Systemic lupus erythematosus (SLE) is a chronic, autoimmune disease of unknown aetiology that affects any organ or tissue. Lupus Nephritis (LN) is the most common cause of kidney involvement in SLE. Approximately 50% of patients with SLE suffer LN at some point in their disease, being a risk factor for morbidity and mortality.
ObjectiveTo provide updated information on LN, evaluating the pathophysiology, clinical manifestations and placing special emphasis on the diagnosis and therapeutic strategies used in clinical practice.
Materials and methodsA narrative review was carried out regarding patients with SLE who developed LN in the Google Scholar, Embase, SciELO, Scopus and Medline databases using the MeSH terms lupus nephritis, biopsy, systemic lupus erythematosus, treatment.
ResultsA total of 50 studies were chosen that met the search requirements. These included 18 original articles, 11 reviews, 9 cases and controls, 7 cohort studies, and 5 experimental studies. The pathophysiology is heterogeneous and genetic and environmental factors contribute to it. Proteinuria, haematuria, and tubular abnormalities are among the main clinical manifestations. There is no single way to treat LN, it varies according to the severity of the disease and the risk of progressive kidney damage; according to the renal biopsy result, standardized by the ISN / RPS classification.
ConclusionsThe purpose of treatment is to improve kidney function, decrease proteinuria, correct immunological markers, avoiding the appearance of complications. To improve the prognosis, new techniques must be developed that will allow us to evaluate the onset of kidney disease activity or its relapse to initiate early management, generating a reduction in mortality and improving quality of life.
El lupus eritematoso sistémico (LES) es una enfermedad crónica, autoinmune, de etiología desconocida, que afecta a cualquier órgano o tejido. La nefritis lúpica (NL) es la causa más frecuente de compromiso renal en LES. Aproximadamente el 50% de los pacientes con LES sufren NL en algún momento de su enfermedad, siendo un factor de riesgo para morbimortalidad.
ObjetivoProveer información actualizada sobre la NL, evaluando la fisiopatología y las manifestaciones clínicas, como también poniendo especial énfasis en el diagnóstico y las estrategias terapéuticas utilizadas en la práctica clínica.
Materiales y métodosSe realizó una revisión narrativa con respecto a pacientes con LES que desarrollaron NL, en las bases de datos Google Scholar, Embase, SciELO, Scopus y Medline, utilizando los términos MeSH nefritis lúpica, biopsia, lupus eritematoso sistémico y tratamiento.
ResultadosSe escogieron 54 estudios que llenaban los requisitos de la búsqueda, 18 fueron artículos originales, 13 revisiones de temas, nueve casos y controles, siete estudios de cohorte y siete estudios experimentales. La fisiopatología es heterogénea y los factores genéticos y ambientales contribuyen a ella. Entre las principales manifestaciones clínicas se encuentran la proteinuria, la hematuria y las anormalidades tubulares. No existe una forma exclusiva de tratar la NL, varía según la gravedad de la enfermedad y el riesgo de daño renal progresivo, de acuerdo con el resultado de la biopsia renal estandarizada por la clasificación de ISN/RPS.
ConclusionesEl objetivo del tratamiento es mejorar la función renal, disminuir la proteinuria y corregir marcadores inmunológicos, evitando así la aparición de complicaciones. Para mejorar el pronóstico deben desarrollarse nuevas técnicas que permitan evaluar el inicio temprano de la actividad de la enfermedad renal o su recaída para dar inicio al manejo temprano, de manera que se genere una reducción en la mortalidad y que la calidad de vida mejore.
Systemic lupus erythematosus (SLE) is an autoimmune disease of unknown aetiology that is characterized by the production of autoantibodies against a wide range of autoantigens, including DNA, RNA, histones, and other nuclear components. It is a multisystem disease, with wide clinical variability, which affects whichever organ or system, such as the skin, mucous membranes, joints, brain, heart, kidneys, lungs and gastrointestinal tract.1
The heterogeneous symptoms of the disease make its characterization in patients very difficult, since the same patterns among them do not exist, which complicates its diagnosis. For this reason, the prevalence of the disease is variable, ranging from one per 100,000 in the Danish population up to 8.7 per 100,000 in Brazil.2 Geographically, there is versatility in the initial presentation of SLE, in such a way that in European patients cutaneous manifestations are more common and in African patients renal manifestations tend to appear more frequently.2 In Colombia, in 2016, the registries reported 41,804 patients with SLE, for an estimated prevalence of 91.9/100,000 subjects (based on a total population of 47,663,162 inhabitants), being more frequent in women (89% of cases), with a 7.9:1 ratio, higher in the age group of 45–49 years.3
The kidneys are among the organs commonly affected by this disease: up to 60% of people with SLE may have renal involvement and between 25 and 30% have renal manifestations at the time of diagnosis, which may or may not be associated with general involvement.4 Lupus nephritis (LN) occurs in approximately 50% of the patients with SLE and is the most common cause, but not the only one, of kidney injury in this pathology. LN is one of the most serious manifestations of SLE, which usually presents in the first 5years of the disease, being one of the predictive factors for morbidity and mortality, and it may even be present in the initial diagnosis.5
This review aims to make a description of LN, evaluating its pathophysiology and clinical manifestations, and placing special emphasis on the diagnosis and therapeutic strategies used in clinical practice that impact both the severity of the disease and the quality of life of the patients.
MethodsLiterature searchA systematic search of articles until December 2020 was performed, in order to evaluate the advances in the treatment of LN and their impact on the remission of the disease. Primary studies, systematic reviews and meta-analyses were searched in the main scientific databases Google Scholar, Embase, SciELO, Scopus and Medline, using the MeSH terms “Lupus nephritis”, “Treatment”, “Biopsy” and “Systemic lupus erythematosus”, linked by the Boolean connector AND, for each of the components of the PICO question (problem, intervention, comparator, outcomes). The search was restricted to articles in Spanish and English.
Selection of articles and extraction of informationAfter completing the search, the articles were compiled in a database built in Microsoft Excel; those that were duplicated were excluded and those that met the inclusion criteria, which contained the keywords in the title or in the abstract, were included. Finally, for the selection of the relevant articles, a consensus was made among all the authors to unify and review the database.
Eligibility criteriaOriginal articles, cohort studies, case-control studies, topic reviews, and experimental studies involving adult patients with SLE who had developed LN were included. The intervention consisted of studies that described the pathophysiology, clinical manifestations, classification, and indications for biopsy, as well as a diagnostic-therapeutic approach for LN. In addition to this, studies reporting the clinical outcomes of patients with LN were also eligible. The analytical studies included all those that considered the exposure, target population, and bias control strategies.
Duplicate articles, without access to full text, case reports and case series were excluded; studies that had not been conducted in the population of interest or that did not present relevant results for that population separately from those of other populations were also discarded.
Data extractionThe studies that fulfilled the inclusion criteria were analyzed for data extraction. Data were extracted independently by 2 of the authors (MCMA and JDRB). Subsequently, the results were reassessed by other authors (AJAH, TRY) in order to analyze the consistency of the data, for which all the titles or abstracts of the publications found were reviewed and the eligibility assessment was carried out. Thereafter, another author (RDA) compiled in Microsoft Excel the information regarding the population, study design, clinical characteristics, treatment and results of all the studies included. The articles retrieved were rejected if they did not meet the eligibility criteria. Finally, another researcher (GAM) verified the information extracted. Any discrepancy or missing information was resolved by consensus. Meta-analysis was not performed due to the high heterogeneity of the studies.
ResultsAfter the initial search with the search terms, 2754 articles were found, the majority in Scopus and Google Scholar. Then, duplicates were removed, the titles and abstracts were examined, and 1590 articles were excluded. In addition, 10 studies that did not include outcomes in remission criteria were excluded in the selection phase. Eligibility criteria were applied to 184 full texts. Likewise, 134 articles whose titles contained the search terms were excluded because it was not possible to find the full texts. Finally, 54 studies that met the search requirements were selected, 18 were original articles, 13 topic reviews, 9 cases and controls, 7 cohort studies and 7 experimental studies. The most relevant aspects found in the literature review are described below.
Epidemiology and pathophysiologyAbout 74% of patients with SLE will develop LN at some point during the course of their disease.6 The prevalence of LN is variable in different regions: in the United States and Canada is 4.8–78.5% per 100,000 inhabitants; in Europe, from 25 to 91% per 100,000 inhabitants; in Australia, from 19 to 63% per 100,000 inhabitants; in China, from 30 to 50% per 100,000 inhabitants; in Japan, from 8 to 18% per 100,000 inhabitants.3 In Colombia, 50 to 55% of adults7 and 75% of children8 with SLE present LN at some point of their evolution.
The heterogeneous pathophysiology of LN is a consequence of the interaction between genetic, environmental, and sociodemographic factors that influence clinical manifestations and renal involvement.9 This commitment ranges from a silent nephritis to a nephrotic syndrome with deterioration of glomerular filtration. The latter has a rapid progression to end-stage renal disease, which determines the need for early diagnosis.
In relation to sociodemographic data, men with SLE tend to have a more aggressive disease, with greater renal and cardiovascular involvement, as well as a higher probability of developing end-stage chronic renal failure (ESRD) than women. Factors such as income, educational level and access to health services are important variables in the prognosis of SLE.10,11 Male gender, early age at diagnosis, poverty and difficult access to healthcare system are among the risk factors for the development of LN.12
There is an enormous racial expression that conditions the appearance or evolution of LN. In a North American multicenter study, the incidence of LN and progression to ESRD is higher in African Americans (51%), followed by Hispanics (43%) and Asians (35%), compared with Caucasian patients (14–23%).13 It has been observed that African-Americans and Hispanics develop a more severe disease and with worse evolution than Caucasians,14–16 with a histopathological characterization that is expressed by proliferative forms and higher levels of serum creatinine and proteinuria at the time of the diagnosis of LN, which leads to a higher probability of IRCT.17
Renal survival 10 years after diagnosis is significantly higher in Hispanic and Caucasian patients than in Afro-descendants (68% vs. 31%).18 However, it is essential to achieve a complete clinical response to treatment to preserve long-term renal health. Mortality from LN occurs in 5%–25% of patients with proliferative LN within 5years after onset.
Genomic association studies have shown the presence of more than 50 genetic polymorphisms that influence the appearance of LN, including apolipoprotein L1, platelet-derived growth factor receptor alpha, and hyaluronan synthase 2.19 Modifications in the alleles that correspond to the major histocompatibility complex, particularly HLA-DR4 and HLA-DR11, generate a protective factor against LN, while DR3 and HLA-DR15 entail a higher risk.20 Other associations such as those of STAT4, PTPN 22 and ITGAM are also present.21 Other studies link allelic variants in immunoglobulin G (IgG) receptors that likely contribute to racial and ethnic disparities in SLE and LN. Further studies are needed to explain the relationship and genetic contribution to the development and risk of LN.22
LN develops in individuals with an unfortunate combination of genetic variants that predispose and compromise the maintenance of immune tolerance to endogenous nuclear material. The production of abnormal alterations in innate and adaptive immunity influences the pathogenesis of the disease; the appearance of autoantibodies constitutes an indispensable requirement. Autoantibodies directed against cellular nuclear antigens, such as DNA, Ro, Smith, C1q, alpha actinin, anti-annexin, ribosomal protein and anti-nucleosome, lead to the formation or immune complexes that accumulate in different renal structures, such as the glomerular basement membrane, mesangial cells, epithelial cells of the proximal tubule, podocytes, endothelial and epithelial glomerular cells; and can also be formed in situ.23 T and B cells contribute to the progression of the disease, while the production of restricted nephritogenic autoantibodies by clonation, recruitment of macrophages and production of proinflammatory cytokines interleukine (IL)-2, IL-8 and alpha interferon lead to the formation of immune complexes which produce inflammation, infiltration, intrarenal injury and development of proteinuria.24
The consequence of the loss of tolerance is self-vaccination and persistence of elevated antinuclear antibodies (ANA) throughout life, indicating persistently active autoreactive T and B cell clones. Only a subgroup of patients develops clinical symptoms, often with infections (viral) or hormonal influences that provide a non-specific stimulus for the expansion of these autoreactive lymphocyte clones.25
Adults continuously lose podocytes, which are not replaced, leading initially to focal-segmental glomerulopathy that can progress later to focal-global glomerulosclerosis. This is a cause of aging, loss of nephrons, and increased incidence of ESRD in the elderly population. A single episode of LN in the first years of life, even if it is well treated and controlled, can lead to significant loss of podocytes and nephrons, which synergizes with age-related loss of nephrons in the future. Thus, a history of LN is a major risk factor for ESRD and exaggerated cardiovascular mortality decades before normal end of life.25
The activity of uncontrolled lupus nephritis accelerates the loss of nephrons and potentiates the risk of early end-stage renal disease and death. Given the hypertrophy that occurs in the remaining nephrons, the glomerular filtration rate (GFR) significantly overestimates the number of nephrons. This implies that a slightly increased creatinine of 1.3mg/dl, which represents a GFR of 45ml/min, may be generated by only 35% of the original nephrons, that is, a more advanced loss of renal mass caused by the systemic autoimmunity associated with loss of the renal reserve and persistent hyperfiltration. The loss of autoregulation of the renal perfusion, which is particularly important in patients with arterial hypertension, a population in which proteinuria, urinary sediments and cells are currently used as biomarkers, does not reflect the number of nephrons. The clinical application of a biomarker that identifies the number of nephrons remains to be identified and validated.25
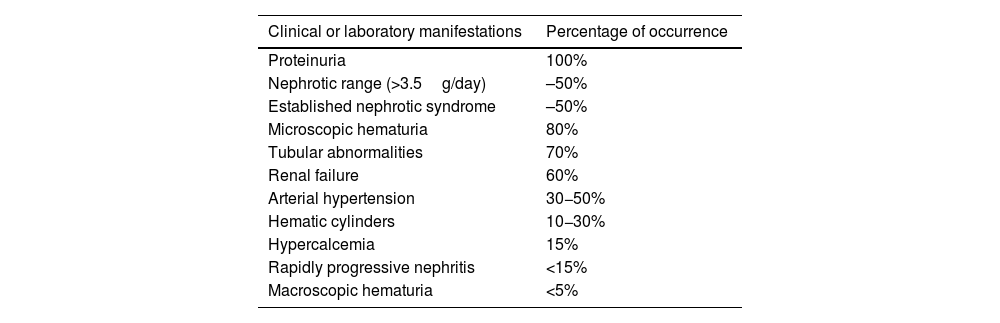
Clinical manifestations and diagnosisLN occurs in patients with known SLE; however, in some cases it presents as an isolated manifestation of the disease, without evident signs of SLE, which in these cases is a medical challenge in the diagnostic approach. The most common clinical characteristics found in LN (Table 1) are proteinuria, abnormal urine sediment due to microscopic hematuria and red blood cell casts, kidney injury and arterial hypertension.4,26
Clinical manifestations of lupus nephritis.
| Clinical or laboratory manifestations | Percentage of occurrence |
|---|---|
| Proteinuria | 100% |
| Nephrotic range (>3.5g/day) | –50% |
| Established nephrotic syndrome | –50% |
| Microscopic hematuria | 80% |
| Tubular abnormalities | 70% |
| Renal failure | 60% |
| Arterial hypertension | 30−50% |
| Hematic cylinders | 10−30% |
| Hypercalcemia | 15% |
| Rapidly progressive nephritis | <15% |
| Macroscopic hematuria | <5% |
Proteinuria is the main manifestation of LN and must be present for the clinical diagnosis of LN. It occurs in 50% in the nephrotic range and the other 50% associated with an established nephrotic syndrome. In patients with already established nephrotic syndrome, the risk of complications arising from LN is higher and is associated with hypercholesterolemia and renal vein thrombosis. Arterial hypertension is more common in those who present more severe forms of LN.
As for kidney injury, it is measured by a decrease in the GFR and an increase in serum creatinine. The presence of 5 leukocytes or erythrocytes (isolated pyuria or hematuria) in a midstream urine sample, especially in the presence of traces of albumin, is indicative of active LN.27 Generally, the magnitude of the kidney damage is not proportional to the amount or intensity of extrarenal symptoms.27
The characteristic course of LN is constituted by flare-up episodes or exacerbations of the disease followed by a period of quiescence. It is important to evaluate lupus activity when LN is suspected, assessing autoimmunity and systemic involvement: central nervous, cardiac, pulmonary or hematological system with hemolytic anemia or severe thrombocytopenia and severe pericardial effusion. Likewise, validated indices of activity such as the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) or the one of the National Institute of Health (NIH) should be evaluated.4,28
The diagnostic criteria of the American College of Rheumatology (ACR)29 and the recommendations of the European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA)30 are defined as the presence of persistent proteinuria>500mg/24h or 3+ in an occasional urine sample or the presence of cell casts (hematic, granular, tubular or mixed). The Systemic Lupus Erythematosus International Collaborating Clinics group (SLICC)31 defines it by the presence of proteinuria≥500mg/24h or proteinuria/creatinuria (UPCR)≥50mg/mmol or erythrocyte casts, and proposes that the presence of a renal biopsy compatible with LN plus the presence of ANA or anti-DNA are sufficient criteria to classify a patient with LN.
Even though the clinical symptoms and laboratory tests guide the diagnosis of LN, percutaneous renal biopsy (PRB) provides certainty and allows its characterization. Thus, the PRB has diagnostic, prognostic, and therapeutic value. PRB is important for the differential diagnosis with diseases such as thrombotic microangiopathy (TMA), antiphospholipid syndrome, minimal change disease, focal segmental glomerulosclerosis, and IgA nephropathy.32 It is the gold standard to determine the diagnosis and the classification of the degree of compromise of renal inflammation and scarring, it is essential for its therapeutic management and prognosis; however, being an invasive procedure with possible complications, it is inadequate for the follow-up series.26
PRB is indicated when there is suspicion of renal involvement, confirmed proteinuria higher than 0.5g/day or protein/creatinine ratio in a morning urine sample higher than 0.5g/day, active sediment (microhematuria/leukocyturia/cylindruria) or when there are changes in the evolutionary-clinical course of a known LN.33 Its prompt execution is associated with a better renal prognosis in the medium and long term. When the treatment is adequate, especially in patients with class iv nephropathy, 5year survival is 90%. It is important to diagnose silent lupus, which is mostly class ii, but there are classes iv and v, mixed forms that can have this type of behavior.34
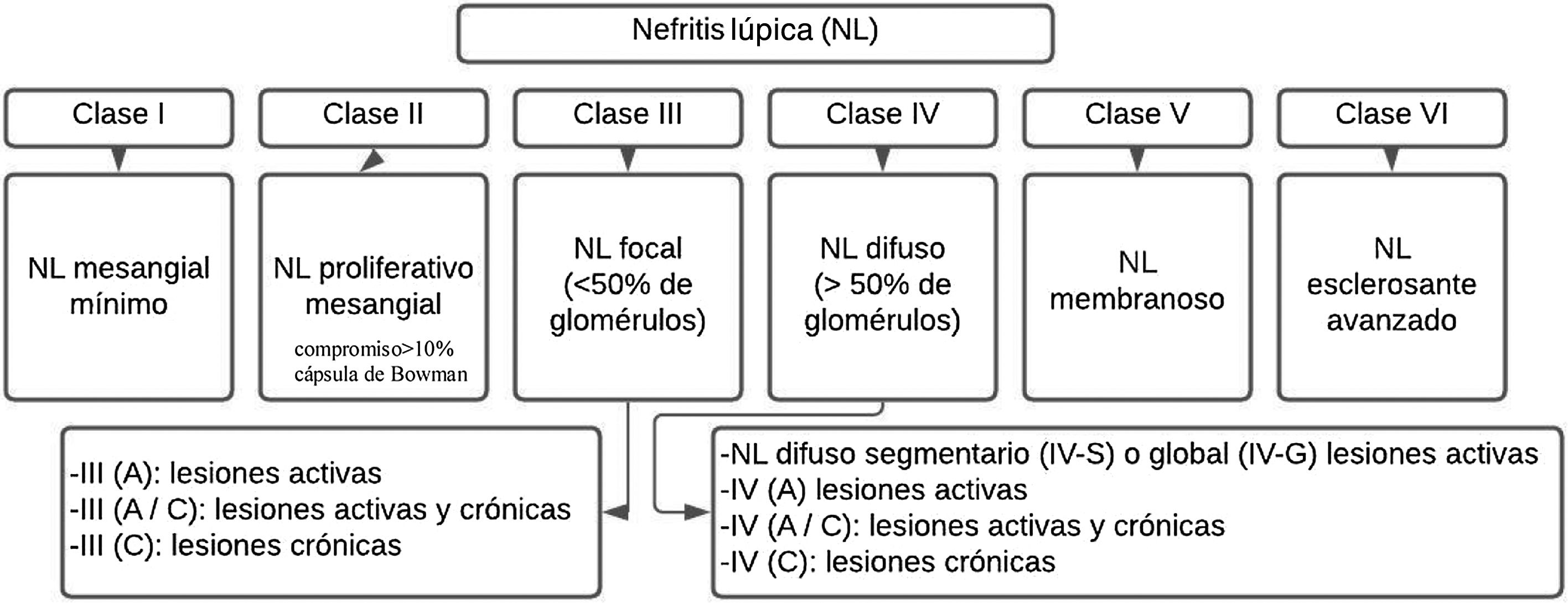
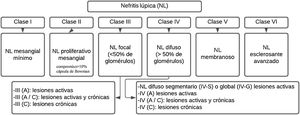
LN is pathologically described using the nomenclature of the International Society of Nephrology/Renal Pathology Society (ISN/RPS) 2003.35 (Fig. 1). This system classifies LN according to the site of accumulation of immune complexes in the glomeruli, by the presence or absence of mesangial or endocapillary proliferation, the general extent of glomerular involvement (focal or diffuse), and glomerular injury (global or segmental), and whether the glomerular lesion is active (inflammatory) or chronic (sclerotic). In this way, treatment decisions are guided.
Generally, patients with disease limited to the mesangium (class ii) do not require specific therapy for their renal disease, but may require immunosuppressive therapy for the extrarenal manifestations of SLE. Likewise, patients with mostly chronic lesion or end-stage damage (class vi) do no need immunosuppression for LN, but they can benefit from renoprotective antiproteinuric measures. Proliferative classes (iii and iv) are often treated with potent immunosuppression, while membranous non-proliferative LN (class v) can be managed conservatively (antiproteinuric therapy) if patients have subnephrotic proteinuria, or with immunosuppression if patients have nephrotic range proteinuria.4
Classes I and IIHistologically, a threshold of 3–4mesangial cells or areas not including the hilar region is proposed, recommendation that is aligned with the Oxford Classification of IgA Nephropathy I–IV, and it should be specified that the nuclei of the mesangial cells are surrounded by matrix.28
Classes III and IVCurrent recommendations consider that the term endocapillary proliferation is inappropriate and should be replaced by endocapillary hypercellularity. The term crescent should be used for a lesion consisting in extracapillary hypercellularity, which is composed of a variable mixture of cells, fibrin and fibrous matrix. Another criterion is a compromise of more than 10% of Bowman’s capsule. On the other hand, the term cellular crescent is defined by a commitment greater than 75% of the cells and fibrin, and less than 25% of the fibrous matrix.28,36
Finally, the fibrous crescent corresponds to cases where there is more than 75% of the fibrous matrix and less than 25% of the cells and fibrin, and the mixed pattern refers to when there is between 25 and 75% of cells and fibrin, and the remaining percentage is occupied by fibrous matrix. It is said that there is adherence when an isolated area of continuity of the material of the extracellular matrix is found between the tuft and the capsule, even when the underlying segment does not have evident sclerosis.35,36 The presence of fibrin associated with glomerular basement, membrane rupture or lysis of mesangial matrix is called fibrinoid necrosis and has the characteristic that it does not require karyorrhexis, like fibrinoid necrosis.35,36
Classes V and VITheir diagnosis is recommended based on the evidence and a phase 2 study is expected for their reclassification; the difficulty lies in the presence of an allowed subendothelial extension without cell deposits and it would have to be classified as class iii. Class vi is rarely seen in our renal biopsies; it has been proposed a reapraisal of this classification since there are difficulties in differentiating globally sclerotic glomeruli resulting from previous active lesions of lupus nephritis vs. non-specific global sclerosis associated with other factors (aging, hypertension, or healed TMA lesions).28
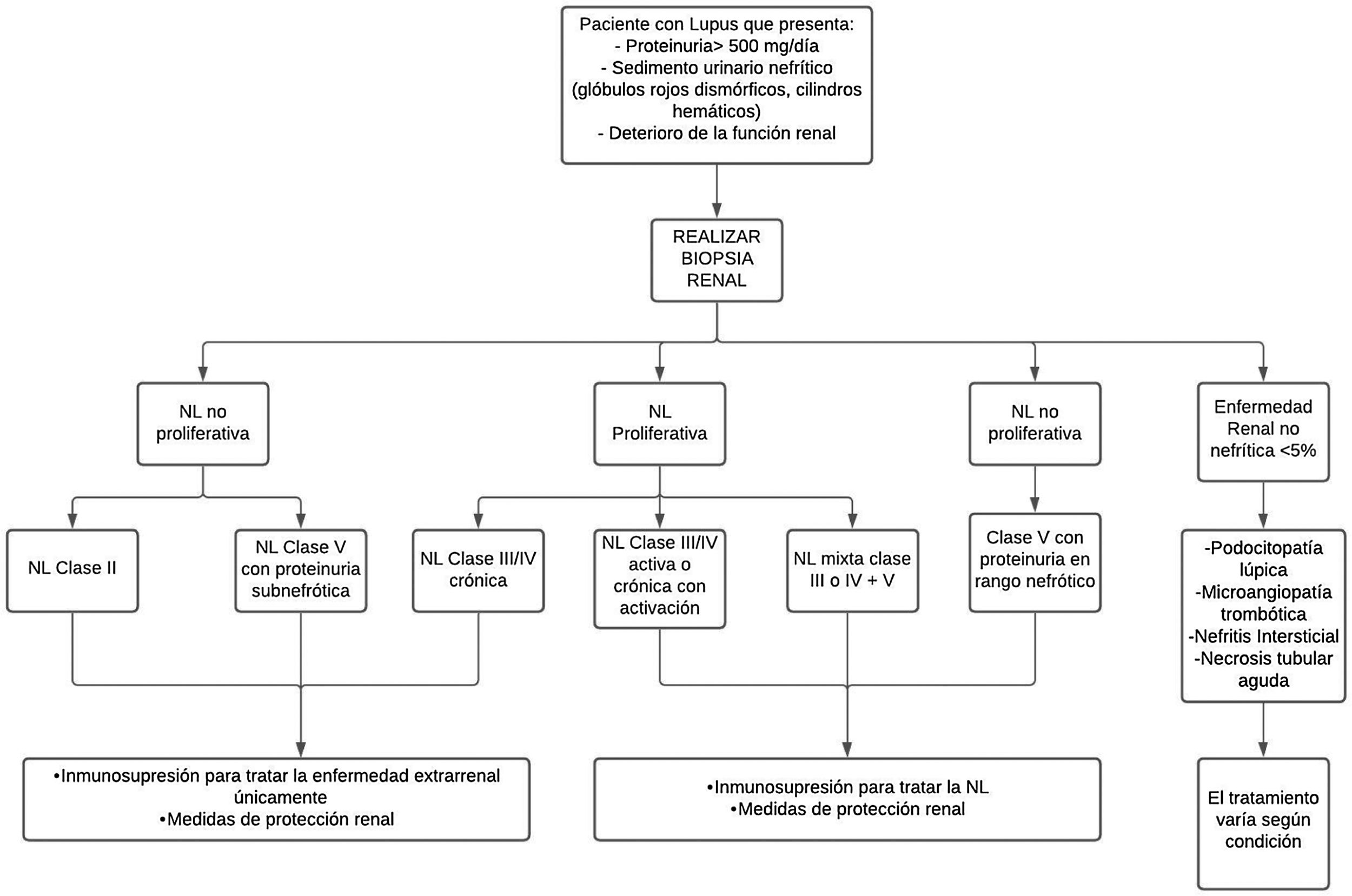
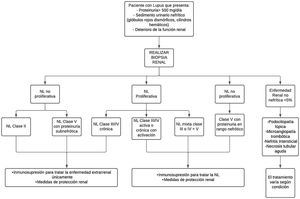
TreatmentAs for treatment, there is no single way to treat LN; it varies depending on the severity of the disease and the risk of progressive kidney damage, according to the result of the renal biopsy standardized by the ISN/RPS classification of LN35 (Fig. 2).
Diagnostic and treatment approach for lupus nephritis.
LN: lupus nephritis.
In patients with non-proliferative LN class ii and class v with proteinuria in the non-nephrotic range, or proliferative class iii/iv only chronic, treatment is carried out with immunosuppressive therapy for extrarenal management of the disease and renal protection measures. This therapy is used for all patients with any form of LN.37 In addition to immunotherapy, antiproteinuric strategies are included.
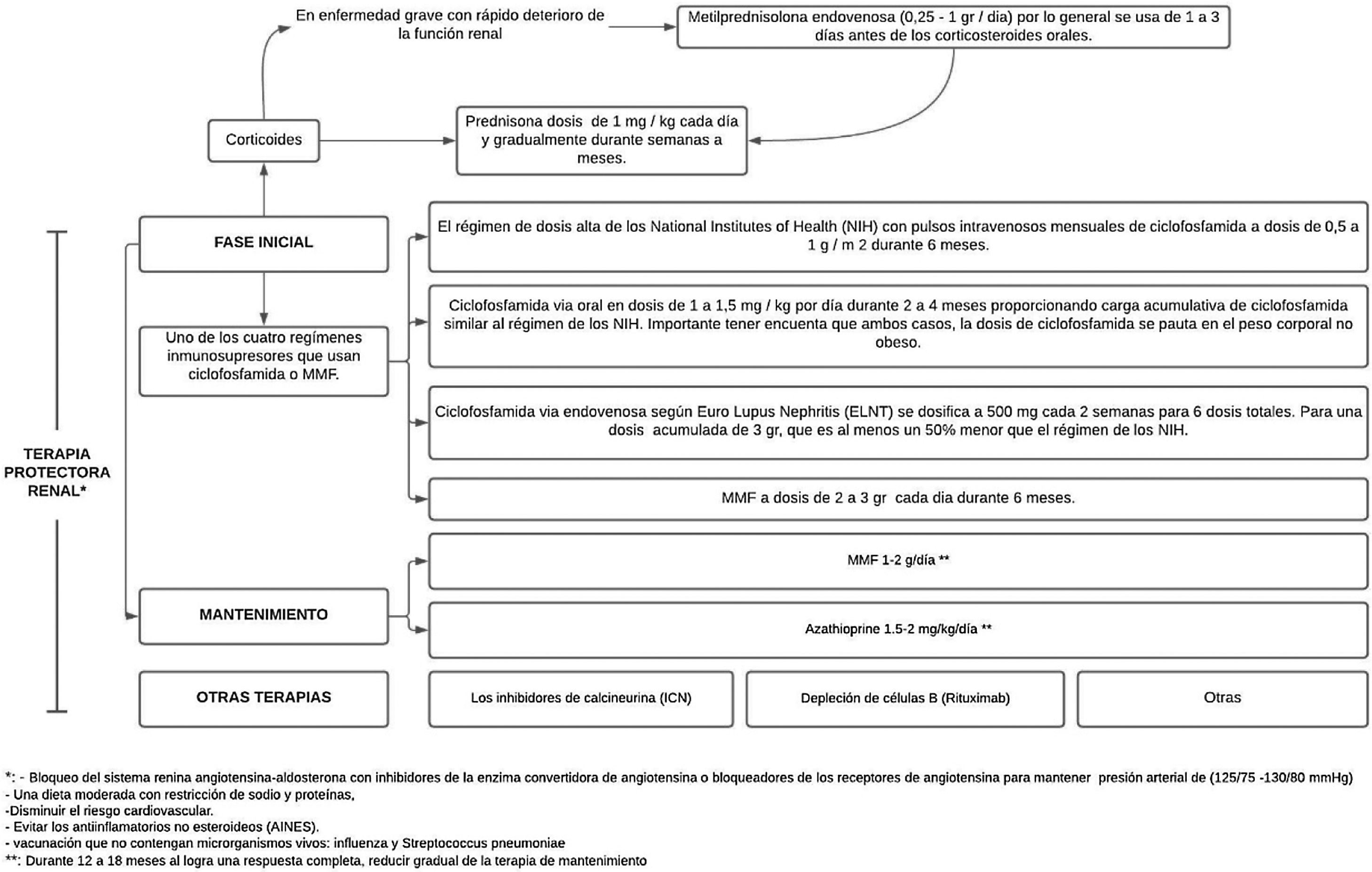
Treatment with statins should be considered based on the lipid levels and the presence of other cardiovascular risk factors. It is necessary to do primary prevention of thrombosis according to the cardiovascular risk at 10 years, recommending the use of low-dose aspirin in the presence of high risk. The protection and prevention of osteoporosis should follow non-pharmacological strategies (exercise, maintenance of the mass index), as well as pharmacological measures, depending on the risk of fracture.38 In women of childbearing age, GnRH analogues should be used to try to preserve ovarian function, especially in patients who receive cyclophosphamide.39
General measuresAmong the general measures, blockade of the renin-angiotensin-aldosterone system with angiotensin-converting enzyme inhibitors or angiotensin receptor blockers is a useful strategy to preserve blood pressure with a goal of 125/75–130/80mmHg.37,38 Likewise, a moderate diet with sodium and protein restriction, and correction of metabolic alterations, mainly of cardiovascular risk, is recommended. It is necessary to avoid non-steroidal anti-inflammatory drugs, as well as the administration of vaccines that do not contain living microorganisms: influenza and Streptococcus pneumoniae.
Immunosuppressive treatmentFor active or chronic active proliferative LN class iii/iv, treatment should be mixed between class iii or iv+v, while for non-proliferative class v with proteinuria in the nephrotic range, management should be with immunosuppression.37 Treatment for LN seeks to improve renal function, decrease proteinuria, correct immunological markers, and prevent or reduce cumulative organ damage, in order to achieve complete remission (CR) or partial remission (PR) at 6months, thus avoiding ESRD, dialysis, transplantation and death, and preserving quality of life.30
Mycophenolate (MMF) or cyclophosphamide, combined with high doses of corticosteroids, are considered the standard treatment for LN, since this combination improves long-term renal survival compared with corticosteroids alone.4,40 These induction regimens are generally accepted as therapeutic standard and are supported by evidence from randomized controlled trials. However, despite the supporting evidence, none of these drugs is approved by the Food and Drug Administration (FDA) and their use in LN is considered off-label, with the exception of corticosteroids.36
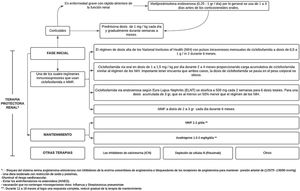
Treatment consist of 2phases, an initial one, which is much more intense and lasts from 3 to 6months, and the maintenance phase with lower drug doses, but which generally lasts several years.41 (Fig. 3).
In a 41-month follow-up of 90 Caucasian patients, no significant differences were found between the 2treatment groups (NIH or ELNT regimen), which left serious doubts on the ability to generalize these results to other racial groups.42 In a subsequent study, a trial of combination therapy of abatacept and cyclophosphamide for LN was conducted, enrolling a racially and ethnically diverse population as follows: 37% African American and 41% Hispanic, and there were similar complete renal response rates at 24 weeks, which supports the use of the ELNT regimen for the initial treatment of LN in various racial and ethnic groups.41
After high-intensity immunosuppressive therapy is given for the first 3–6months, it is replaced with MMF (or a lower dose of MMF if it was used for induction) or azathioprine to maintain suppression of autoimmunity and inflammation and thus prevent flare-ups. MMF is the preferred treatment for maintenance of remission, but there are no data on the optimal duration of the therapy or a definition of a low disease activity state that would predict and allow a safe withdrawal of treatment; by performing repeated biopsies 6–12 months after the induction, an attempt has been made to investigate and it has been demonstrated an alarming discrepancy between the clinical and the histological response.40
The objectives of the maintenance phase are to continue immunosuppressive therapy in order to achieve a complete renal response and prevent renal exacerbations while minimizing the potential toxicity of long-term exposure to immunosuppressive drugs,41 as has been demonstrated with cyclophosphamide, which is associated with significant toxicity, specifically increasing the risk of premature ovarian insufficiency and future malignancy.37
The doses used are MMF 1−2g/day or azathioprine 1.5−2mg/kg/day for 12–18 months. In case of obtaining a complete response, maintenance therapy should be gradually reduced. If a partial response is achieved, treatment should be continued for an indefinite term, but it should be considered to repeat the biopsy in order to determine if active lesions are still present.37 IL-17 and IL-23 may be alternative biomarkers for diagnosing LN, monitoring the activity, and predicting the response to treatment in patients with active LN.43
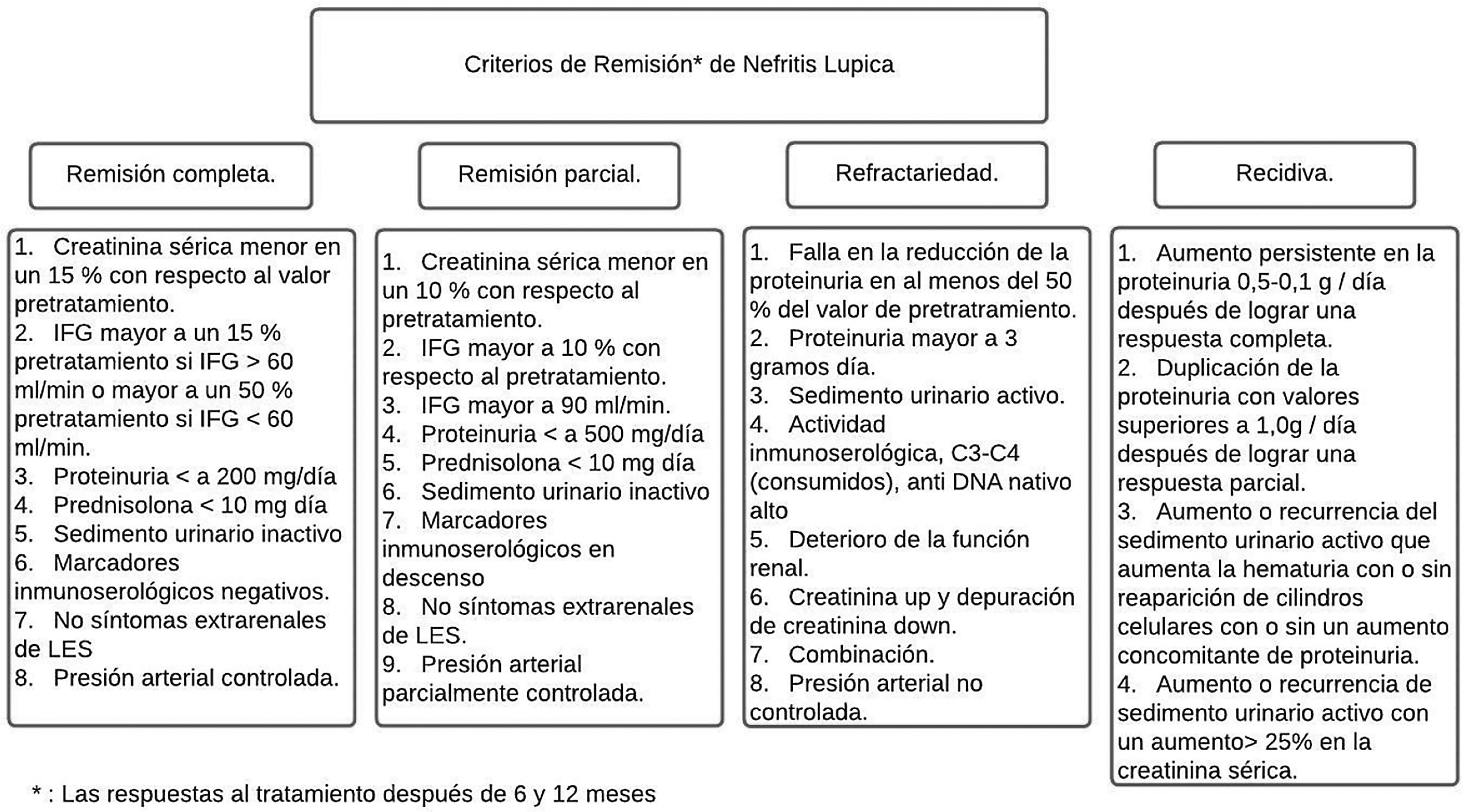
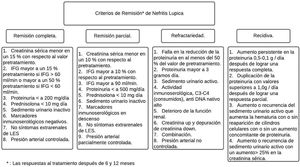
Between 20 and 70% of patients with LN are refractory to standard immunosuppressive therapy.43,44 Most of the criteria for CR or PR are a combination of clinical indices that include serum creatinine, proteinuria, and red blood cells in urine (Fig. 4). The Kidney Disease and Improving Global Outcomes (KDIGO) guideline on glomerulonephritis defines CR as the return of serum creatinine to previous baseline and a decrease in the UPCR ratio to <500mg/mmol. In PR, there is evidence of stabilization (±25%) or improvement, but not normalization, in serum creatinine, and a decrease>50% in the UPCR. In clinical practice, a PR is expected at 3–6months, and the clinical parameters are usually assessed every 4weeks in the first 6months. The foregoing with certain variables that need to be considered, such as the relationship of the biopsy after remission and what it reveals, the use of therapies with calcineurin inhibitors (CNI) and the lack of adherence of the patients with the final results.37,45
Remission criteria in lupus nephritis according to the American College of Rheumatology.
Alternative therapies such as multitherapy, which combines tacrolimus or cyclosporine+MMF: 0.05mg/kg/day of tacrolimus (minimum target level 4−6ng/ml) or 3−5mg/kg/day of cyclosporine (the level is not well established)+500−1000mg of MMF 2times a day for 6months are considered. Rituximab is administered intravenously at 1000mg on days 1 and 14 in 2doses.37,44
Calcineurin inhibitorsCNIs attenuate inflammation by preventing the release of inflammatory cytokines from leukocytes and also block T-cell activation, which is why they could have an effect in maintaining remission. They have been used as a part of a multitarget approach for the treatment of LN, added to a regime of MMF and corticoids, and have been demonstrated to be superior to cyclophosphamide in inducing remission at 6months. CNIs plus corticosteroids alone have also been used for LN induction and have been found to be as effective as MMF for proliferative LN. At this time, the studies of CNI should be considered with caution. Unexpectedly, in the multitarget study, patient withdrawals due to adverse events were higher in the CNI group than in the cyclophosphamide group. Therefore, the effects of acute respiratory infections should be studied in cohorts with greater racial and ethnic diversity.4
Given that proteinuria is the main contributor to current NL response criteria and CNIs can affect proteinuria by mechanisms non-related to immune modulation, it is not clear that proteinuria is an appropriate valuation criterion to compare CNIs with drugs with different mechanisms of action and should be interpreted with caution. Perhaps is preferable in this context to consider a renal biopsy to verify histological improvement or resolution.4,37,44
According to the Aurora 1 study, a phase 3, double-blind, randomized, multicenter, placebo-controlled trial, voclosporin, a CNI, together with MMF and low-dose steroids, led to a complete renal response rate clinically and statistically superior than MMF and low-dose steroids alone, with a comparable safety profile, thus becoming a promising treatment option in the setting of active LN.45
Depletion of B cells in LNB cells play a prominent role in the pathogenesis of LN, through a variety of mechanisms that include autoantibody production, antigen presentation, cytokine production, and interactions with T cells. Therefore, their selection has become a biologically formidable therapeutic strategy.41,46
Rituximab is an anti-CD20 monoclonal antibody that depletes B cells from the pre-B cell to the memory B cell stage. It is important to highlight that plasma cells and pro-B cells are saved due to the unexpression of CD20. The evaluation trial of LN with rituximab was a randomized controlled trial of 144 patients with proliferative LN that evaluated the induction therapy with rituximab vs. placebo in the setting of MMF and steroids. Although the trial was unable to demonstrate a statistically significant difference between the 2groups in the renal response rate at 52 weeks, more patients treated with rituximab achieved a renal response (57% vs. 46%). In a secondary analysis, more patients treated with this monoclonal antibody achieved at least a 50% reduction in proteinuria at 78 weeks. This observation raises the possibility that trials of longer duration are needed to fully discern the differences between these treatments.46
Rituxilup is the first large-scale randomized controlled trial on LN, which studies a treatment regimen completely free from oral steroids. If it can be demonstrated that a steroid-free regimen is successful, the eventual patients can forget the multiple well-described toxicities of the use of steroids and their unwanted effects in the long-term. This would be a revolutionary advance in the lupus community.41
Obinutuzumab is an anti-CD20 monoclonal antibody that, unlike rituximab, has greater antibody and phagocytosis-dependent cytotoxicity, better effects of direct death of B lymphocytes and less subjection to complement-dependent cytotoxicity. The Nobility trial demonstrated positive results for obinutuzumab as add-on therapy to steroids and MMF when administered intravenously every 6months for a period of 76 weeks, with an effect size of 22% for a complete renal response.47
Finally, belimumab, a humanized anti-BAFF/BLYS antibody that inhibits the maturation of B cells, was recently approved for use in LN. A phase 3, multinational, multicenter, randomized, double-blind, placebo-controlled trial, in which 448 patients with active LN proven by biopsy participated, disclosed the strengths of belimumab, revealing a renal response of primary efficacy (43% vs. 32%; OR 1.6; 95% CI: 1.0–2.3; p=0.03) and a complete renal response (30% vs. 20%; OR 1.7; 95% CI: 1.1−2.7; p=0.02), demonstrating that patients who received belimumab plus the standard therapy had a more marked improvement in renal parameters than those who received standard therapy alone.48
ConclusionDespite the general improvement in the care of patients with SLE and an increase in the 5 and 10year survival rates for LN, its prognosis remains unsatisfactory, especially in certain ethnic groups such as African Americans and Hispanics.49–51 To improve the prognosis, new techniques to assess the early onset of kidney disease activity or its relapse must be developed, thus making it possible to initiate timely management.52 Late diagnosis of LN is associated with a higher frequency of renal failure and renal replacement therapy, making early diagnosis even more important.53
Current laboratory indicators to detect and assess LN, such as proteinuria, protein/creatinine ratio, anti-dsDNA, complement levels, and active urinary sediment, lack the ability to differentiate between activity and renal damage in LN, which is a cardinal marker for planning a treatment strategy.54 A biomarker is a biological, biochemical, or molecular substance that can be qualitatively or quantitatively detected by laboratory techniques and that correlates with the pathogenesis of the disease at various points. In the case of LN, the ideal biomarker should have the following properties:
- 1.
Be specific for the detection of renal involvement in patients with SLE.
- 2.
Establish a correlation with the activity or renal damage.
- 3.
Be efficient for longitudinal serial monitorization of the disease status.
- 4.
Be superior to the parameters currently used to predict future renal events and thus be able to avoid progressive renal damage.
- 5.
Be capable of measuring the severity of renal involvement.
- 6.
Be validated in 2 or more independent cohorts.
- 7.
Be easy to perform, with minimal needs of infrastructure and inexpensive.54
Timely diagnosis and treatment reduce long-term sequelae, improving the quality of life and the prognosis of the disease, with a positive impact on the morbidity and mortality of patients. Different molecules have been studied to try to make an earlier and more efficient evaluation of LN. Despite the high number of new biomarkers that have been explored to predict and assess LN, few have been rigorously validated in large-scale longitudinal studies in different ethnic populations. To date, none of these new biomarkers has been standardized for daily clinical practice or has replaced conventional biomarkers for monitoring the progression of the disease and predicting renal flares.39 It is important to know the new alternative therapies, since in the near future they will be used increasingly and earlier in patients who do not respond or present side effects in treatment consisting of regimens based on MMF or prednisone.
FundingNone.
Conflict of interestNone.