Systemic lupus erythematosus (SLE) is the prototype of systemic autoimmune disease with clinical characteristics that show geographic variations. However, these differences between regions have not been fully described; therefore, the objective of this study is to describe the clinical and sociodemographic characteristics of Cuban patients with SLE.
Patients and methods149 patients with SLE and 151 with other systemic autoimmune diseases were studied. Sociodemographic and clinical characteristics according on the criteria of the American College of Rheumatology of 1997 were identified. To evaluate the associations between clinical manifestations and SLE, a logistic regression analysis was performed; the odds ratio (OR) was calculated with its corresponding 95% confidence interval and the method of multiple correspondence analysis was also used. By an analysis of the configurations of frequency the typical combinations of criteria related to the patients with SLE were identified.
ResultsThe most frequent criteria in SLE were immunological disorders (85.2%). ANA positive (85.2%) arthritis (78.5%), photosensitivity (77.2%), and malar rash (61%). The renal involvement and immunological disorders criteria were the best (highest OR) at discriminating SLE patients. The combination of only three criteria (malar rash, positive ANA, and immunological disorder) could be enough to classify a homogeneous population.
ConclusionsThis study enabled us to determine the main clinical characteristics of patients with SLE in Cuba. This information could be useful to improve the efficiency of SLE diagnosis and facilitate more specific treatments.
El lupus eritematoso sistémico (LES) es el prototipo de enfermedad autoinmune sistémica, con características clínicas que muestran variaciones geográficas. Sin embargo, estas diferencias entre regiones no están completamente descritas, por lo cual, el objetivo de este trabajo es describir las características clínicas y sociodemográficas de pacientes cubanos con LES.
Pacientes y métodosSe hizo un estudio con 149 pacientes con LES y 151 con otras enfermedades autoinmunes sistémicas. Se identificaron sus características sociodemográficas y clínicas, basadas principalmente en los criterios del Colegio Americano de Reumatología de 1997. Para evaluar las asociaciones entre las manifestaciones clínicas y el LES se llevó a cabo un análisis de regresión logística, se calculó la odds ratio, con su correspondiente intervalo de confianza al 95%, y se empleó la técnica de análisis de correspondencia múltiple. Mediante un análisis frecuencial de las configuraciones, se identificaron las combinaciones típicas de criterios relacionadas con los pacientes con LES.
ResultadosLos criterios más frecuentes en el LES fueron: alteraciones inmunológicas (85,2%), ANA positivo (85,2%), artritis (78,5%), fotosensibilidad (77,2%) y rash malar (61%). Los criterios afección renal y alteraciones inmunológicas son los que mejor (mayor valor de la odds ratio) discriminan a los pacientes con LES. La combinación de solamente tres criterios (rash malar, ANA positivo y alteraciones inmunológicas) podría ser suficiente para clasificar a una población homogénea.
ConclusionesEl estudio permitió conocer las principales características clínicas de pacientes con LES en Cuba. Esta información puede ser útil para mejorar la eficacia del diagnóstico del LES y favorecer la aplicación de tratamientos más específicos.
Systemic lupus erythematosus (SLE) is a complex multisystem inflammatory chronic disease, with a great variety in the incidence and prevalence. The etiology of SLE is not fully understood, but both genetic predisposition and non-genetic triggers are considered.1
Recently, it has been demonstrated association between genetic markers with specific susceptibility to clinic manifestation of SLE, like the hematologic, renal and dermatological manifestation, without exact understanding on the external elements interacting in their development in subjects genetically predisposed. It is postulated, that environmental triggers, including drugs, viral infections, ultraviolet light, socioeconomic and nutritional factors, into others, can be identified into the clinical pattern of the disease2,3 and may also explain the some discrepancies observed in the incidence, clinic behavior and prognostics of the disease in different regions around the world.4,5
So, North America the highest reported incidence and prevalence of SLE, while Africa had the lowest incidence and Australia the lowest prevalence.6 Respect the clinical manifestation, Europeans and their descendants in other parts of the world generally show more frequently mucocutaneous manifestations, in particular photosensitivity, compared to most other ethnic groups. At the same time, the African descendent develop renal disease faster and more often during the course of the disease, with a higher level of activity and accumulated damage.7
However, these differences among patients from different countries are not understood at all and neither has been published. Reports from Europe and United State of America are predominant,6 and are scarce in patients from our Caribbean region.
In general, in Latin America, the clinical manifestations of these patients are very heterogeneous, depending on ancestry population component (Amerindian, European, African and mestizo).8 For example, Latin American mestizos SLE patients have more renal and myocardial involvement as well as a higher level of disease activity as compared to Caucasians from Latin American.9 For this reason, the results obtain in a country are not similar to others, and it is necessary that each region to carry out their own studies.
In this respect, one of the studies that best characterize the diseases in Latin America is the one developed by the Latin American Group of Study on SLE (GLADEL), in which 27 Cuban patients were included.8 Nevertheless, the majority of reports that characterize the disease describe it without making any comparison with carrier patients of other autoimmune diseases which are transcendental in making the differential diagnosis.10,11
The most widely used classification criteria for SLE are those proposed by the American College of Rheumatology (ACR-97)12 however, when foreign references are used, the genetic and environmental differences among areas of different socioeconomic development should be taken into account in order to avoid a mistaken assessment of the healthy problems.
This paper aims at characterizing Cuban patients with SLE according to the criteria suggested by the ACR-97.
This study compares the clinic manifestations among patients with SLE and other autoimmune diseases with the purpose of identifying patterns of reference that allow to improve the SLE diagnosis and the application of more specific treatments for reducing or delaying the complications described for this disease.
Material and methodA prospective study was conducted from January 2016 to December 2019. During this period 652 patients were studied in psichoneuroimmunology service at National Institute of Nephrology Dr. Abelardo Buch Lopez, in Havana, Cuba, all with the probably diagnosis of an autoimmune disease. The patients were grouped, considering the clinical and laboratory criteria as patients with SLE (meeting of the ≥4 ACR-97 criteria for SLE) and controls no SLE (patients with criteria to others systemic autoimmune disease). Subjects were excluded if they were pregnant, if their SLE diagnosis was made before they were 18 years old, or were unable to give their valid consent.
149 patients with SLE and 151 controls (Sjögren's Syndrome, Rheumatoid arthritis, Systemic Scleroderma, Chronic cutaneous lupus, Mixed disease of connective tissue) were identified. A formulary was applied, including demographic aspects (age, gender, skin color) and clinical aspects (disease duration in age, all the ACR-97 classification criteria presented up to the moment of the study, history of fever without cause identified, alopecia, Raynaud phenomenon, and relative with autoimmune diseases).
Laboratory assaysSerum antinuclear antibodies (ANA), antibodies against extractable nuclear antigens (ENAS): anti double-stranded DNA, anti-Smith, anti-ribonucleoprotein, anti SS-A, Anti SS-B, anti Scl-70, anti Jo-1 and antiphospholipid antibodies were measured by enzyme immunoassays (ELISA Orgentec Diagnostika GmbH, Germany). Others analysis included hemogram, creatinine clearance, 24-h proteinuria and study of urinary sediment were performed all by methods well established in the laboratory. The diagnosis of renal disease was assessed by trained nephrologists based on the clinical criteria, laboratory test and the histology of the results of kidney biopsy.
The design of the study was approved by the ethics committee of the National Nephrology Institute. All patients and control subjects gave their consent to participate in the study after having been informed about its purpose.
Statistic methodDescriptive analyses were performed to the clinical variables in Cuban patients with SLE and test for comparison between groups as well as tables of contingency were built to each group of criteria (presence or absence) and to the variable that identify the group. A test of Chi-Square of homogeneity was done to check the proportions of the populations.
An analysis of logistic regression was performed to evaluate the type of dependence of the clinical manifestations. The Odds Ratio at 95% and corresponding confidence interval (CI) at 95% was applied to analyze the effect of each criterion. The technique of analysis for Multiple Correspondence was perform to visualize the associations between the clinical criteria tested and the disease.13
Finally, an analysis of frequency of the configurations was done, considering the variables with higher clinical and statistical relevance in order to identify typical combinations of criteria related to patients with SLE. A significance of p<0.0 was considered.
ResultsSociodemographic characteristicsThe 94% of patients with SLE were women, the rate female/male was 15:1, with a mean of age of 47±11 years old; the mean of the disease duration was of 8±8 years old. The control group showed a 92.% of women with a mean age of 47±12, the rate female/male was 12:1 and the mean of the disease duration was 6±7 years. Patients with white skin color predominated in both groups with a frequency of 59% and 64% respectively. Except from the years of evolution of the disease, the rest of the characteristics showed no significant differences between the groups.
Clinical and serological manifestations according to the ACR-97 criteriaTable 1 shows the number and percentage of individuals for each criterion in each group and the probability value P (level of significance) of the Comparison tests of proportions between the groups.
ACR-97 criteria in patients with SLE and controls.
| Criterion | Controls | SLE | p-Value | ||
|---|---|---|---|---|---|
| N=151 | % | N=149 | % | ||
| Malar rash | 36 | 23.8 | 91 | 61 | .0000* |
| Discoid rash | 22 | 14.6 | 52 | 34.9 | .0001* |
| Photosensitivity | 58 | 38.4 | 115 | 77.2 | .0000* |
| Oral ulcers | 20 | 13.2 | 48 | 32.2 | .0001* |
| Arthritis | 111 | 73.5 | 117 | 78.5 | .3105 |
| Serosites | 2 | 1.32 | 22 | 14.8 | .0000* |
| Renal disease | 4 | 2.6 | 53 | 35.6 | .0000* |
| Alterations of CNS | 7 | 4.6 | 13 | 8.8 | .1514 |
| Hematologic disorder | 3 | 2 | 14 | 9.4 | .0058* |
| Immunologic disorder | 40 | 26.5 | 120 | 80.5 | .0000* |
| ANA | 55 | 36.4 | 127 | 85.2 | .0000* |
ANA, antinuclear antibodies; CNS, central nervous system.
The serositis, the hematologic and CNS disorders, were less frequent in both groups. The Chi-Square test to measure homogeneity; verify significant difference between the proportions to the majority of the criteria, except to the arthritis and the nervous system disorders.
Table 2 shows the effects of the evaluated criteria from the OR results and their corresponding CI at 95%, by means of an analysis of logistic regression. Except from the serositis variable, the rest of the criteria showed a significant positive effect (p<0.05) about the possibility of being ill of SLE, these findings reaffirm the results of the proportions comparison analysis. The variables Malar rash, discoid lesion, photosensitivity, oral ulcers, renal affection, positive ANA and immunological alterations were greatest positive significance (p=0.000) associated to the SLE diagnosis. Among them, renal and immunologic disorder were the variable with highest OR values and, consequently, the ones that best explain the differences between the control group and the patients with SLE. The presence of arthritis, hematological and CNS alterations were also relevant, significant but with a lower level of association with the presence of SLE, compared to the other variables that turned out to be significant.
ACR-97 criteria in SLE patients from the analysis of logistic regression.
| Criterion | Coefficient | p-valor | 0R | CI 95% |
|---|---|---|---|---|
| Malar rash | 4.7 | .00* | 111.1 | 16.3 - 758.9 |
| Discoid rash | 43. | .00* | 74.1 | 10.2 - 536.8 |
| Photosensitivity | 4.9 | .00* | 132.5 | 13.3 - 1315.2 |
| Oral ulcers | 3.6 | .00* | 35.6 | 4.8 - 268.2 |
| Arthritis | 2.7 | .00* | 15.3 | 2.4 - 98.9 |
| Serositis | 7 | .4 | 1126.2 | 0 - 120922 |
| Renal Disorder | 8.5 | .00* | 5275.3 | 132.8 - 209525 |
| Hematologic Disorder | 4.2 | .00* | 69.3 | 4 - 1213.2 |
| Positive ANA | 4.7 | .00* | 112.2 | 16.9 - 743.3 |
| Immunologic Disorder | 6.3 | .00* | 557.4 | 56.3 -5518.7 |
OR, odds ratio; CI, confidence interval; ANA, antinuclear antibodies; CNS, central nervous system
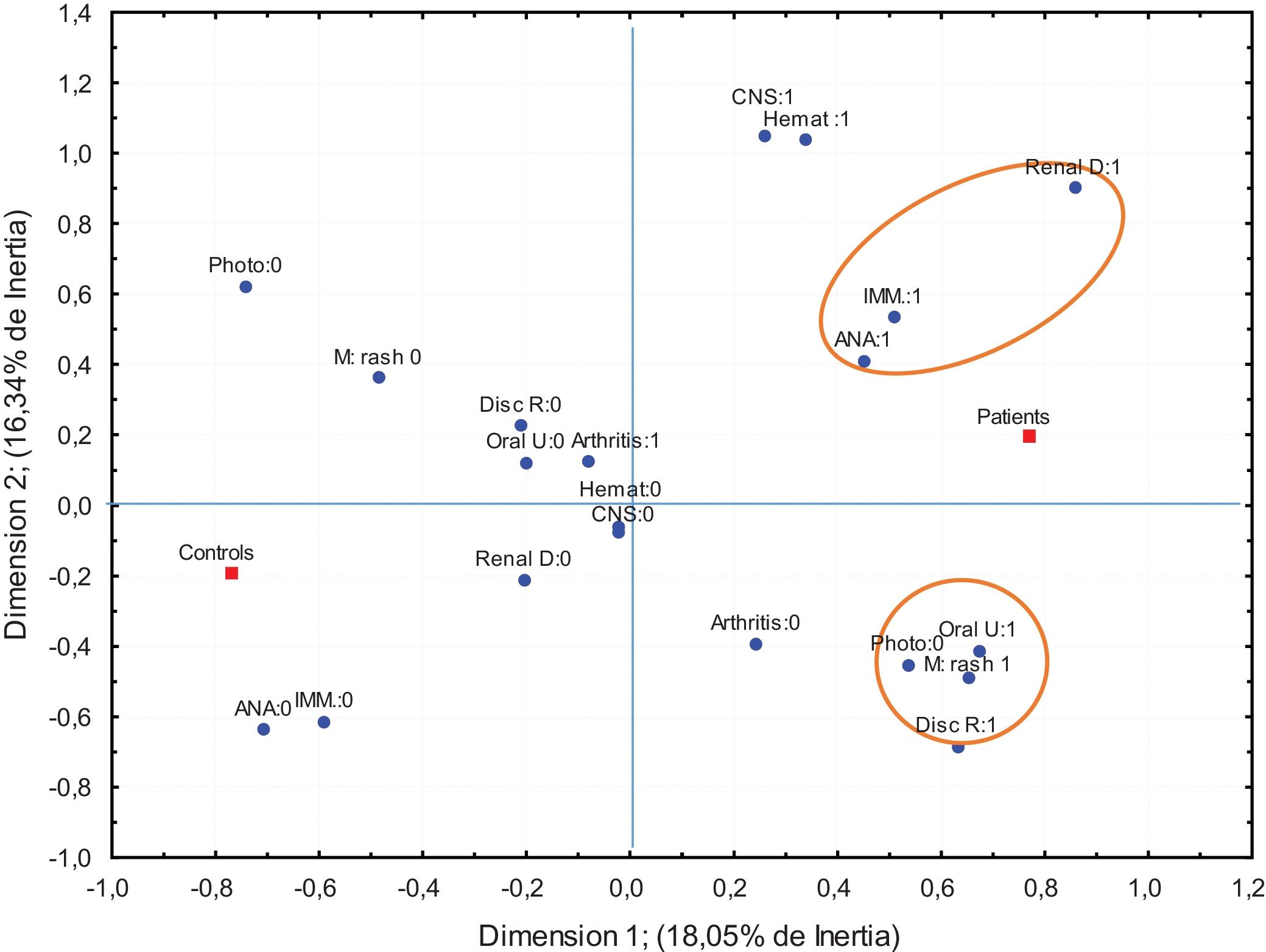
Given that, in the classification of lupus according to the ACR-97 criteria, the accumulative effects among them charge more value, the association among them was studied by means of a Multiple Correspondence Analysis for the variables that turned out to be significant in the logistic regression analysis. The results are shown in Fig. 1.
Multiple correspondence analysis ACR-97 criteria. These graphic show the two dimension map of the association between a criteria and group (controls and patients). Number 0: absence of category number 1: presence of the category. Discoid R: discoid rash, Photo: photosensitivity, oral u: oral ulcer, M rash: malar rash, Imm: immunologics disorder, CNS: Central nervous systemic. Renal D: renal disorder, Hemat: Hematologic disorder. ANA: positive antinuclear antibody.
It is interesting that the presence of kidney affectation, positive ANA, and immunological alterations is very closed to the category representing the group of patients with SLE. On the other hand, the absence of these criteria is closer to the categories representing the control group. In the same way, the categories that reflect the mucocutaneous alterations like rash malar, oral ulcers and discoid injury with the photosensibility are also closed to each other in the group of patients (same side of the axis). So, it can be assumed that these two subgroups of manifestations are significantly correlated in the group of patients with SLE.
From the clinical and statistical view point, the variables selected to the Frequencies Analysis of the configurations were: Renal affectation, positive ANA Immunologic disorder and malar rash. This analysis allowed to determine whether the categories patterns of variables (configurations) in each group show a different frequency than expected.
Table 3 shows the typical configurations that determine the differences between groups. Observe that, not only the presence of the four characteristics constitutes a discriminatory configuration (disproportionate rates in the groups compared), but also a configuration with the presence of only 3 manifestations constitutes a typical configuration among SLE patients. These configurations with only three or four clinical manifestations could be interpreted as specific combinations that allow to identify with relative clarity to possible patients with SLE. However, other configurations that represent the combined presence of three clinical manifestations are highly unlikely to occur in none of the two groups, therefore, they are unable to discriminate.
Combination of categories of and observed frequencies in patients with SLE and controls.
| Malar rash | Renal disorder | ANA | Immunologic disorder | Observed frequencies | |
|---|---|---|---|---|---|
| Discriminatory configurations | SLE controls | ||||
| + | + | + | + | 22 | 0 |
| − | + | + | + | 22 | 0 |
| + | − | + | + | 38 | 0 |
| Not discriminatory configurations | |||||
| + | + | + | − | 1 | 0 |
| + | + | − | + | 1 | 0 |
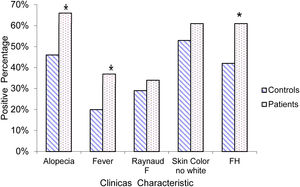
Other characteristics, which although not part of the ACR-97 classification criteria, due to their clinical relevance, was considered important to include in this study is shown in Fig. 2.
DiscussionSeveral studies demonstrate that social and demographic characteristic are interrelated with genetic and environmental factors to influence in the natural history of SLE, which are also responsible in great measure of the heterogeneity of the disease, evidenced by their clinical and serological expression in different countries.2,3
In this study the rate female/male was higher compared to other studies reporting a superior prevalence in woman (values between 9–12:1).14 The evolution of the disease observed was adequate to the characterization of the disease, since the majority of these patients, after onset, show symptoms over the year.15
Photosensitivity was one of the criteria with the highest prevalence in the control group. It was superior, even when compared with reports of patients with Lupus in other regions such as Saudi Arabia 22%,16 Europe 22.9%,17 and Oman 12%18; probably due to genetic factors and/or the major expositions of our patients to sunlight in the Caribbean area. In correspondence, a high frequency of these criterion was observed in SLE patients, similar to other reports in other similar populations, example 60.9% in African Americans from the islands of South Carolina19 and 64.3% in the Brazilian population.20
Arthritis is one of the most common manifestations in rheumatic diseases. It is relevant because of its incidence and prevalence in the general population, the associated morbidity and mortality, disability and invalidity. In Cuba, this disease is considered into the first 10 causes of health problem.21 This manifestation was very frequent in the control group; it was not useful to discriminate among patients with SLE and patients with other autoimmune diseases, only when it is studied in conjunction with other criteria does it acquire its clinical relevance in the diagnosis of SLE(0R ›1).
In patients with SLE, arthritis was one of the criteria with most prevalence. It is known that it constitutes one of the first clinical manifestations and it is estimated that arthritis is present in 90% of these patients.22 In this study, the frequency was 78%, very similar to the one reported in other regions of the world.10,11
Renal disorder, a prognostic factor influencing to this disease course, in which genetic and socioeconomic factors are postulated, without there being a consensus on which of them is the most important.23,24 The frequency of kidney involvement found is very similar to that reported in the GLADEL studies for the white population (36.7%),8 and for patients treated in private health centers in Argentina with 41%25 and lower than the low-income Amerindian population 48.7%.26 This could be a reflection of the free access of the Cuban population to health services.
The serositis and the CNS disorder showed a low frequency similar to what was reported by the studies perform Gladel.8 There are reports stating that these signs are more frequent in Asian population, mainly in Japanese people.27
On the other hand, the significant association observed between mucocutaneous variables and photosensitivity, supports the decision taken by Systemic Lupus International Collaborating Clinics Classification Criteria (SlLCC) 201228 to excluding photosensitivity from the criteria in other to avoid duplicity of information. Likewise, the association between kidney disease, ANA positivity and immunological alterations, capable of classifying a patient as lupic, reaffirms the new criterion postulated by SLICC, which states that a biopsy compatible with lupus nephritis, together with. The presence of precisely these auto antibodies is enough to classify patients with SLE.28
From the genetic view point, the SLE has an strong association due the high heritability (∼66%) and concordance with homocigotic twin and the most of 52 genetic locus describes as associate with SLE.24
In this study, a significant association was found between the presence of a family history with an autoimmune condition and not the color of the skin. These apparently contradictory findings support the argument that differences in phenotypes do not always reflect differences in genetic information.
In Cuban population, have been demonstrated la heritability to genes from Caucasia and African populations, which are observed either in individuals black and white.29
The febrile syndrome is a significant feature in our patients. It appear in a 42% patients with inflammatory manifestation, been necessary to exclude other causes to make a decision from the therapeutic view point.30
The ACR-97 classification criteria have been used worldwide. However many groups have attempted to identify additional criteria to aid in the classification of the disease. with greater sensitivity and specificity for early SLE. Recently in 2019, new SLE classification criteria were developed with support by both the European League Against Rheumatism (EULAR) and the ACR (EULAR/ACR 2019).31 In our work we focus mainly on the ACR-97 criteria, however we include certain characteristics such as the presence of alopecia, nonspecific fever, that are part of the items of the EULAR/ACR 2019 criteria, demonstrating the strong association between these characteristics in SLE patients. On the other hand, the need for a positive ANA as a mandatory requirement for the classification of patients with SLE, proposed by theses criteria is in correspondence with our finding that all combinations of criteria that were classifiable for the disease include the presence of a positive ANA.
Cuba is a country with certain geographical and ethnic peculiarities, with a great racial and cultural mixture, which leads to SLE in the Cuban population exhibiting its own characteristics. An adequate characterization of patients with SLE from each region or country, to a more precise diagnosis of SLE patients and the distinction of their clinical manifestation respect other autoimmune diseases as well as to contribute to the diagnosis criteria at earlier state of the disease.
FundingThis research received no financial support from agencies of the public sector or commercial sector.
Conflict of interestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
To. DrCs. Sergio Arce Bustabad memory, pioneer and guide of this research.