Patients with systemic lupus erythematosus (SLE) have a higher frequency of traditional cardiovascular risk factors (CVR). This, combined with the presence of non-traditional cardiovascular risk factors, increases the probability of cardiac events by five times.
ObjectiveTo determine the prevalence of CVR factors in a population of patients with SLE.
Material and methodsA descriptive, cross-sectional, observational study in 51 patients with the diagnosis of SLE.
ResultsA lupus dyslipoproteinaemia pattern was reported, of which 52.9% had hypo-alpha-lipoproteinaemia, 49% hypercholesterolaemia, 35.3% hypertriglyceridaemia, and 19.6% with an elevated c-LDL. The comorbidities found were, 31.4% with obesity, 27.5% with high blood pressure, and 6% suffered from diabetes mellitus. Predominant non-traditional CVR factors were associated with disease activity, with 90.1% taking glucocorticoids, 70.6% had low levels of complement C3, 41.2% had low levels of complement C4, 66.7% had a CRP>2mg/l, 56.9% had a SLEDAI-2K score greater than 4 points, 29.4% had more than 10 years of disease duration, and 25.5% had lupus nephritis. As regards the presence of antibodies associated with CVR, 58.8, 9.8, 74.8 and 3.9% had anti-Smith antibodies, lupus anticoagulant, anti-beta2glycoprotein I, and positive anticardiolipin, respectively.
ConclusionsPatients with SLE have a pro-inflammatory and atherogenic state, increasing the risk of developing cardiovascular diseases, and therefore a higher incidence of traditional risk factors, such as the presence of factors that promote chronic inflammation.
Los pacientes con lupus eritematoso sistémico (LES) tienen mayor frecuencia de factores de riesgo cardiovascular (RCV) tradicionales, esto sumado a la presencia de factores de RCV no tradicionales, aumenta la probabilidad de eventos cardiacos hasta 5 veces.
ObjetivoDeterminar la frecuencia de los factores de RCV en una población de pacientes con LES.
Materiales y métodosSe realizó un estudio descriptivo, transversal, observacional, en 51 pacientes con diagnóstico de LES.
ResultadosSe reportó el patrón lúpico de dislipoproteinemia ya que el 52,9% presentó hipoalfalipoproteinemia, 49% hipercolesterolemia, 35,3% hipertrigliceridemia y 19,6% elevación de c-LDL. Con respecto a las comorbilidades el 31,4% presentó obesidad, 27,5% hipertensión arterial y 6% diabetes mellitus. Los factores de RCV no tradicionales que predominaron fueron los asociados con la actividad de la enfermedad, el 90,1% tomaba glucocorticoides, 70,6% presentó niveles bajos de C3, 66,7% tuvo PCR>2mg/l, 56,9% tenía más de 4 puntos de SLEDAI-2K, 41,2% presentó niveles bajos de C4, 29,4% tenía más de 10 años de duración de la enfermedad, 25,5% tenía nefritis lúpica. Con lo que respecta a la presencia de anticuerpos asociados a RCV el 58,8, 9,8, 74,8 y el 3,9% presentaron anti-Smith, anticoagulante lúpico, anti-beta 2 glicoproteína I, anticardiolipinas positivas, respectivamente.
ConclusionesLos pacientes con LES presentan un estado proinflamatorio y aterogénico, aumentando el riesgo de desarrollar enfermedades cardiovasculares tanto por mayor incidencia de los factores de riesgo tradicionales, como por la presencia de factores que promueven una inflamación crónica.
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown etiology which is characterized by the activation of polyclonal T and B lymphocytes, production of autoantibodies and formation of immune complexes that cause damage to tissues and organs.1
Patients with SLE exhibit a higher incidence of atherosclerosis than the general population,2 as well as an increased risk of acute myocardial infarction, and they have a cardiovascular risk (CVR) 5–6 times higher than the general population.3
Patients with SLE have a higher frequency of traditional CVR factors.4,5 They have dyslipidemia with an atherogenic lipid profile called «dyslipoproteinemia lupus pattern», characterized by elevated levels of total cholesterol, triglycerides, low density lipoprotein (LDL-c), and A lipoprotein, as well as decreased levels of high density lipoprotein (HDL-c).6 The patients with SLE also have more frequently diabetes mellitus (DM) due to the significant decrease in insulin sensitivity and to the high prevalence of metabolic syndrome.7,8 In the same way, they have a higher frequency of systemic arterial hypertension (SAH), cigarette smoking and sedentary lifestyle.6
Despite the foregoing, the high CVR cannot be explained solely by the traditional risk factors. The effect of chronic inflammation in the development of atheromatous plaque and accelerated atherosclerosis is well known, and therefore it is considered the presence of other CVR factors associated with the disease, the disease activity and the treatment used, known as nontraditional risk factors. Among them there are the high levels of C-reactive protein (CRP) which have been associated with the presence of carotid atheromatous plaques.9 Elevated basal levels of CRP are predictors of mortality and it has been observed that levels higher than 2mg/l are a CVR factor independently of other risk factors.10
The disease activity has been associated with higher CVR, it can be measured by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), which consists of a systematic review (physical examination, interrogation and laboratory analyses), and it determines with some certainty the degree of activity at a given moment which needs to be manifest at least 10 days before its carrying out.11 An increase of 6 points in the SLEDAI index during one year is correlated with an increase of 5% in the CVR. It has been found a relationship between higher hypertriglyceridemia, hypercholesterolemia and hypoalphalipoproteinemia and a higher activity index.6
Lupus nephritis has been associated with accelerated atherosclerosis.6,12 Up to 40% of the deaths in patients with lupus nephritis are due to cardiovascular causes,13 which can be due to the higher tendency to SAH and dyslipidemia with atherogenic pattern.
It has been found that patients with SLE who have atheromatous plaque show higher frequency of anti-Smith (anti-Sm), anti-ribonucleoprotein and anticardiolipin (aCL) antibodies compared with those patients without atheromatous plaque,6 as well as of atherosclerosis.14 The aCL have been associated with a cross-reaction against apoA-1, which is an important component of the HDL-c complex, thus increasing the atherogenic risk.15,16 The beta-2 glycoprotein I (B2GPI) impedes the uptake of the oxidized LDL-c by the macrophages, thus avoiding the formation of foam cells, so the presence of antibodies against it facilitates the formation of atheromatous plaque.17 The lupus anticoagulant (LA) increases the CVR, as it is associated with more thrombotic events and increased risk of acute myocardial infarction, which increase with the presence of other CVR factors.18
Patients taking glucocorticoids increase the CVR due to their increase in the blood pressure values and the insulin resistance.19
The degree of lupus activity and the risk of developing nephropathy, with the consequent increase of the arterial blood pressure and the CVR, can be determined by the levels of C3 and C4 complement.20 The lupus activity almost always implies active kidney disease with decreased levels of C3 and C4 complement, in such a way as the low levels of C3 and C4 complements can be indirectly related to a higher CVR.
SLE itself is a risk factor for the development of cardiovascular diseases. There is an increased incidence of the traditional risk factors in these patients; however, there are also other non-traditional risk factors that condition a chronic inflammatory process, making that systems such as the Framingham risk scale do not adequately reflect the CVR. In the patients with SLE there is a synergy between the traditional and non-traditional CVR factors, so it is necessary to be acquainted with these factors and to have a strict control of them; for this reason, this research had the purpose of determining the traditional and non-traditional risk factors in patients with SLE, and their association with the Framingham risk scale.
Materials and methodsA descriptive, cross-sectional, observational and retrospective study was conducted. The study population was integrated by the patients with diagnosis of SLE according to the classification criteria of the Systemic Lupus Erythematosus International Collaborating Clinics21 2012, who attended the outpatient clinic of the Rheumatology Service of the High Specialty Regional Hospital Dr. Gustavo A Rovirosa Pérez, between June 2014 and June 2015, for a total of 65 patients, of which only 51 met the inclusion criteria. The clinical records were reviewed, excluding those patients with a previous diagnosis of cardiovascular disease (ischemic heart disease, cerebrovascular disease, deep vein thrombosis, pulmonary thromboembolism and heart failure). The traditional CVR factors (DM, SAH, cigarette smoking, obesity, total cholesterol, HDL-c and LDL-c and triglycerides) were recorded as variables, as well as factors associated with SLE (duration of the disease, disease activity by SLEDAI-2K, ultra-sensitive CRP levels, use of glucocorticoids, C3 and C4 complement levels, presence of lupus nephritis, and presence of anti-Sm, aCL, LA and anti-B2GPI antibodies).
Each patient signed an informed consent authorizing the participation in the study, previous approval by the Bioethics Committee of the Hospital.
Measurement of anthropometric variablesWith the patient barefoot and in light clothing, we proceeded to determine the weight with a Health o meter brand scale with a maximum capacity of 227kg, previously calibrated (according to the manufacturer's specifications), the results were expressed in kg. With the patients in standing position, the height was measured by means of a height rod integrated to the scale with a maximum height of 213cm; the results were expressed in meters. The body mass index (BMI) was calculated with these results using the formula weight/height2 (kg/m2), classifying the results within the ranks of low weight: <18.5kg/m2; normal: 18.5–24.9kg/m2; overweight: 25–29.9kg/m2; type I obesity: 30–34.9kg/m2; type II obesity: 35–39.9kg/m2; and type III obesity: ≥40kg/m2.
With the patient sitting, after 20min of rest, the blood pressure was taken using a mercury column sphygmomanometer of the American Diagnostic Corporation brand, adequate for the width of the arm of each patient.
Determination of biochemical parametersAfter fasting for 8h, venous blood samples (10ml) were taken from the antecubital region in the morning (8:00–9:00h), to measure the values of triglycerides, total cholesterol, HDL-c, glucose, and ultrasensitive CRP. The samples were analyzed in the automated equipment ILab 350 (instrumentation laboratory). The levels of LDL-c were obtained indirectly using the Friedewald formula.
As positive risk factors were considered glucose levels >100mg/dl, due to the relationship that exists between CVR and the pre-diabetes glycemic levels,22 the concentrations of lipids considered by the ATP III guidelines23 as high or low in the case of HDL-c, that is, triglycerides>200mg/dl, LDL-c>160mg/dl, HDL-c<40mg/dl; and concentrations of ultrasensitive CRP>2mg/l.10
Determination of disease-related factorsThe time of duration of the disease was recorded, the disease activity was assessed using the SLEDAI-2K, classifying it as inactivity or mild activity <4 points, moderate from 4 to 8 points, and severe >8 points,11 the presence of lupus nephritis was determined according to the definition of the SLICC group21: presence of proteinuria ≥500mg/24h or proteinuria/creatinine ≥50mg/mmol, presence of red cell casts or presence of renal biopsy compatible with lupus nephropathy plus the presence of anti-DNA-ds or antinuclear (ANA) antibodies.
The determination of the antibodies was carried out by means of the ELISA technique (Orgentec Diagnostika, Germany), expressing as normal cut-off values those recommended by the manufacturer, which were negative for anti-Sm, ANA, LA, for anti-DNA-ds of 0–5.2UI/ml, aCL IgG 0–23 and IgM 0–11, anti B2GPI IgG 0–20 and IgM 0–15. The serum concentrations of the complement proteins were determined by the nephelometry technique (Beckman Coulter Izasa, California, United States) considering as normal ranks, C3 of 90–180mg/dl, C4 10–40mg/dl and CH50 200–288U/ml.
Determination of cardiovascular riskThe CVR was calculated using the modified Framingham score,24 which assesses the risk that a person has of suffering from a cardiovascular event over the next 10 years, taking as variables the age, gender, levels of total cholesterol and HDL-c, cigarette smoking, presence or absence of DM, levels of systolic blood pressure and the use of antihypertensive drugs. Considering as low risk values <1%, moderate 1–5% and high >5%.
Statistical analysisA statistical analysis was carried out using the SPSS 22.0 package for Windows, considering as a significant result a p-value ≤0.05. The normal distribution of the continuous variables was evaluated using the Kolmogorov–Smirnov test. The continuous variables with normal distribution were presented as mean and standard deviation, and those without normal distribution were presented as median and interquartile range.
The categorical variables were presented as frequencies and percentages, and were compared using the Chi-square test. Finally, the odds ratio was calculated in order to determine the specific weight that the significant traditional and non-traditional risk factors had on the CVR.
ResultsDuring the established period, 65 patients with diagnosis of SLE attended the rheumatology outpatient service; only 51 met the inclusion criteria. 92.1% of the studied population was of female gender.
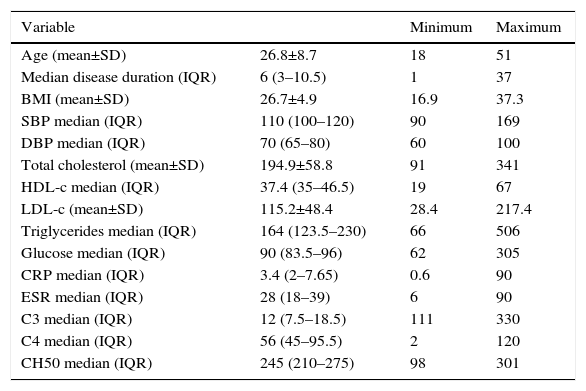
The general characteristics are shown in Table 1. The mean age at diagnosis was 26.84±8.8 years. Regarding the BMI, the normal weight predominated with 39.2%, followed by overweight with 31.5%, 23.5% had type I, obesity 3.9% type II obesity and 1.9% exhibited low weight. 15.7% had systolic blood pressures higher than 140mmHg, and 7.8%, had diastolic blood pressures over 90mmHg.
General characteristics of the studied patients with systemic lupus erythematosus.
| Variable | Minimum | Maximum | |
|---|---|---|---|
| Age (mean±SD) | 26.8±8.7 | 18 | 51 |
| Median disease duration (IQR) | 6 (3–10.5) | 1 | 37 |
| BMI (mean±SD) | 26.7±4.9 | 16.9 | 37.3 |
| SBP median (IQR) | 110 (100–120) | 90 | 169 |
| DBP median (IQR) | 70 (65–80) | 60 | 100 |
| Total cholesterol (mean±SD) | 194.9±58.8 | 91 | 341 |
| HDL-c median (IQR) | 37.4 (35–46.5) | 19 | 67 |
| LDL-c (mean±SD) | 115.2±48.4 | 28.4 | 217.4 |
| Triglycerides median (IQR) | 164 (123.5–230) | 66 | 506 |
| Glucose median (IQR) | 90 (83.5–96) | 62 | 305 |
| CRP median (IQR) | 3.4 (2–7.65) | 0.6 | 90 |
| ESR median (IQR) | 28 (18–39) | 6 | 90 |
| C3 median (IQR) | 12 (7.5–18.5) | 111 | 330 |
| C4 median (IQR) | 56 (45–95.5) | 2 | 120 |
| CH50 median (IQR) | 245 (210–275) | 98 | 301 |
HDL-c: high density lipoprotein; LDL-c: low density lipoprotein; C3: C3 complement; C4: C4 complement; CH50: hemolytic complement 50; SD: standard deviation; BMI: body mass index; CRP: C-reactive protein; IQR: interquartile range; DBP: diastolic blood pressure; SBP: systolic blood pressure; ESR: erythrocyte sedimentation rate.
With regard to the C3 complement, 70.6% had low concentrations, 27.5% normal, and 1.9% high. 56.9% had normal C4 complement, 41.2% low and 1.9% high. Finally, el 76.5% had normal concentrations of CH50, 13.7% high, and 9.8% low.
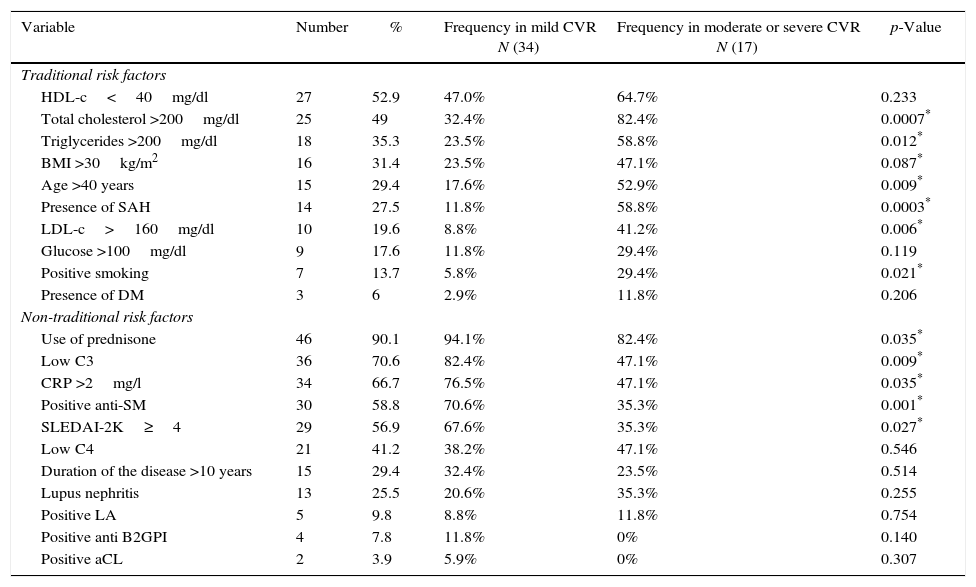
In Table 2, can be seen the frequency of the traditional and non-traditional risk factors, as well as their relationship with the CVR <1% (mild) or >1% (moderate or severe). The traditional risk factors which predominated were, in order of frequency, hypoalphalipoproteinemia, hypercholesterolemia, hypertriglyceridemia and obesity. 68.6% of the studied patients had dyslipidemia. The predominant non-traditional risk factors were the use of glucocorticoids, 53% used more than 20mg/day of prednisone; secondly, low levels of C3 were found, followed by CRP >2mg/l and positive anti-Sm. According to the activity index, 43.1% of patients had mild activity, 27.5% had moderate activity, and 29.4% severe. 78.4% had positive ANA and 47% had positive anti-DNA-ds.
Frequency of the traditional and non-traditional risk factors present in the patients with SLE and their relationship to cardiovascular risk.
| Variable | Number | % | Frequency in mild CVR N (34) | Frequency in moderate or severe CVR N (17) | p-Value |
|---|---|---|---|---|---|
| Traditional risk factors | |||||
| HDL-c<40mg/dl | 27 | 52.9 | 47.0% | 64.7% | 0.233 |
| Total cholesterol >200mg/dl | 25 | 49 | 32.4% | 82.4% | 0.0007* |
| Triglycerides >200mg/dl | 18 | 35.3 | 23.5% | 58.8% | 0.012* |
| BMI >30kg/m2 | 16 | 31.4 | 23.5% | 47.1% | 0.087* |
| Age >40 years | 15 | 29.4 | 17.6% | 52.9% | 0.009* |
| Presence of SAH | 14 | 27.5 | 11.8% | 58.8% | 0.0003* |
| LDL-c>160mg/dl | 10 | 19.6 | 8.8% | 41.2% | 0.006* |
| Glucose >100mg/dl | 9 | 17.6 | 11.8% | 29.4% | 0.119 |
| Positive smoking | 7 | 13.7 | 5.8% | 29.4% | 0.021* |
| Presence of DM | 3 | 6 | 2.9% | 11.8% | 0.206 |
| Non-traditional risk factors | |||||
| Use of prednisone | 46 | 90.1 | 94.1% | 82.4% | 0.035* |
| Low C3 | 36 | 70.6 | 82.4% | 47.1% | 0.009* |
| CRP >2mg/l | 34 | 66.7 | 76.5% | 47.1% | 0.035* |
| Positive anti-SM | 30 | 58.8 | 70.6% | 35.3% | 0.001* |
| SLEDAI-2K≥4 | 29 | 56.9 | 67.6% | 35.3% | 0.027* |
| Low C4 | 21 | 41.2 | 38.2% | 47.1% | 0.546 |
| Duration of the disease >10 years | 15 | 29.4 | 32.4% | 23.5% | 0.514 |
| Lupus nephritis | 13 | 25.5 | 20.6% | 35.3% | 0.255 |
| Positive LA | 5 | 9.8 | 8.8% | 11.8% | 0.754 |
| Positive anti B2GPI | 4 | 7.8 | 11.8% | 0% | 0.140 |
| Positive aCL | 2 | 3.9 | 5.9% | 0% | 0.307 |
LA: lupus anticoagulant; aCL: anticardiolipin; Anti-SM: anti-Smith; B2GPI: beta-2-glycoprotein-I; HDL-c: high density lipoprotein; LDL-c: low density lipoprotein; C3: C3 complement; C4: C4 complement; DM: diabetes mellitus; SAH: systemic arterial hypertension; BMI: body mass index; CRP: C-reactive protein; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000.
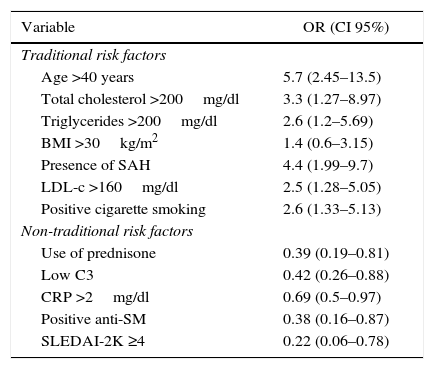
Concerning the CVR, 66.7% had low risk, 25.5% moderate and 7.8 severe. The odds ratio was established in order to determine the specific weight of each risk factor in severe CVR, which is summarized in Table 3.
Specific weight of the traditional and non-traditional risk factors in the moderate-severe cardiovascular risk in patients with systemic erythematosus lupus.
| Variable | OR (CI 95%) |
|---|---|
| Traditional risk factors | |
| Age >40 years | 5.7 (2.45–13.5) |
| Total cholesterol >200mg/dl | 3.3 (1.27–8.97) |
| Triglycerides >200mg/dl | 2.6 (1.2–5.69) |
| BMI >30kg/m2 | 1.4 (0.6–3.15) |
| Presence of SAH | 4.4 (1.99–9.7) |
| LDL-c >160mg/dl | 2.5 (1.28–5.05) |
| Positive cigarette smoking | 2.6 (1.33–5.13) |
| Non-traditional risk factors | |
| Use of prednisone | 0.39 (0.19–0.81) |
| Low C3 | 0.42 (0.26–0.88) |
| CRP >2mg/dl | 0.69 (0.5–0.97) |
| Positive anti-SM | 0.38 (0.16–0.87) |
| SLEDAI-2K ≥4 | 0.22 (0.06–0.78) |
Anti-SM: anti-Smith; LDL-c: low density lipoprotein; C3: C3 complement; SAH: systemic arterial hypertension; CI: confidence interval; BMI: body mass index; CRP: C-reactive protein; OR: odds ratio; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index 2000.
Due to the high risk of cardiovascular disease in SLE, a proper assessment of the CVR is essential, the Framingham risk score evaluates a number of CVR factors to estimate the likelihood of developing coronary disease within the next 10 years. This score is very useful in daily practice, in the general population, to stratify the risk and determine the therapeutic intervention; however, in patients who have chronic inflammation, as in the case of SLE patients, these scales underestimate the CVR. This was demonstrated in our study, as expected, the traditional factors were more frequent and there were significant differences for a moderate to severe CVR; however, what draws attention is that the patients who had mild risk, exhibit a higher frequency of non-traditional CVR factors, being significant the use of prednisone, low C3, CRP >2mg/dl, positive anti-SM and SLEDAI-2K ≥4 points. The importance of this lies in the fact that the non-traditional factors increase the morbidity and mortality from cardiovascular causes in patients with autoimmune or inflammatory diseases. When establishing an odds ratio between these factors and severe CVR, a negative association was found, which confirms the little usefulness of this type of risk scales, not including factors associated with the disease and inflammation, which are even most frequently found in patients with CVR staged as mild. However, the results of this study should be analyzed with caution, given the sample size and the type of cross-sectional study.
This is because the analyses of the OR such as of protection or risk in this type of studies should be carried out in a cautious way, since if it there was a longer time of follow-up, as well as a larger sample size, they might be different from those currently observed.
In our population, when determining the Framingham risk we found results similar to those reported by O’Neil et al.25 where only 3.57% of the patients with SLE presented severe risk. This underestimated risk, since despite the fact that cardiovascular events are up to 5 times more frequent and less than 5% are classified at high risk of suffering an event within the next 10 years, is due to the presence of non-traditional CVR factors, which are not part of the variables studied in these scales. For that reason, the European League Against Rheumatism recommends multiplying by 1.5 the value of CVR in patients with SLE.26
There are other scales used in this type of population, such as the Reynolds Risk Score,27 which, in addition to the traditional risk factors, also uses high levels of CRP; however, although it has been observed that it estimates a greater risk than the Framingham scale, the majority still remains below 10% of risk, which is still not of great significance, since this cut-off is the one used for the beginning of the treatment.
Another method is the SLE cardiovascular risk equation,28 which has shown to estimate much better the 10-year risk in patients with SLE compared with the Framingham Scale, this is because it uses variables that are associated to the disease activity, as is the case of SLEDAI, low levels of C3, and history of lupus anticoagulant, which, as we observed in our study, are frequent factors and may even have a greater presentation in patients with low risk calculated by Framingham.
The lupus pattern of dyslipoproteinemia was observed in our study population. In a study carried out in our country was found that 48.4% of the adult population has hypoalphalipoproteinemia, 42.3% hypertriglyceridemia and 27.1% hypercholesterolemia.29 The hypoalphalipoproteinemia and the hypercholesterolemia found in our population were higher than the reported in the general population, this may be due to the fact that the Mexican population is among those with higher frequency of this type of lipid alterations, as well as of metabolic syndrome.30 The percentage of dyslipidemia in the patients of our study was lower than the reported by Wijaya et al.31 (75.3%) in Indonesian patients with SLE, this is because the values used in this study as cut-off for dyslipidemia were lower than those used by us, since we considered the high cut-off values according to ATP III. The dyslipidemia in our population was higher than that found by Amaya et al.32 in Colombia (18.1%); as previously mentioned, the Mexican population has a higher frequency of hypoalphalipoproteinemia and hypertriglyceridemia, compared with other populations of Latin America.30
In a study conducted by Bravo et al.,33 in 24 patients with SLE they found an average BMI of 26.7kg/m2, 50% had normal weight, 20.8% overweight and 29.2% obesity; in a study carried out by Navarro et al.20 the average BMI was 28.2kg/m2, 40.9% had normal weight, 40.9% obesity and 18.2% overweight, the means of these two studies coincided with what was reported in our population.
Patients with SLE have greater insulin resistance. This is due to the high incidence of metabolic syndrome in this population, chronic inflammation, and the use of glucocorticoids; however, only 5% of patients with SLE develop DM.34 This frequency coincides with what was reported in our population.
Between 30% and 48% of patients with SLE exhibit a higher prevalence of SAH than the general population. This is probably due to the use of glucocorticoids and the development of lupus nephritis.35 The frequency of SAH in our population was lower than the expected.
As part of the treatment for the control of the disease activity, patients with SLE use glucocorticoids, as was also observed in our study where more than 90% received them, this may be due to the fact that our population had high activity data, since 56.9% had low or moderate activity, and 66.7% had elevated CRP. In our population the non-traditional CVR factor that prevailed was the use of glucocorticoids, which favors the development of atherosclerosis since it increases the frequency of SAH, dyslipidemia, hyperglycemia, and central obesity with increased BMI.19
There was a high frequency of CRP >2mg/dl in our population, this increases the CVR, because it participates in the atherogenic process, since it induces the production of inflammatory cells and decreases the expression of nitric oxide synthase.36
Regarding the antibodies, the frequency of anti-Sm found in our population was much higher than the reported in the literature, which is 25%37; however, with respect to the antiphospholipid antibodies, the reported in our population was found far below than expected, since the frequency of LA in patients with SLE is 25%, 23% have positive aCL, and 20% anti B2GPI.37
Another non-traditional CVR factor that predominated in our population was the time of evolution of the disease longer than 10 years, since almost 30% presented it; this increases the risk of accelerated atherosclerosis, because there is a greater exposition to the inflammation secondary to the disease activity, with the subsequent endothelial damage.
The frequency of lupus nephritis found in our population coincides with the expected which is 27%.37 Our population had a high frequency of hypocomplementemia and disease activity, which speaks of chronic inflammation and greater CVR.
The results obtained in our study highlight the importance of the adequate control of the disease, which reduces the non-traditional risk factors, likewise, the moderate use of glucocorticoids is important to reduce their indirect effects on the traditional risks, as well as prioritize the use of drugs in which it has been observed a beneficial factor on the CVR, as is the case of hydroxychloroquine, which improves the lipid profile, reduces the disease activity and the risk of lupus nephritis.38 With regard to the use of statins, it has been observed that in rheumatic diseases such as rheumatoid arthritis, the use of these lipid-lowering drugs reduces the clinical and biological parameters of inflammation and is associated with improvement of the endothelial dysfunction.39 As established in the ACC/AHA guidelines for the treatment of high cholesterol published in 2013,40 the diabetic patients with LDL-c between 70 and 189mg/dl and a CVR ≥7.5% should receive treatment with statins; if we take into account that those patients with rheumatic diseases have the same CVR that the patients with DM, the use of statins could be considered in them.
Among the limitations of our study there is the number of patients included, likewise, regarding the use of glucocorticoids, the determination of the average dose employed would have had a better translation in the results; however, the strict selection of the patients without previous cardiovascular events, as well as the determination of the frequencies of all the non-traditional factors known in this type of population, should be considered as a strength of the study.
ConclusionWith the use of drugs that allow an adequate control of the disease and with the moderate use of glucocorticoids, which has decreased the infectious processes; the cardiovascular disease has become one of the main problems in the patients with SLE. The traditional methods to estimate the CVR in the general population, underestimate the risk in patients with inflammatory or autoimmune diseases. For this reason, it is important to design strategies to reduce the non-traditional risk factors, and thereby, to decrease the morbidity and mortality from cardiovascular complications.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare that there are no conflicts of interest in the publication of this work and that we have followed the protocols established in our institution for the publication of this material.
Please cite this article as: Batún Garrido JAJ, Radillo Alba HA, Hernández Núñez E. Riesgo cardiovascular en lupus eritematoso sistémico. Rev Colomb Reumatol. 2016;23:242–249.