Systemic lupus erythematosus (SLE) is an autoimmune disease that affects multiple organs, and renal involvement is found in 50% of patients and is variable according to racial and ethnic group. It is estimated that 10% of patients with lupus nephritis (NL) develop end-stage renal disease (ERT), and once progression occurs, 80% of patients are negative for activity markers.
However, although it is rare, the reactivation of the disease can occur in advanced renal involvement, and it is important to diagnose it in a timely manner to define the cause and treat it, avoiding complications.
The clinical case is presented of a 45-year-old patient, with ERT on peritoneal dialysis, who was in remission of the disease and subsequently developed lupus activity.
El lupus eritematoso sistémico (LES) es una enfermedad autoinmune que afecta múltiples órganos; el compromiso renal se encuentra en el 50% de los pacientes y es variable de acuerdo con el grupo racial y étnico. Se estima que el 10% de los pacientes con nefritis lúpica (NL) desarrollan enfermedad renal terminal (ERT) y, una vez que se da la progresión, el 80% de los pacientes negativizan los marcadores de actividad.
Sin embargo, aunque es inusual, la reactivación de la enfermedad puede presentarse en compromiso renal avanzado y es importante diagnosticarla oportunamente para definir la causa, tratarla y evitar complicaciones.
Presentamos el caso clínico de una paciente de 45 años, con ERT en diálisis peritoneal, que se encontraba en remisión de la enfermedad y posteriormente desarrolló actividad lúpica.
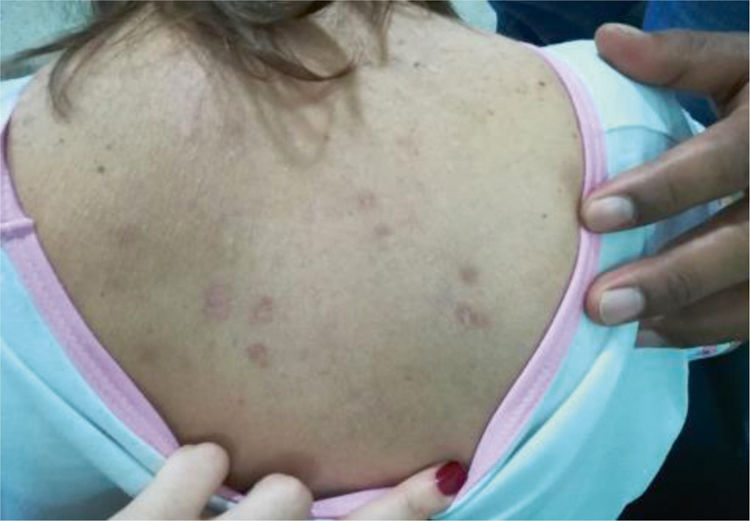
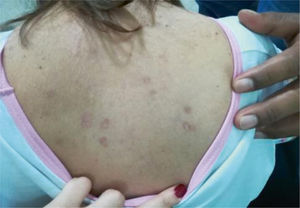
This article discussed a case of a female, 45-year old patient, housewife, with a history of systemic lupus erythematous (SLE) for over 20 years, with no previous immunosuppressive therapy, with lupus nephritis (LN) class IV, which progressed to end-stage renal disease (ESRD); the patient had been on peritoneal dialysis for the last 8 years. Additionally, the patient presents hypertension, secondary hypothyroidism, and nonrevascularizable coronary artery disease, with moderate mitral regurgitation from ischemia. The patient came to the institution because of 2 days of overt rectal bleeding, desquamative plaques on her body (Fig. 1), dizziness and dyspnea.
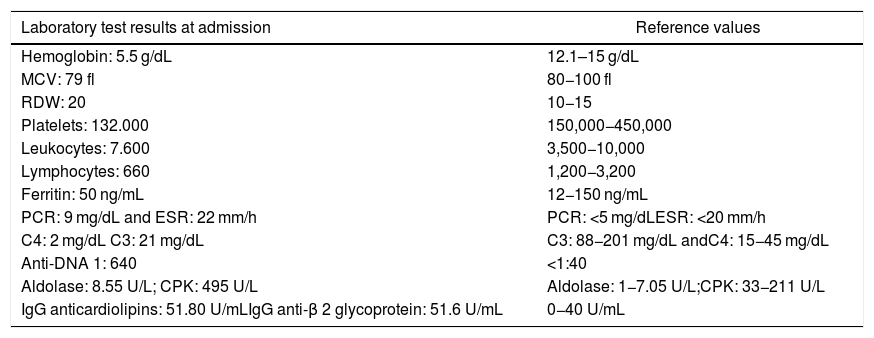
The tests conducted confirmed severe heterogeneous microcytic anemia, thrombocytopenia and iron deficiency, so a red blood cells transfusion was required (Table 1). A colonoscopy reported colitis with round button-shape ulcers suggestive of amebiasis; however, due to the skin lesions described, cytomegalovirus (CMV) or other opportunistic infection was suspected, so the patient was prescribed metronidazole for 7 days and ganciclovir; the latter was discontinued after four days because the special colon biopsy stains and the serum viral load were negative for CMV. Other opportunistic infections such as histoplasma were ruled out with antigen detection and colon biopsy. Furthermore, no hemolysis and vitamin deficit were documented to account for the hematological alterations.
Most relevant tests.
| Laboratory test results at admission | Reference values |
|---|---|
| Hemoglobin: 5.5 g/dL | 12.1–15 g/dL |
| MCV: 79 fl | 80−100 fl |
| RDW: 20 | 10−15 |
| Platelets: 132.000 | 150,000−450,000 |
| Leukocytes: 7.600 | 3,500−10,000 |
| Lymphocytes: 660 | 1,200−3,200 |
| Ferritin: 50 ng/mL | 12−150 ng/mL |
| PCR: 9 mg/dL and ESR: 22 mm/h | PCR: <5 mg/dLESR: <20 mm/h |
| C4: 2 mg/dL C3: 21 mg/dL | C3: 88−201 mg/dL andC4: 15−45 mg/dL |
| Anti-DNA 1: 640 | <1:40 |
| Aldolase: 8.55 U/L; CPK: 495 U/L | Aldolase: 1−7.05 U/L;CPK: 33−211 U/L |
| IgG anticardiolipins: 51.80 U/mLIgG anti-β 2 glycoprotein: 51.6 U/mL | 0−40 U/mL |
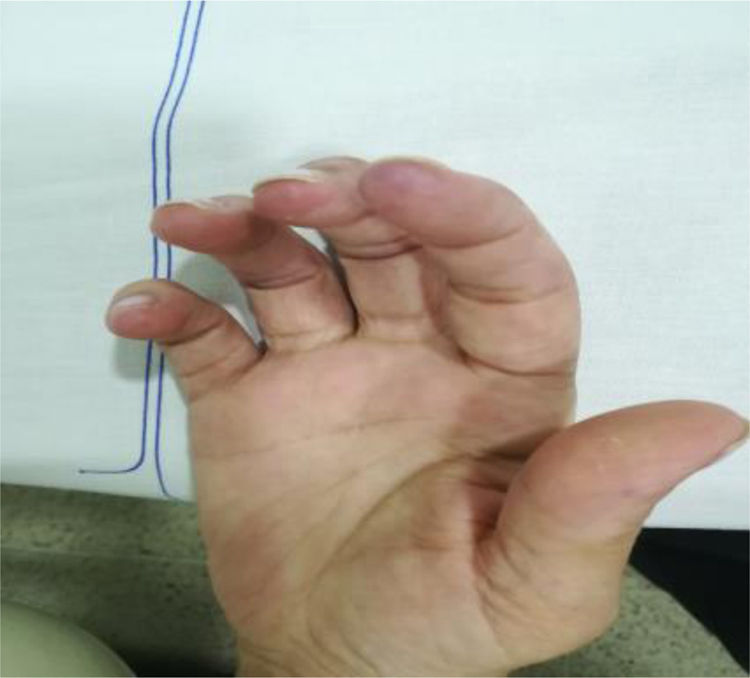
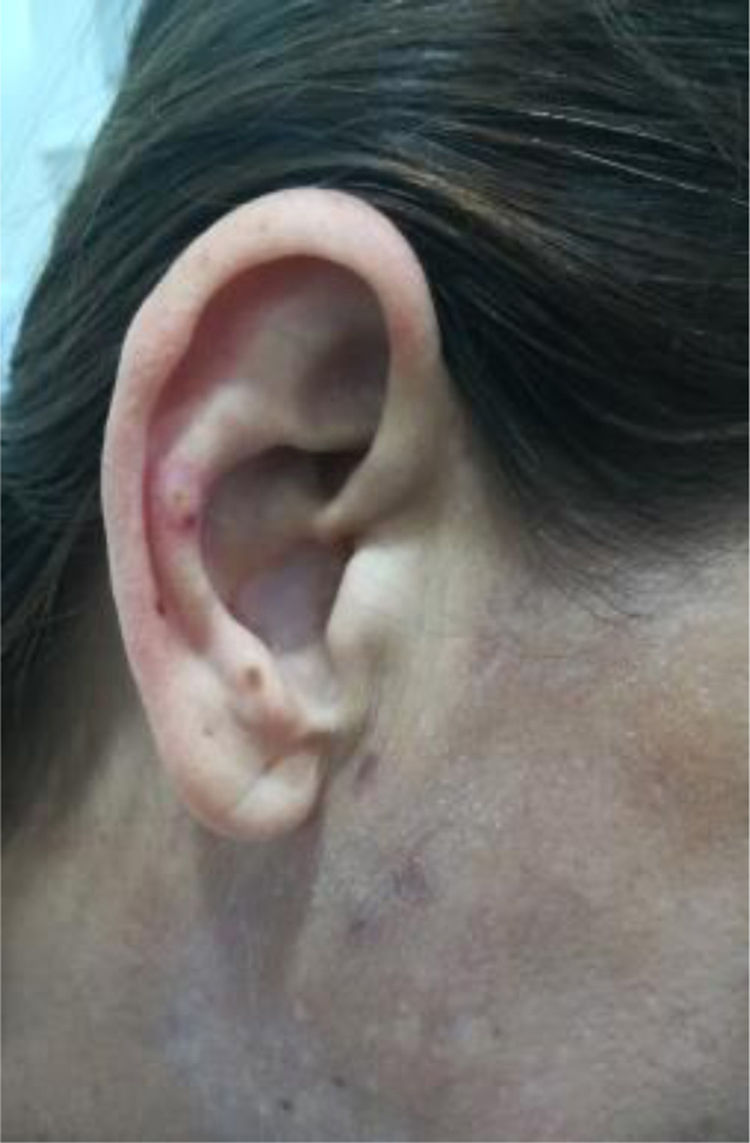
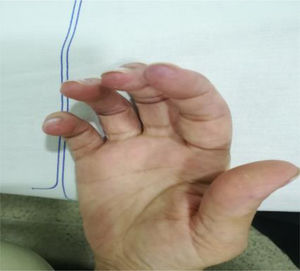
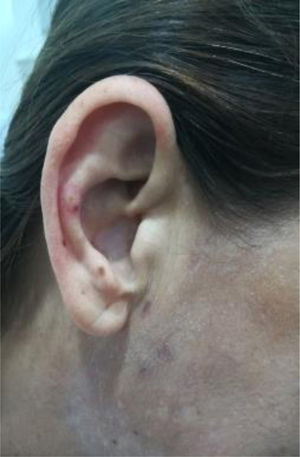
The clinical condition continued to progress over the days following the treatment of the infection, with arthralgias, myalgias, non-painful mouth ulcers, pleural serositis, pernio-like lupus lesions (Fig. 2), discoid lupus in the pinna (Fig. 3), ulcers in the lower extremities, and Raynaud’s phenomenon.
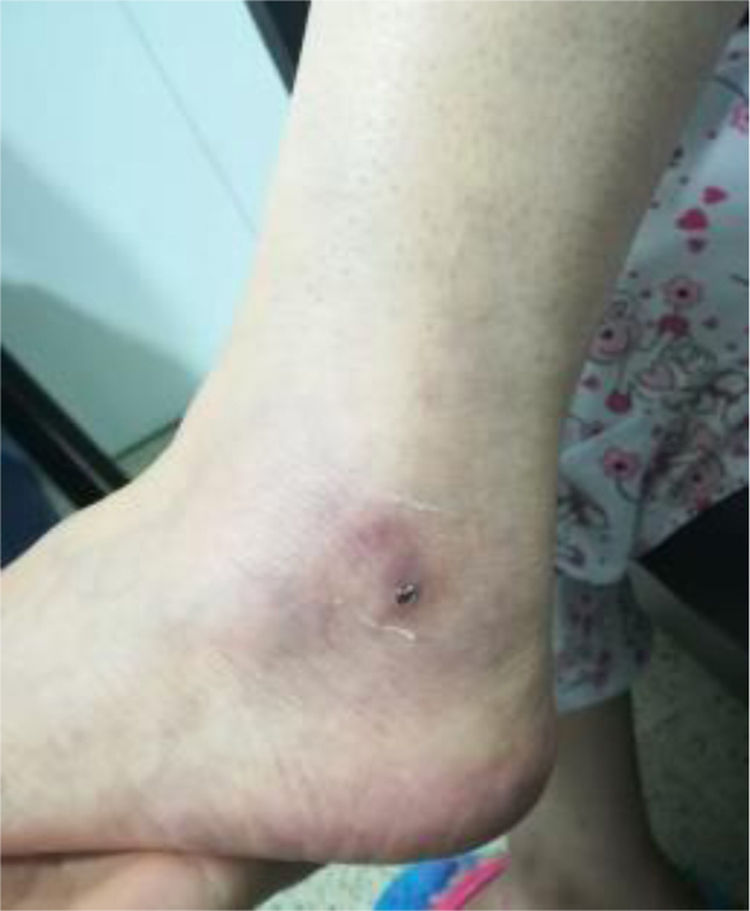
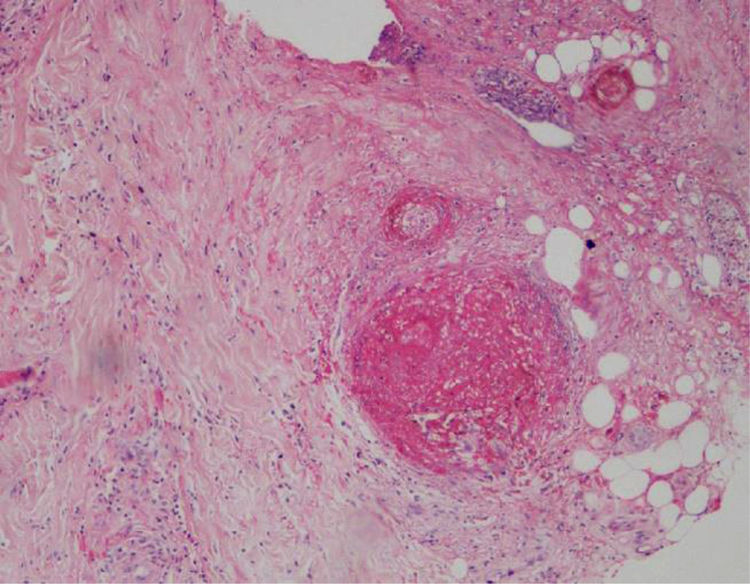
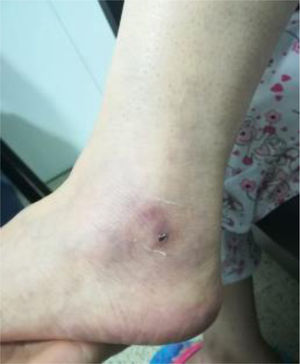
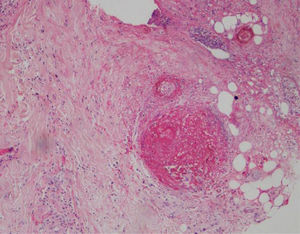
A biopsy of the left ankle was performed which reported thrombotic vasculopathy (Figs. 4 and 5).
Based on this clinical and paraclinical scenario, lupus reactivation was considered and the appropriate tests were ordered (Table 1), which revealed hypocomplementemia, positive anti-DNA, aldolase-associated myositis and elevated CPK, in addition to positive antiphospholipid syndrome markers (positive lupus anticoagulant, IgG anticardiolipin and IgG anti-B2 glycoprotein).
The conclusion was that the patient experienced a severe reactivation based on a Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) of 24, and probable secondary antiphospholipid syndrome (based on the presence of positive antiphospholipid antibodies and manifestations consistent with thrombotic vasculopathy). Prednisolone treatment was initiated at a dose of 10 mg, which was then escalated up to 30 mg per day, associated with azathioprine 100 mg/day, nifedipine for Raynaud’s management and ASA 100 mg for managing the APS; the patient experienced clinical and symptom improvement, once the therapeutic management was initiated.
DiscussionSLE is an autoimmune, chronic inflammatory disease, affecting primarily females, and has a variable course with multiple clinical and serological manifestations.1
Renal involvement is clinically evident in 60% to 80% of the patients with SLE and is a significant cause of morbidity and mortality. Up to 10% of the patients with LN develop ESRD, leading to higher mortality, as compared to patients without ESRD.2–14
Fries et al. were the first to describe the «burn-out» phenomenon of the SLE activity in end-stage LN.5 These authors compared the data from 13 patients, before and after the start of dialysis; initially, 92% had positive anti-DNA and 100% developed hypocomplementemia. After initiating dialysis, 85% had negative anti-DNA and that population required a lower dose of steroids. In this cohort, only one patient (8%) continued to present symptoms of activity.2,5 Further studies reported that the clinical and serological lupus activity becomes negative in up to 80% of the patients, following progression to ESRD.2 However, there is contradictory information in the literature, since there were a number of patients with ESRD who continued with some lupus activity.
Franccine et al. in a Brazilian cohort found that up to 39% of the patients with ESRD continued with activity and the characteristics most strongly associated with this outcome were: young age at the time of diagnosis, being a female, and having more than five years undergoing renal replacement therapy.15 Other authors such as Krane et al. found that being a young black woman, from a low socioeconomic level, was associated with persistent lupus activity in the population with ESRD.14 González et al. evidenced that having a lower C3 and being younger was associated with this process of reactivation during renal replacement therapy.4 Moreover, Young et al. reported 45 patients with SLE and renal disease, in whom the SLEDAI score increased over the 59-month follow-up, after the initiation of peritoneal dialysis.2,8 Barrera et al., in a retrospective case control study identified independent factors associated with lupus activity, including age of introduction of the renal replacement therapy, IgM-type anticardiolipin titers, and the C4 levels, which were correlated with the level of activity in this group of patients.3
This article discusses the case of a patient with lupus reactivation after 8 years of renal replacement therapy; she presented with serositis, hematological involvement, myositis, thrombotic vasculopathy, skin and oral involvement, in the presence of GI infection.
According to the literature, many of the characteristics present in this patient were associated with this condition: she had more than 5 years undergoing dialysis, was a female, and was diagnosed with SLE at a young age. The age of initiation of the renal replacement therapy was around 38 years old (a bit higher than the mean age reported in other trials such as González et al. which was 22.4)7 and she was an active smoker, which is a well-studied risk factor associated not just with accelerated atherosclerosis, but also with a severe disease activity as reflected in this patient’s SLEDAI scores.16
ConclusionsNotwithstanding the trend to a declining SLE activity in chronic kidney disease undergoing dialysis, as the years go by, the risk of reactivation does not go away; therefore, it is important to be aware of the variables predisposing to disease reactivation, such as gender, age of presentation, infections based on the associated immunodeficiency status, serum markers, and patient-specific risk factors (smoking, atherosclerotic disease, etc.).
FundingThere was no special financing for the development of this case report.
Conflict of interestsThe authors have no conflict of interests to disclose.
Ethical responsibilitiesRight to privacy and informed consent. The authors declare that this article does not reveal any patient information and was approved by signing the informed consent.
Please cite this article as: Gómez Gómez N, Velásquez Franco CJ, Lozano Pineda F, Caro Palacio J. Actividad lúpica en paciente con enfermedad renal terminal: reporte de caso. Rev Colomb Reumatol. 2021;28:64–68.