C1q nephropathy was first described in 1985 as a process of glomerulonephritis with mesangial C1q deposit. The histology is similar to lupus nephritis, and was initially described as being seronegative renal lupus. However, these two entities are now considered different pathological processes. Its association with rheumatoid arthritis is unusual, and there are no cases with a similar presentation reported in the literature. In this article, the case is presented of a man who developed both these conditions.
La nefropatía C1q, se describió por primera vez en 1985, como un proceso de glomerulonefritis con depósito mesangial de C1q, histológicamente similar a la nefritis lúpica, siendo inicialmente descrita como lupus renal seronegativo, sin embargo, estas dos entidades se consideran actualmente como procesos patológicos diferentes. Su asociación con artritis reumatoide es inusual y la literatura no reporta casos con presentación similar. A continuación, presentamos el caso de un hombre que desarrolla estas dos entidades.
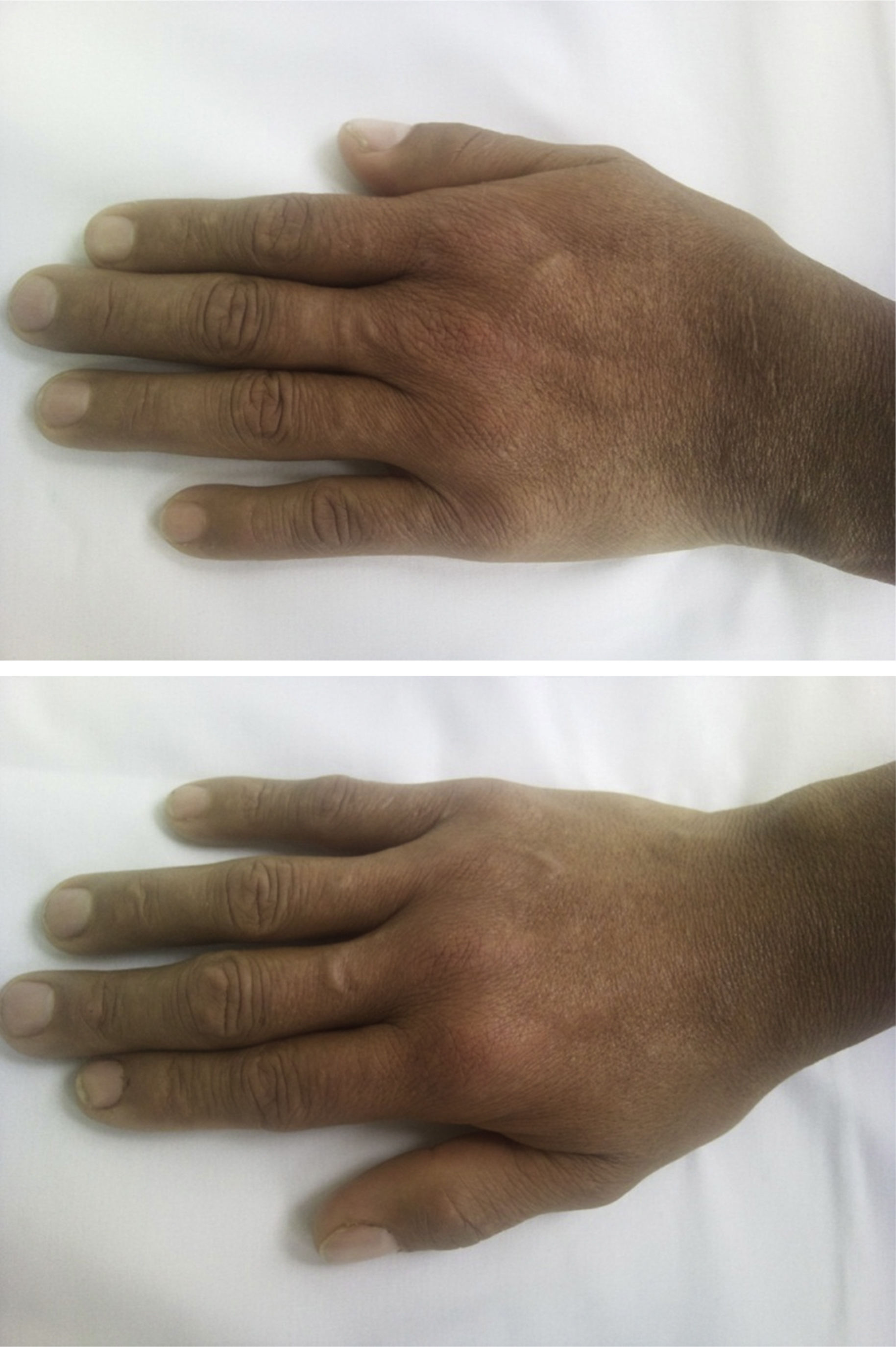
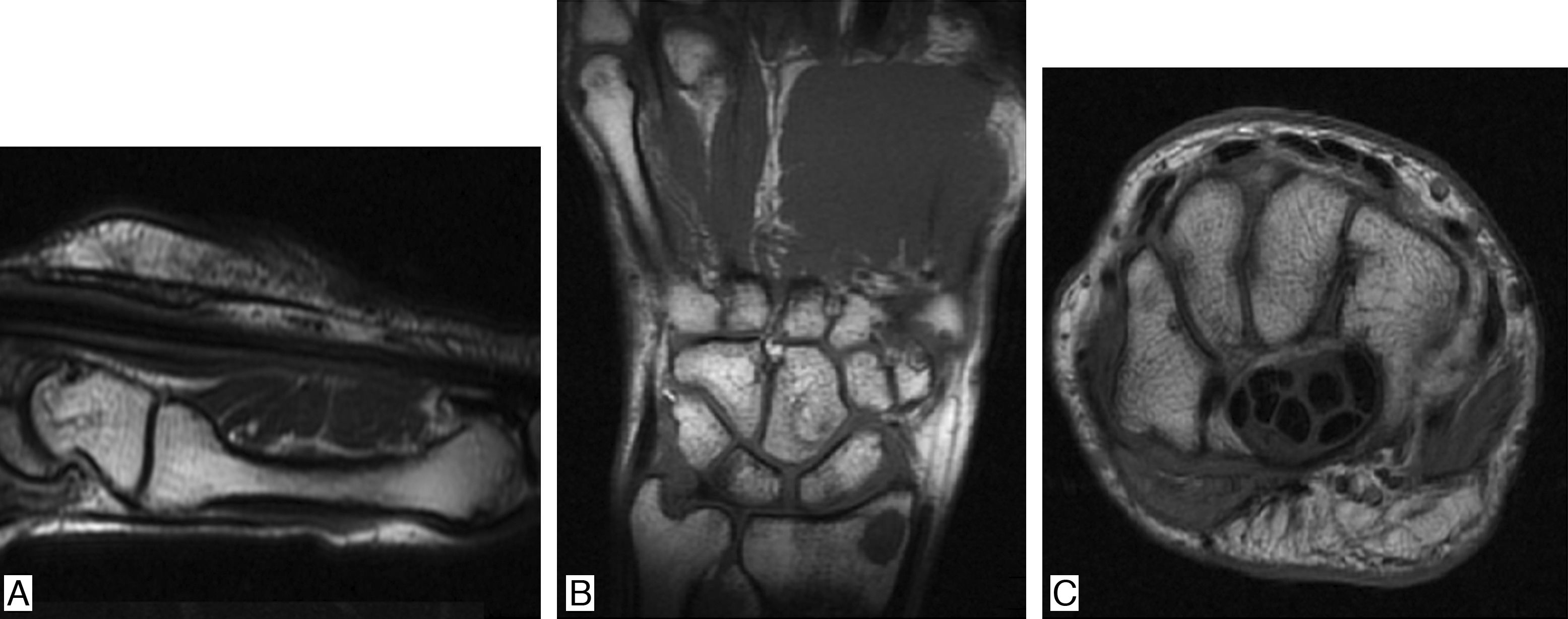
It is a 38-year-old man who in 2011 presented nephrotic syndrome and arterial hypertension, and was taken to kidney biopsy, which documented C1q nephropathy. The patient received treatment with 6 monthly boluses of cyclophosphamide and subsequently was changed to azathioprine, presenting gastrointestinal intolerance to the latter drug, and for this reason was changed to mycophenolate, with adequate control of the nephropathy. Systemic lupus erythematosus (SLE) was ruled out, since the patient did not present clinical stigmata of this disease and the immunological studies were normal (negative ANAS, ENAS and anti-DNA, normal complement, negative lupus anticoagulant, anticardiolipins and B2 glycoprotein). Three years after the onset of the nephropathy, presents arthralgias on hands of inflammatory characteristics, associated with episodes of synovitis and morning stiffness during 40min. Physical examination shows synovitis and pain in 10 joints (bilateral 2–5 PIP and wrists) (Fig. 1). Laboratory data are collected finding: ESR 67mm/h, CRP 25.7mg/dl (normal between 0 and 1mg/dl), positive high titer of anti-cyclic citrullinated peptide (anti-CCP) (98.9mg/dl, normal value up to 20mg/dl), high positive rheumatoid factor (89.7mg/dl, normal value up to 14mg/dl). Given the history of nephropathy and the establishment of a clinical picture of inflammatory arthropathy, studies were extended in order to rule out SLE. Samples were taken for ANAS, ENAS, anti-DNA, IgG and IgG antiocardiolipin antibodies, IgG and IgM antibeta2 glycoprotein antibodies and VDRL, all with negative results, as well as normal complement. The nuclear magnetic resonance showed in the right hand cortical erosions in all bones of the carpus and distal radius as well as in the head of the 2nd, 3rd and 5th metacarpal bones, with incipient synovitis in metacarpophalangeal joints of the 1st, 2nd, 3rd and 4th fingers. In the left hand there were mild periarticular synovial inflammatory changes of the carpus with mild thickening of the periarticular synovial, both dorsal and ventral, with incipient generalized synovitis in metacarpophalangeal joints (Fig. 2). Since there was not any clinical or paraclinical finding that would suggest the presence of SLE and in the presence of highly positive acute-phase reactants, anti-CCP and rheumatoid factor, it was made the diagnosis of rheumatoid arthritis (RA), deciding to optimize its immunomodulatory management with methotrexate 15mg every week, with which the patient presents adequate control of his joint symptoms and enters into clinical remission of RA.
C1q nephropathy is a glomerular disease with a low frequency of presentation, characterized by proteinuria (from mild proteinuria to proteinuria in the nephrotic range) with poor response to the administration of glucocorticoids, unknown etiology and histopathological findings determined by the great immune component which characterizes it. It was first described in 1985 by Jennette and Hipp, who published a detailed review or the immunofluorescence staining pattern in 800 consecutive renal biopsies, finding C1q in 36% of the biopsies, with a mean fluorescence intensity of 1.6+ in a scale of 6 points (0.0–4+). The intensity and the C1q staining pattern in combination with standard immunofluorescence microscopy and the detailed ultrastructural analysis identified a subset of patients that was called C1q nephropathy. By definition, hundred per cent of these patients had C1q staining with a mean fluorescence intensity of 3.6+ in a predominantly mesangial pattern, with scattered granular staining in some capillary loops.1
This type of nephropathy occurs with a pattern of glomerulonephritis characterized by predominant deposits of mesangial C1q, but with a histological pattern that resembles lupus nephritis. The distinctive immunopathological characteristic is the dominant or codominant immunostaining for C1q with mesangial electron-dense deposits, without clinical or paraclinical evidence of SLE.2 The literature reports a very variable prevalence with values ranging from 0.2% to 16%.3
The first reports of C1q nephropathy arose from renal biopsies of patients with diagnosis of SLE, in whom the only manifestation of lupus commitment was renal, but with the characteristic of being serologically negative and, therefore, without meeting the classification criteria for SLE, being called, at that time, “seronegative lupus”. The findings in the renal biopsies in these first cases demonstrated the presence of dominant or codominant C1q deposits, especially in the mesangium, in the clinical context of patients without any other manifestation of SLE.4 Subsequent clinical-pathological correlation studies in children and young adults with this type of kidney damage evidenced the presence of severe nephrotic-range proteinuria, with very poor response to management with glucocorticoids and a high recurrence rate.5,6
C1q is a protein critical for the function of the classic activation pathway of the complement system. In human beings the normal serum concentrations of C1q range from 100 to 180mg/l. It is a complex biological molecule with a molecular weight of approximately 459kD and is made up by 18 polypeptide chains: 6A chains, 6B chains and 6C chains. Each chain is encoded by a separate gene located on chromosome 1 between p36.3 and p34.1.7
The clinical spectrum of the disease is broad, with histopathological patterns ranging from minimal change disease, membranoproliferative glomerulonephritis, to focal and segmental glomerulosclerosis, with varied clinical presentations, that include hematuria associated with proteinuria which can range from mild to nephrotic-range.8
Renal involvement in RA is relatively frequent, with a prevalence between 5 and 50% in the different series.9 The most common disorders are: membranous nephropathy, secondary amyloidosis, focal mesangial proliferative glomerulonephritis, rheumatoid vasculitis and analgesic nephropathy.10 In the histopathological study of our case predominates the pattern of membranous involvement without the presence of amyloidosis and without any other associated comorbidity. Membranous nephropathy is associated with treatment with D-penicillamine or gold salts. The incidence described is 1% with D-penicillamine and 1–3% with parenteral gold with development of proteinuria, usually, in the first 6–12 months of treatment.11 The suspension of treatment generates slow resolution of the proteinuria in approximately 9–12 months, due to the subepithelial location of the immune deposits that limits the access of phagocytic cells.11,12
The chronic inflammation in RA determines an increase in the production of acute phase protein serum amyloid A. A fragment of serum amyloid protein is deposited generating nephrotic syndrome. Currently, it is rare due to the therapeutic effectiveness in the control of inflammation that determines, in addition, resolution of the deposits and the proteinuria in the patients who present it.13 Helin et al., describe mesangial proliferative glomerulonephritis as a finding in 40 out of 110 patients with RA, who underwent biopsy, which usually presents with hematuria with or without proteinuria,10 but these cases, unlike the one we are presenting, are of patients who have a history of RA and later develop renal involvement. Among the findings that can be documented in patients with RA that suggest renal involvement, proteinuria with or without hematuria stands out, therefore, the detection of renal disease should not only include the measurement of creatinine but also sensitive methods to detect glomerular and tubular proteinuria.14 Microalbuminuria can be used as a simple and sensitive test to detect subclinical renal dysfunction in patients with kidney damage induced by RA.15 These correlations between clinics and laboratories are not absolute and do not exclude the need for a renal biopsy, in some selected cases, to establish a diagnosis.
Our patient presented a clinical picture of symmetrical inflammatory arthralgia in the hands, associated with morning stiffness with elevation of acute phase reactants, anti-CCP and rheumatoid factor, all positive in high titers and with the presence of erosions in hands, so it is considered that RA in the context of a patient with a history of C1q nephropathy. When reviewing the literature we did not find, to date, the report of similar clinical cases.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Benavides MF, Fernández-Ávila DG, Gutiérrez JM. Hombre con nefropatía C1q y artritis reumatoide: reporte de un caso. Rev Colomb Reumatol. 2017;24:247–250.