2004;50:892–90025 |
| Undifferentiated connective tissue disease | 8% | Muro Y, et al. High concomitance of disease marker autoantibodies in anti-DFS70/LEDGF autoantibody-positive patients with autoimmune rheumatic disease. Lupus. 2008;17:171–656 |
| Systemic lupus erythematosus | 2% | Watanabe A, et al. Anti-DFS70 Antibodies in 597 Healthy Hospital Workers. Arthritis Rheum. 2004;50:892–90025 |
| Systemic sclerosis | 0.6% | Muro Y, et al. High concomitance of disease marker autoantibodies in anti-DFS70/LEDGF autoantibody-positive patients with autoimmune rheumatic disease. Lupus. 2008;17:171–656 |
These results show that the prevalence of these autoantibodies is higher in healthy population than in those with autoimmune diseases.
The LEDGF (responsible for the DFS-70 pattern) is a survival factor isolated and identified in the laboratory in 1995,29 and it tends to increase with the signals of environmental stress, such as alcohol, UVB irradiation, certain viruses and some cytotoxic drugs. Its main function is to protect the cells in an environment of stress, by binding elements that are promoters of many related genes induced by these deleterious factors, including the genes of heat shock proteins (Hsps) and stress-related proteins (STRP) which are activated,. In addition, it has been documented that the STRPs protect the cells from various stresses and the Hsps negatively regulate apoptosis by interaction with the system associated with the caspase-dependent cell death. Therefore, in the absence of LEDGF the cells become more sensitive in unfavorable microenvironments, and it is for this reason that the presence of this protein leads to better cell survival.23,30,31 Furthermore, it has been identified as a cofactor for the replication of the human immunodeficiency virus through its interaction with the viral integrase.22,31,32
Methods for detection of anti-DFS70The anti-DFS70 antibodies can be detected by different tests including IIF (representing 12% of the results of positive Hep2), immunoblot, enzyme linked immunosorbent assay (ELISA) and chemiluminiscence, being IIF the most widely used.28 In recent years has been developed a new immunoadsorption technique, in which the serum samples of the patients are diluted with a buffer containing the recombinant DFS70 antigen, so that the possible antibodies are immunoadsorbed before the samples are deposited in the wells of Hep-2 cells,19,22 generating a negative fluorescence in the presence of anti-DFS70.
Clinical relevance of the anti-DFS70 antibodyTo date, there are numerous studies that talk about the possible clinical relevance of this antibody in different diseases such as interstitial cystitis, atopic dermatitis and prostate cancer, among others. However, these associations are not conclusive to include this antibody as part of the routine diagnosis of these diseases.21,33–35 In addition, ocular diseases such as cataracts and atypical retinal degeneration, among others, are also associated with the presence of this antibody, which can be explained by the function that has been attributed to the DFS/LEDGF in the protection of the pigment epithelial cells of the retina and the lens from stress factors or mutated forms of rhodopsin.29,36,37 It should be noted that some diseases that have ocular manifestations, such as sarcoidosis or Vogt-Koyanagi-Harada syndrome (VKH), present high concentrations of this antibody,24 which reinforces the association of the anti-DFS70 with this type of diseases, an association that must be confirmed with more studies. Regarding the organ-specific autoimmune diseases, such as diabetes mellitus type 1, autoimmune hepatitis and autoimmune thyroiditis, among others, there is not enough evidence that links these diseases with the presence of the anti-DFS70 antibody. However, a high prevalence of this antibody is found in the sera of patients with autoimmune thyroiditis.19,38 There is not enough evidence that allows to establish the role of this antibody in this type of diseases.
Anti-DFS70 antibody in systemic autoimmune diseasesThese diseases, also known as associated autoimmune rheumatic diseases (AARD), are negatively associated with the presence of the anti-DFS70 antibody. Below are explained the two AARDs in which the importance of this antibody for their diagnostic process has been better demonstrated.22
Systemic lupus erythematosusSLE is a SID that occurs with a wide range of clinical manifestations that make that in many occasions its diagnosis in early stages represents a challenge for the clinician, since they are not only nonspecific but they do not occur in all patients with this diagnosis.39 Just to give an example, the parvovirus B19 infection can simulate, to a large extent, the initial symptoms of SLE.40 As a consequence of the foregoing, underdiagnosis or wrong diagnosis are common, even leading patients with symptoms suggestive—but not conclusive—of SLE and positive ANA to unnecessary treatments that could even result in possible adverse effects.41
It has been observed recently that the presence of the anti-DFS70 antibody in patients with SLE is scarce compared with healthy individuals.20,22,42 Therefore, the anti-DFS70 antibody, in the absence of autoantibodies specific for SLE or non-organ-specific antibody, could serve as an exclusion criterion for the diagnosis of SLE, which in the clinical scenario would help to focus and reduce the costs of additional tests in patients who have positive ANA and non-specific clinical symptoms such as arthralgias, myalgias, fatigue or rash.20,43
Systemic sclerosisIt is a heterogeneous disease of the connective tissue and the microcirculation characterized by fibrosis and vasculopathy, which can affect the skin, the intestinal tract, the lungs and the kidneys.20,44 The main characteristics are scleroderma (dermatofibrosis) of variable intensity and extension and microcirculatory alterations, mainly in the fingers of the hands and feet.20 The ANA are common in this disease, reaching a sensitivity of up to 85% determined by IIF, without being part of the diagnostic criteria for this disease.45,46 However, and although it is not part of the diagnostic criteria, it is common to request the ANA test for the initial approach to patients with early systemic sclerosis or patients without cutaneous manifestations, since the positivity of the ANA is a strong indicator to look for the presence of the specific autoantibody of this disease.46,47 Like in SLE, the frequency of anti-DFS70 antibodies is found in very low proportions in patients with a diagnosis of systemic sclerosis.20 Therefore, it would be possible to use the anti-DFS70 as a rule out criterion, since having a positive anti-DFS70, in the absence of non-organ-specific antibody of systemic sclerosis, the development of this disease would be unlikely.
Anti-DFS70 antibody in healthy individualsThe prevalence of anti-DFS70 in AARD is relatively low, which has led to the development of multiple studies in which healthy population is compared with another type of AARD to observe how this antibody behaves in healthy people. It has been evidenced that the anti-DFS70 is more prevalent in healthy individuals than in patients with AARD.25,28,38,48–50 The presence of this antibody has been determined in young people, finding that it is more prevalent in individuals under 35 years of age,25 as well as its presence in the child population, where it was found a prevalence slightly lower than in adults. For example, in a study conducted by Schmeling et al.,51 a prevalence of 2.1% of this antibody was found in healthy children. However, other studies show that, although the frequency is low, the persistence of the antibody can continue without being associated in the future with the development of an AARD, so it can be a follow-up marker in this type of patients.52 In complement, some studies conducted in Latin America show the same behavior of the antibody described above, confirming its high prevalence in healthy individuals and in different population groups.53,54
ConclusionThe anti-DFS70 antibodies are increasingly gaining importance in the diagnostic process of certain diseases, mainly in SLE and systemic sclerosis, not because of their diagnostic value but rather for the negative relationship that exists between the antibody and these diseases, since it could provide a helpful tool as a marker for the exclusion of AARD when dealing with patients with non-specific symptoms attributable to these diseases. On the other hand, it is a low-cost tool, which would allow it to become an accessible, fast and reliable exam to provide a better approach of patients with suspected autoimmune diseases. Although there are many reports that support these conclusions, studies for their standardization and application in the clinical practice are still necessary.
Conflict of interestNone of the authors has any conflict of interest.
Please cite this article as: Aragón C-C, González JD, Posso-Osorio I, Naranjo-Escobar J, Puerta G, Echeverri A, et al. Anticuerpos anti-DFS70: un nuevo autoanticuerpo útil en la exclusión de patologías autoinmunes. Rev Colomb Reumatol. 2018;25:104–111.