To review the definitions, associated factors, as well as the methods for the measurement and determination of adherence to oral disease modifying drugs in rheumatoid arthritis.
MethodsA search of the literature was carried out in the PUBMED databases up to December 2017 using MeSH terms: (((“Arthritis, Rheumatoid” [Mesh] AND “Medication Adherence” [Mesh]) OR “Patient Compliance” [Mesh]) AND “Antirheumatic Agents” [Mesh]). Only articles that included an adult population and were in Spanish or English were reviewed.
ResultsFrom the 387 articles found, 43 were included for general review (definitions of adherence, compliance, concordance and persistence, components, classification and dimensions of adherence, risk factors related to non-adherence, description of direct and indirect methods for measuring adherence). Only 9 articles measured adherence and included information about risk factors related to non-adherence to oral treatment in rheumatoid arthritis.
ConclusionsThe adherence to pharmacological treatment in rheumatoid arthritis is sub-optimal and is associated with less effectiveness in the control of inflammatory activity. The main factors related to low adherence include problems of drug access and availability, increased activity and duration of the disease, polypharmacy, use of medications for prolonged periods, socioeconomic stratum, ethnicity, adverse drug reactions, perception of ineffectiveness of the medication, and concomitant diseases. It is necessary to incorporate the systematic measurement of pharmacological adherence within clinical practice. It is also important to identify the most frequent risk factors associated with low adherence, in order to design strategies aimed at improving patient adherence and achieve better clinical outcomes.
Revisar definiciones, factores asociados a adherencia, métodos para medición y determinación de adherencia a medicamentos modificadores de la enfermedad orales en artritis reumatoide.
MétodosSe realizó una búsqueda de la literatura en las bases de datos de Pubmed hasta diciembre de 2017 mediante términos MeSH (((«Arthritis, Rheumatoid» [Mesh] AND «Medication Adherence» [Mesh]) OR «Patient Compliance» [Mesh]) AND «Antirheumatic Agents» [Mesh]) de artículos que estuvieran en idioma español o inglés e incluyeran solo población adulta.
ResultadosDe un total de 387 artículos encontrados, 43 se incluyeron para la revisión general, con información sobre definiciones de adherencia, cumplimiento, concordancia y persistencia, componentes, clasificación y dimensiones, factores de riesgo relacionados con la no adherencia al tratamiento, descripción de los métodos de medición. Solo 9 artículos midieron adherencia e incluyeron información sobre factores relacionados con adherencia a medicamentos orales en artritis reumatoide.
ConclusionesLa adherencia al tratamiento farmacológico en artritis reumatoide es subóptima y se relaciona con menor efectividad en el control de la actividad inflamatoria. Los principales factores relacionados con baja adherencia incluyen problemas de acceso y disponibilidad del medicamento, mayor actividad y duración de la enfermedad, polifarmacia, uso de medicamentos por periodos prolongados, bajo estrato socioeconómico, etnia, reacciones adversas por medicamentos, percepción de inefectividad de la medicación y enfermedades concomitantes. Es necesario incorporar de forma sistemática la medición de adherencia farmacológica dentro de la práctica clínica rutinaria y la identificación de los factores de riesgo más frecuentes asociados a una baja adherencia con el fin de diseñar estrategias encaminadas a mejorar la adherencia de los pacientes y lograr mejores desenlaces clínicos.
Rheumatoid arthritis (RA) is a systemic autoimmune disease of chronic course1 whose most important clinical expression is articular,2,3 although it may exhibit extra-articular involvement, such as the presence of rheumatoid nodules, pulmonary involvement, vasculitis or systemic comorbidities.3,4 Its prevalence is higher in middle-aged people and in older adult women.5 It has an incidence of 0.32–0.38% and a prevalence close to 1%1,5; it is estimated that 1.3–1.5 million adults in the United States of America suffer from the disease.6 In Colombia, it has been described a prevalence of 0.9 patients per 100 inhabitants, with a female/male ratio of 4:1.7 Recurrent joint pain, inflammation and deformity are developed during the course of the disease, which not only affects the functionality, but also the ability to work and the quality of life both of patients and their families, in addition to increasing the morbidity and mortality.1,5,8
In the global burden of disease study, RA ranked 42nd among the conditions that produce greater disability, accounting for 0.49% (0.36–0.62%) of the total years of life lived with disabilities.9 In addition, it is estimated that the total economic burden of the disease is $ 5.8 billion dollars annually.10
In 2009 was started the implementation of the Treat to Target (T2T) strategy, through which algorithms are created to simplify the different strategies and pharmacological combinations in the treatment or RA, since they are focused on concrete therapeutic goals.11–14 At present, the goal of treatment proposed in the guidelines for management of RA of the European League Against Rheumatism (EULAR) consists in achieving remission or a period of low disease activity.12 A fundamental principle of this strategy is that the treatment must be a shared decision between the patient and the rheumatologist14; therefore, the specialist must involve the patient in the context of the T2T and in the strategy that will be implemented to reach it.13
Unfortunately, patient's adherence to the chronic treatment of RA is suboptimal.15 The World Health Organization (WHO) reports that, in developed countries, the adherence of people with chronic pathologies is around 50%,15,16 this means that half of the people suffering from chronic diseases comply with the treatment recommendations.15,16
Non-adherence to treatment reduces the effectiveness of drugs, delays the recovery from symptoms, allows the progression of the disease and creates the need to add new interventions and medications to achieve an optimal control of the symptoms with the risk of generating pharmacological interactions and adverse events, as well as the increase in costs.15,17 In addition, it affects the quality of life and generates an increase in the number of relapses, disability and mortality.5,15,18 It has been described that in North America the cost of lack of adherence is estimated at 300 billion dollars per year represented in avoidable costs.19
MethodologyA literature search of reviews published until December 2017 that included only adults was conducted in the PUBMED databases. The search strategy was carried out using the MeSH terms: (((“Arthritis, Rheumatoid” [Mesh] AND “Medication Adherence” [Mesh]) OR “Patient Compliance” [Mesh]) AND “Antirheumatic Agents” [Mesh]). Only articles in Spanish or English were reviewed.
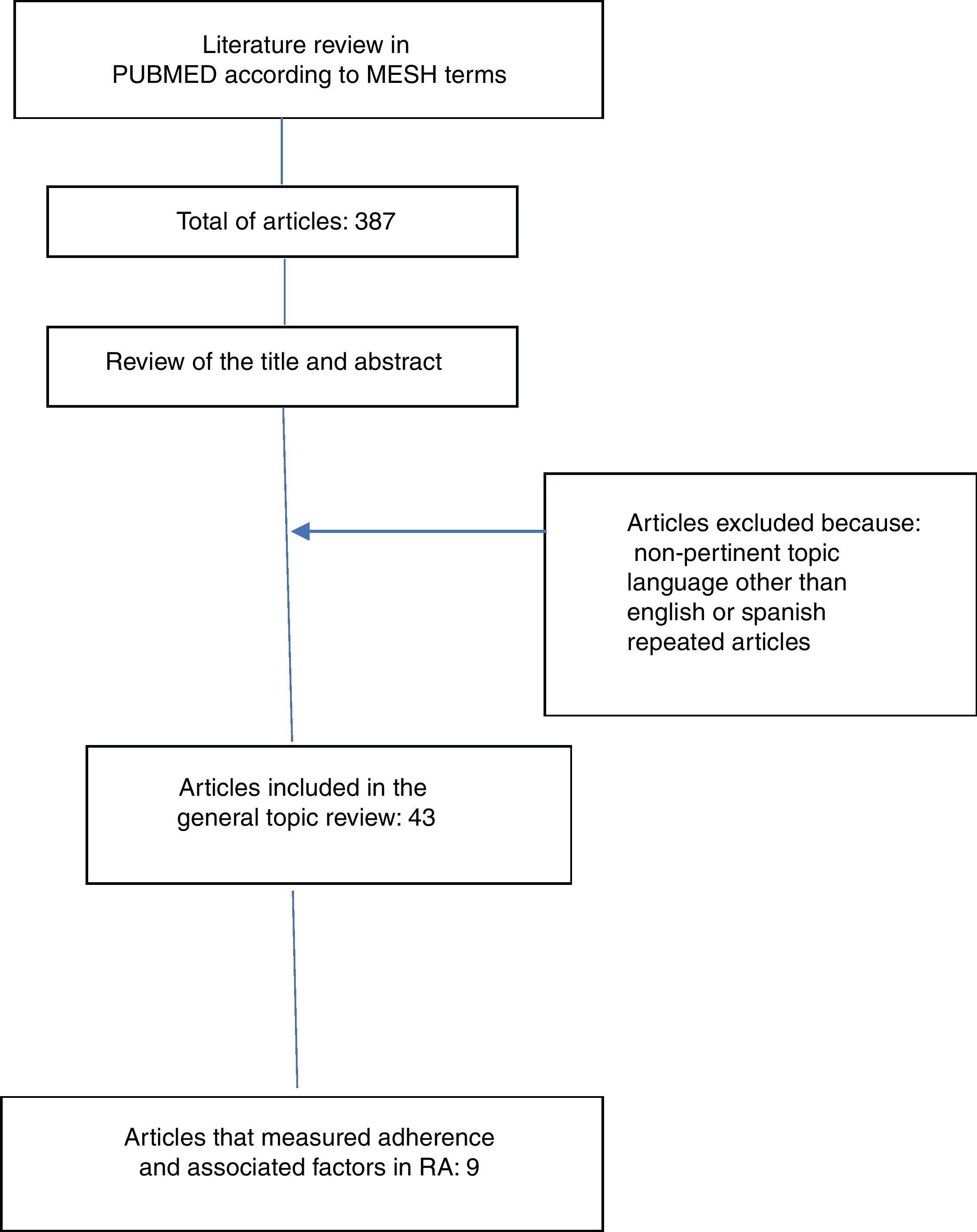
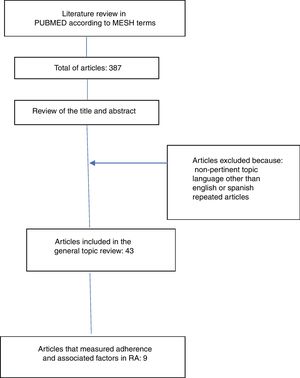
ResultsThe literature search yielded 387 articles, of them, 43 were finally included for the review. 344 articles were excluded due to non-relevance of the topic, language other than English or Spanish and because they were repeated articles (Fig. 1).
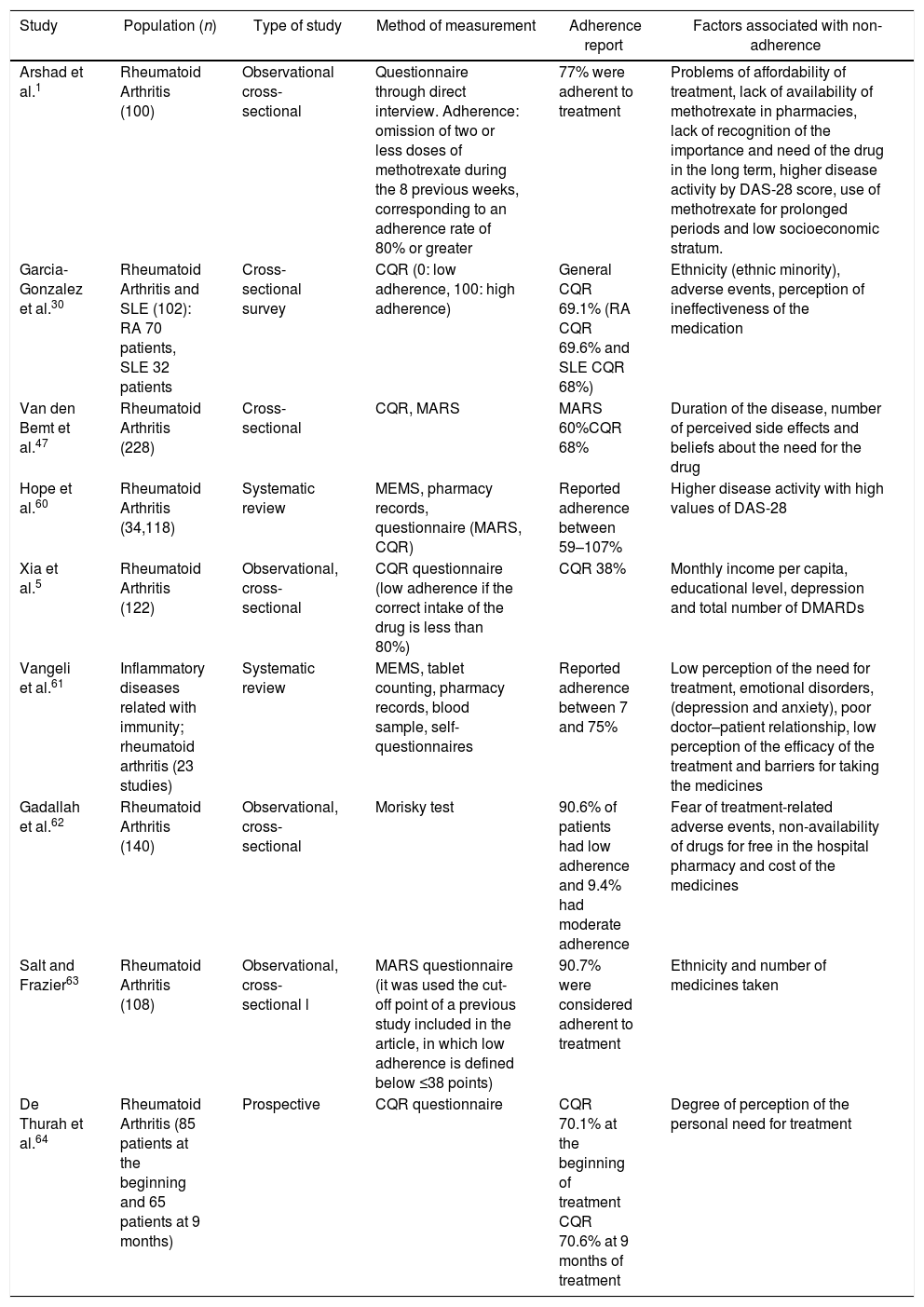
The information collected from the 43 articles included: definitions (adherence, compliance, concordance and persistence), components, classification and dimensions of adherence, risk factors related to adherence to treatment, description of direct and indirect methods for the determination of adherence and determination of the global adherence to oral disease-modifying treatment. Only 9 articles measured adherence and included information on factors related to adherence to oral medications in RA (Table 1).
Characteristics and results of the studies that evaluated the degree of adherence to pharmacological treatment in rheumatoid arthritis.
| Study | Population (n) | Type of study | Method of measurement | Adherence report | Factors associated with non-adherence |
|---|---|---|---|---|---|
| Arshad et al.1 | Rheumatoid Arthritis (100) | Observational cross-sectional | Questionnaire through direct interview. Adherence: omission of two or less doses of methotrexate during the 8 previous weeks, corresponding to an adherence rate of 80% or greater | 77% were adherent to treatment | Problems of affordability of treatment, lack of availability of methotrexate in pharmacies, lack of recognition of the importance and need of the drug in the long term, higher disease activity by DAS-28 score, use of methotrexate for prolonged periods and low socioeconomic stratum. |
| Garcia-Gonzalez et al.30 | Rheumatoid Arthritis and SLE (102): RA 70 patients, SLE 32 patients | Cross-sectional survey | CQR (0: low adherence, 100: high adherence) | General CQR 69.1% (RA CQR 69.6% and SLE CQR 68%) | Ethnicity (ethnic minority), adverse events, perception of ineffectiveness of the medication |
| Van den Bemt et al.47 | Rheumatoid Arthritis (228) | Cross-sectional | CQR, MARS | MARS 60%CQR 68% | Duration of the disease, number of perceived side effects and beliefs about the need for the drug |
| Hope et al.60 | Rheumatoid Arthritis (34,118) | Systematic review | MEMS, pharmacy records, questionnaire (MARS, CQR) | Reported adherence between 59–107% | Higher disease activity with high values of DAS-28 |
| Xia et al.5 | Rheumatoid Arthritis (122) | Observational, cross-sectional | CQR questionnaire (low adherence if the correct intake of the drug is less than 80%) | CQR 38% | Monthly income per capita, educational level, depression and total number of DMARDs |
| Vangeli et al.61 | Inflammatory diseases related with immunity; rheumatoid arthritis (23 studies) | Systematic review | MEMS, tablet counting, pharmacy records, blood sample, self-questionnaires | Reported adherence between 7 and 75% | Low perception of the need for treatment, emotional disorders, (depression and anxiety), poor doctor–patient relationship, low perception of the efficacy of the treatment and barriers for taking the medicines |
| Gadallah et al.62 | Rheumatoid Arthritis (140) | Observational, cross-sectional | Morisky test | 90.6% of patients had low adherence and 9.4% had moderate adherence | Fear of treatment-related adverse events, non-availability of drugs for free in the hospital pharmacy and cost of the medicines |
| Salt and Frazier63 | Rheumatoid Arthritis (108) | Observational, cross-sectional l | MARS questionnaire (it was used the cut-off point of a previous study included in the article, in which low adherence is defined below ≤38 points) | 90.7% were considered adherent to treatment | Ethnicity and number of medicines taken |
| De Thurah et al.64 | Rheumatoid Arthritis (85 patients at the beginning and 65 patients at 9 months) | Prospective | CQR questionnaire | CQR 70.1% at the beginning of treatment CQR 70.6% at 9 months of treatment | Degree of perception of the personal need for treatment |
Over time, three terms that must be taken into account when evaluating the intake of medications have been described: “concordance”, “compliance” and “adherence”.20,21 However, some authors use the terms “adherence” and “compliance” indiscriminately to describe the degree in which a patient takes his medication as prescribed.22
The term “concordance” is defined as the extent to which patients are successfully supported for decision-making on issues related to drugs and their intake.21,22 On the other hand, the term “compliance” makes reference to the extent to which the record of administration of the drugs referred by the patient corresponds to the regimen prescribed by the physician.21,22 The term “adherence” refers to the degree to which the behavior of the patients is consistent with the recommendations agreed by the prescriber.21,22 In this way, it can be understood that the term “adherence” supposes an agreement or cooperation of the patient with the recommendations or advice of the prescriber, while “compliance” suggests that the patient passively follows the instructions of his physician.22
Currently, the term used is “adherence to treatment”, which according to the WHO is defined as the degree to which the behavior of the patient in terms of medication, following of a diet or changes in lifestyle is consistent with the recommendations agreed with the healthcare professional.16,23 It is also defined as the extent to which a patient acts according to the prescribed interval and dosage regimen.10 Based on the foregoing, a consumption equal to or greater than 80% of the prescribed medication in a proper manner has been defined as a cut-off point to determine an adequate or satisfactory adherence.1,24,25
Another term that should be taken into account is the “medication persistence”, which refers to the compliance with the treatment recommendations during the time of prescription,2 that is, complying with the interval of duration of the treatment from the moment in which it is started until the interruption of the therapy.10
The adherence has three main components.26 The first one is the initiation, in which the patient takes the first dose of the medicine that was prescribed by the health professional,21 accepts the need for the drug and adjusts the prescription schedule to his daily life.26 The second component is the implementation, in which it is determined that the dose used by the patient corresponds to the dosage prescribed by the health professional from the first to the last dose.27 And finally there is the interruption component, in which no more doses of the prescribed drug are taken.21
Non-adherence to treatment can be classified into primary and secondary.15Primary non-adherence occurs when a patient does not follow the initial prescription of the physician due to multiple factors, for example, socioeconomic factors.28 On the other hand, the secondary non-adherence occurs when the patient interrupts treatment early due to factors such as the presence of adverse drug reactions, lack of efficacy of the drug or when there is not a timely response to the treatment.15,28
Risk factors related to the adherence to treatmentThe WHO has described five dimensions involved in the adherence to treatment, which are grouped into socioeconomic factors, factors related to health care systems and medical equipment, factors related to comorbidities, factors related to treatment and factors related to the patient.16,21,23 Some authors have described that factors such as the disease activity, comorbidities, the doctor–patient relationship and age can influence the adherence to pharmacological treatment.29 It has even been documented that non-adherence to treatment occurs more frequently in ethnic minorities compared with Caucasian patients.30 Some factors related to the physician are also determined by the knowledge of the disease and the relationship with the medical team.31 In addition, it is important to recognize patients’ beliefs about treatment as a decisive factor for adherence.15,32
The risk factors related to non-adherence to treatment can be classified as modifiable (based on the behavior) and non-modifiable (age, gender).19
Non-adherence to treatment from the patient's perspective can be classified as non-intentional (involuntary) or intentional.25 The involuntary non-adherence occurs when the patient has the intention to take the prescribed treatment but cannot do it due to different circumstances, such as forgetting to take the medication, lack of understanding of the recommendations given during the prescription or inability to pay for pharmacological treatment.21,25,33,34 The intentional non-adherence is determined by the patient decision to discontinue treatment or by the modification of the dosage regimen that had been prescribed by the health professional. This type of non-adherence may be influenced by the beliefs of the patients about the effectiveness of the recommendations of health professionals, their own knowledge about the disease and their ability to achieve health goals.21,25,33,34
Description of the direct and indirect methods for determining adherenceThere are different methods to evaluate the adherence, which are classified into direct and indirect.25,35 The direct methods make reference to the measurement of the drug through biological samples for the determination of drugs/metabolites in serum and the direct observation of the intake of the drugs.21,25,36 Although none of these direct methods is 100% reliable, they have the advantage that they present a low index of bias.37
Biological studies of measurement of drugs/metabolites in serum are more accurate, but are not available for the study of all drugs. These methods have the disadvantage of being more expensive, invasive and impractical for the patients and their interpretation depends on the pharmacokinetics of each individual and the pharmacological interactions21,25,38,39; in addition, they reflect results of short-term adherence and may overestimate the long-term adherence because they may be influenced by the effect of “white coat adherence”.37 On the other hand, direct observation is carried out by administering the medication or observing the patient while he is ingesting it; however, this method is only practical for single-dose treatments or for patients attending a hospital or infusion center.21
The indirect methods include the realization of interviews, self-questionnaires, control of drug dispensation, tablet counting, evaluation of therapeutic results and use of electronic devices.21,25,37
The pharmacy records provide information about dispensed medications, and are objective, low-cost, non-invasive methods37; however, they cannot assess whether the patient actually took the drug or the precise moment when he did it.40 Through these records it is possible to measure the days without medication or the persistence of the treatment.40 The tablet counting is a low-cost, non-invasive, practical method, but it may overestimate adherence because there may be manipulation by the patient and it is a method that depends on the correct number of tablets dispensed.37 This method is useful to estimate the general or average adherence but does not allow to establish the daily adherence or the adherence per dose.40
The electronic monitoring devices, such as the Medication Event Monitoring System (MEMS), are currently the gold standard test in the determination of adherence,25,41 since they are more accurate and objective methods and allow to obtain additional information on the dosing interval.37,38 The disadvantage of these devices is that they are expensive, do not allow direct observation of the patient or to determine how much of the medication was ingested.25,40
The self-reported measures are useful given their low cost, the ability to provide information about the patient's beliefs, and the ease of implementation, non-invasiveness and reproducibility.42,43 These instruments include diaries and self-report questionnaires.42,43 Several questionnaires have been used in rheumatic diseases, including the Compliance Questionnaire in Rheumatology44 and the Medication Adherence Report Scale (MARS).45,46 However, it should be taken into account that the self-report methods are subjective, often overestimate adherence when compared with tablet counting or monitoring by electronic devices and may be influenced by recall and notification bias; in addition, patients tend to answer questionnaires in a way that people feel is socially acceptable.40,41,47 The scales or questionnaires are used because they have good reliability, can be answered quickly and have been validated for different diseases.48
The Morisky test49 is a questionnaire which consists of four questions to identify the reasons for the lack of adherence, it has a consistency (Cronbach's) of α=0.61, a sensitivity of 81% and a specificity of 44%; however, it has not been validated in RA.37 The MARS questionnaire has been used in a variety of diseases such as psychosis, asthma, chronic obstructive pulmonary disease, RA and diabetes and it has items that are focused on determining the intentional non-adherence (preference to avoid, forget, adjust and stop taking medicines) and when the patient forgets to take the medication.45,46,50,51
The Compliance Questionnaire on Rheumatology (CQR) is a self-administered 19-item questionnaire that is used to measure the adherence to a therapeutic regimen, identifying factors that contribute to suboptimal adherence.35 It was developed specifically for the area of rheumatology in RA, polymyalgia rheumatica and gout in the Maastrich University Hospital44 with a sensitivity of 98%, a specificity of 67% and a Cohen's kappa of 0.71 in the detection of low adherence.44 The stepwise discriminant analysis revealed that 3 items correctly classified 84% of all cases, with a sensitivity of 99%, a specificity of 80% and kappa of 0.78.44 In a further study validated in patients with rheumatologic inflammatory diseases with MEMS as comparator in 6 months of follow-up, it had a sensitivity of 62%, a specificity of 95% and an expected kappa of 0.78 to detect non-adherence.24 The likelihood ratio for detecting low adherence was 11.6.24 Therefore, it is a valid and reliable scale used in clinical practice for patients with RA.
A Colombian group performed the transcultural adaptation and validation in Colombia in 233 patients with RA.52 The cut-off point of the CQR to establish adherence to treatment was 80.7, with which a sensitivity of 80.2% (95%CI: 71.9–86.9%) and a specificity of 72.3% (95%CI: 63.1–80.4%) were obtained.52 According to this cut-off point, it was established that 43.8% (n=102) of patients were adherent to oral antirheumatic therapy.52
Global adherence to oral disease modifying treatment in rheumatoid arthritisApproximately 50% of people with chronic pathologies do not have adherence to the medical pharmacological treatment and up to 50% of the drugs prescribed for a long term are not taken as indicated in the medical precription.16,47,53 It has been identified that between 10 and 30% of patients with RA are intolerant to methotrexate, and for this reason they discontinue treatment periodically.54,55 In addition, patients are fearful of adverse drug reactions; for example, the ocular toxicity caused by chloroquine,56 the development of cancer with the use of biotechnological drugs 57 or are afraid to take a drug that is also used as an antineoplastic agent (methotrexate).58
Specifically in AR, the adherence rate described on average is low, reported between 30 and 80%,5,18 although there are reports that estimate that the adherence varies between 33% (underutilization) and 107% (excessive use).10 The variability of the data is secondary to the different measurement methods used in the studies.59
A cross-sectional observational study determined the adherence to methotrexate in 100 patients with RA who had received therapy for at least 2months and evaluated the possible factors that could intervene in the pharmacological adherence.1 It was identified that the non-adherence rate was 23%, related to the greater disease activity determined by the DAS-28 score (p<0.001), the use of methotrexate for prolonged periods (p<0.001) and the low socioeconomic stratum (p<0.0001). Problems of affordability of treatment, lack of availability of methotrexate in the pharmacies, lack of recognition of the importance and need for a long term of the drug were evidenced as variables involved in non-adherence. In addition, it was found that an important motivational factor was the fact of having an adequate education and counseling by the attending physician (p<0.001) and that the lack of family support is a variable that influence the non-adherence to treatment (p<0.001).1
A study that assessed the self-informed adherence in patients with RA and systemic lupus erythematosus was also conducted in 102 patients with ethnic diversity (43% Hispanic, 32% African-American and 25% Caucasian) through the CQR questionnaire (0: low adherence; 100: high adherence).30 The average CQR score was 69.1±10.5 (moderate adherence) and it was evidenced that one third of the patients reported that they never forgot to take their medications, while 40% reported that they discontinued their drugs due to side effects and 20% due to lack of effectiveness. 23% of patients belonging to ethnic minorities had problems to take their medications compared to 11% of patients of white ethnicity (p=0.03). Factors such as lower educational levels and side effects were associated with lower adherence.30
A descriptive study evaluated the adherence rates in 228 patients with RA who used disease-modifying antirheumatic drugs (DMARDs) through the CQR and MARS methods; 68% (CQR) and 60% (MARS) of patients were adherent to pharmacological treatment. The duration of the disease, the number of perceived side effects, and the beliefs about the need for the medication were weakly related to the adherence.47
A systematic review of 2015 evaluated the rate of adherence to methotrexate and the possible predictors of adherence.60 The adherence rate was estimated between 59 and 107%, it had a suboptimal response (59–63%) in 2 studies, optimal in other 2 studies (80–88%), and in one study it was reported an adherence of 107% (overutilization). It was determined that high values of DAS-28 were related to a decreased adherence, suggesting that a more severe disease activity is associated with a lower degree of adherence to treatment. Some modifiable factors to improve the adherence to treatment were found, such as the beliefs that exist around the treatment, the self-efficacy with regard to the intake of medications and the styles of coping with the disease.60
A descriptive cross-sectional study evaluated the degree of adherence to DMARDs in Chinese patients with RA, reporting that 38% of patients were adherent to treatment with DMARDs according to the CQR scale.5 It was found that the monthly income per capita (OR=2.515; p=0.01), the depression (OR=4.305; p=0.01) and the total number of DMARDs (OR=1.843; p=0.05) were variables related to results of non-adherence to treatment.5
Another systematic review determined that the adherence rate varied between 7 and 75% for rheumatic conditions, between 4 and 72% in inflammatory bowel disease and between 8 and 87% in psoriasis.61 It was concluded that psychosocial factors were associated with the development of non-adherence to treatment, including low perceptions of the need for treatment (beliefs about the intake of medications, concerns and efficacy), emotional disorders (depression and anxiety), poor doctor–patient relationship, low perception of the efficacy of the treatment and barriers to take the medications.61
A cross-sectional descriptive study conducted in Egypt and the Middle East region evaluated the pharmacological adherence in 140 patients who suffered from RA.62 It was found that 90.6% of the participants had low adherence and 9.4% had a moderate adherence rate.62 The variables related to non-adherence were the fear of side effects, the lack of availability of medicines for free in the hospital pharmacy and the cost of the drugs.62 Younger patients (p=0.002) with better satisfaction (p=0.02) had better adherence to treatment, in addition to the fact of having the treatment on time (p=0.001) and lower disease activity (p=0.02).62
The adherence to DMARDs was evaluated in a cross-sectional descriptive study.63 It was estimated that about 90% of the individuals reported adherence; however, the only variable that showed a statistically significant difference between the adherent and non-adherent groups was the ethnicity (p=0.04), given that the individuals of non-Caucasian race reported a significantly lower adherence to DMARDs.63 With the logistic regression model was identified that the ethnicity (OR=3.34; p<0.05) and the number of medications taken (OR=1.7; p<0.05) were predictors related to the lack of adherence to medications.63
In another study, the adherence to methotrexate was evaluated at the beginning of the treatment (85 patients) and 9 months later (65 patients) through the CQR. The authors report an adherence of 70.1% and 70.6% in the first and the second evaluation, respectively. The main objective of this study was to evaluate the relationship between personal beliefs about the need for treatment and the concerns of the individual about methotrexate, finding that only the perception of need for treatment is related to low adherence.64
Finally, in a study that evaluated the beliefs about the need for medications, the factors that are related to specific beliefs about the drugs and their correlation with adherence to treatment in 600 patients with RA, it was identified that three quarters of the patients who suffer from RA have positive beliefs about the need to take medications, but there are factors related to the potential for adverse events, especially in the long-term.65 Adherence was not measured in this study.
ConclusionsAdherence to pharmacological treatment is a term that involves the patient in the decisions related to the treatment and that should be used to evaluate the behavior of patients in relation to the intake of medicines and the recommendations in terms of healthy lifestyle habits which are provided by health professionals. The adherence to drug treatment has been determined through direct and indirect methods. The direct methods are more accurate but are not available to evaluate all drugs; they are expensive and invasive and vary according to the pharmacokinetics of each individual. The indirect methods, although subjective, are not invasive, inexpensive and easy to implement. It has been identified that the adherence to pharmacological treatment in RA is suboptimal and is related with lower effectiveness in the control of the inflammatory activity. The main factors related to low adherence to the oral disease modifying drugs are the problems of affordability of treatment, lack of availability of the drug in pharmacies, higher disease activity by DAS-28 score, longer duration of the disease, the number of medications, use of medications for prolonged periods, low socioeconomic stratum, ethnicity, adverse drug reactions, perception of ineffectiveness of the medication and concomitant diseases.
It is necessary to incorporate in a systematic manner the measurement of pharmacological adherence within the routine clinical practice and the identification of the most frequent risk factors associated with low adherence in order to design strategies aimed at improving the adherence of patients and achieving better clinical outcomes.
FinancingThe writers received funding from the Colombian Association of Rheumatology for the realization of a project related to the measurement of pharmacological adherence in patients with rheumatoid arthritis in Colombia. This revision is a first product of the project.
Conflict of interestThe authors do not have any conflict of interest.
Please cite this article as: Rincón Rincón JR, Jaimes Fernández DA, García Casallas JC, Beltránd A, Télleze A, Fernández-Ávila DG, et al. Métodos para la medición de la adherencia a medicamentos modificadores de la enfermedad orales en artritis reumatoide y factores asociados con baja adherencia farmacológica. Rev Colomb Reumatol. 2018;25:261–270.