Organizing pneumonia is a rare clinico-pathological syndrome. This cryptogenic or secondary condition is of unknown origin, and may be infectious, or associated with autoimmune diseases, cancer, drugs, or radiation.
Case descriptionThe case is presented of a 52-year-old patient who was diagnosed with organizing pneumonia secondary to anti-synthetase syndrome.
DiscussionIt is intended to make known that not all pulmonary consolidative clinical pictures correspond to infectious processes. In this case, an organizing pneumonia secondary to anti-synthetase syndrome is documented. Despite being a disorder that is classified as an idiopathic inflammatory myopathy, it manifests as an interstitial lung disease with predominantly respiratory symptoms.
La neumonía organizativa es un síndrome clínico-patológico poco frecuente, dentro del cual se desconoce la etiología de la denominada neumonía criptogénica o secundaria, que puede ser infecciosa o asociada con enfermedades autoinmunes, cáncer, fármacos o radiación.
Descripción del casoSe presenta el caso de una paciente de 52 años a quien se le diagnostica neumonía organizativa secundaria a síndrome antisintetasa.
DiscusiónSe busca dar a conocer que no todos los cuadros clínicos de consolidación pulmonar corresponden a procesos infecciosos. En este caso se documentó una neumonía organizativa secundaria a síndrome antisintetasa, la cual a pesar de ser una patología que se cataloga como una miopatía inflamatoria idiopática, se manifestó como una enfermedad pulmonar intersticial con síntomas predominantemente respiratorios.
The term organizing pneumonia refers to a clinicopathological syndrome characterized by diffuse interstitial involvement of the small airway, mainly affecting distal bronchioles, respiratory bronchioles, alveolar ducts, and alveolar walls, which ends in excessive fibrotic proliferation and granulation tissue.1,2
Organizational pneumonia is a rare entity, with an incidence of 1.97 per 100.000 inhabitants, mainly affecting older adults between 67 years of age.3 It can present as a cryptogenic organizing pneumonia where a clear cause is not established and can correspond to 53–55%,3,4 and the rest presents as secondary organizing pneumonia, that have been linked to infectious processes (bacterial and viral), autoimmune diseases, drugs, cancer and associated with radiation.
Antisynthetase syndrome is a recently classified disease that belongs to the group of idiopathic inflammatory myopathies, the most recent diagnostic criteria for the diagnosis of the disease are based on the Solomon criteria5: requires the presence of antisynthetase antibody, associated with two major criteria or one major and two minor criteria; the major criteria are: myositis (by Bohan and Peter criteria) and diffuse interstitial lung disease. Minor criteria: arthritis, Raynaud's phenomenon or mechanic's hands.
The case of an organizing pneumonia secondary to antisynthetase syndrome is presented, which, although it is a myopathic disease, in this case debuted with predominantly respiratory symptoms.
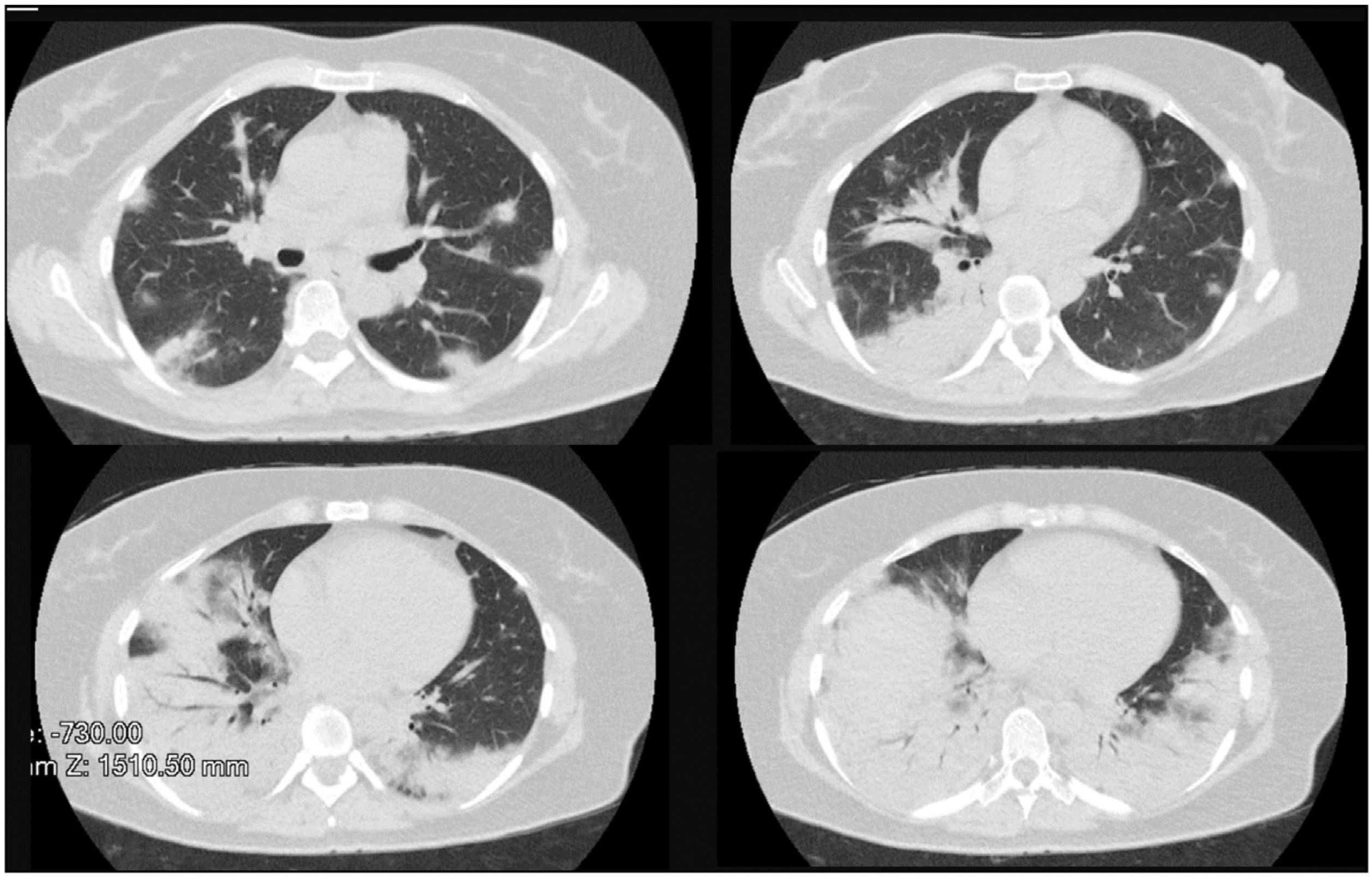
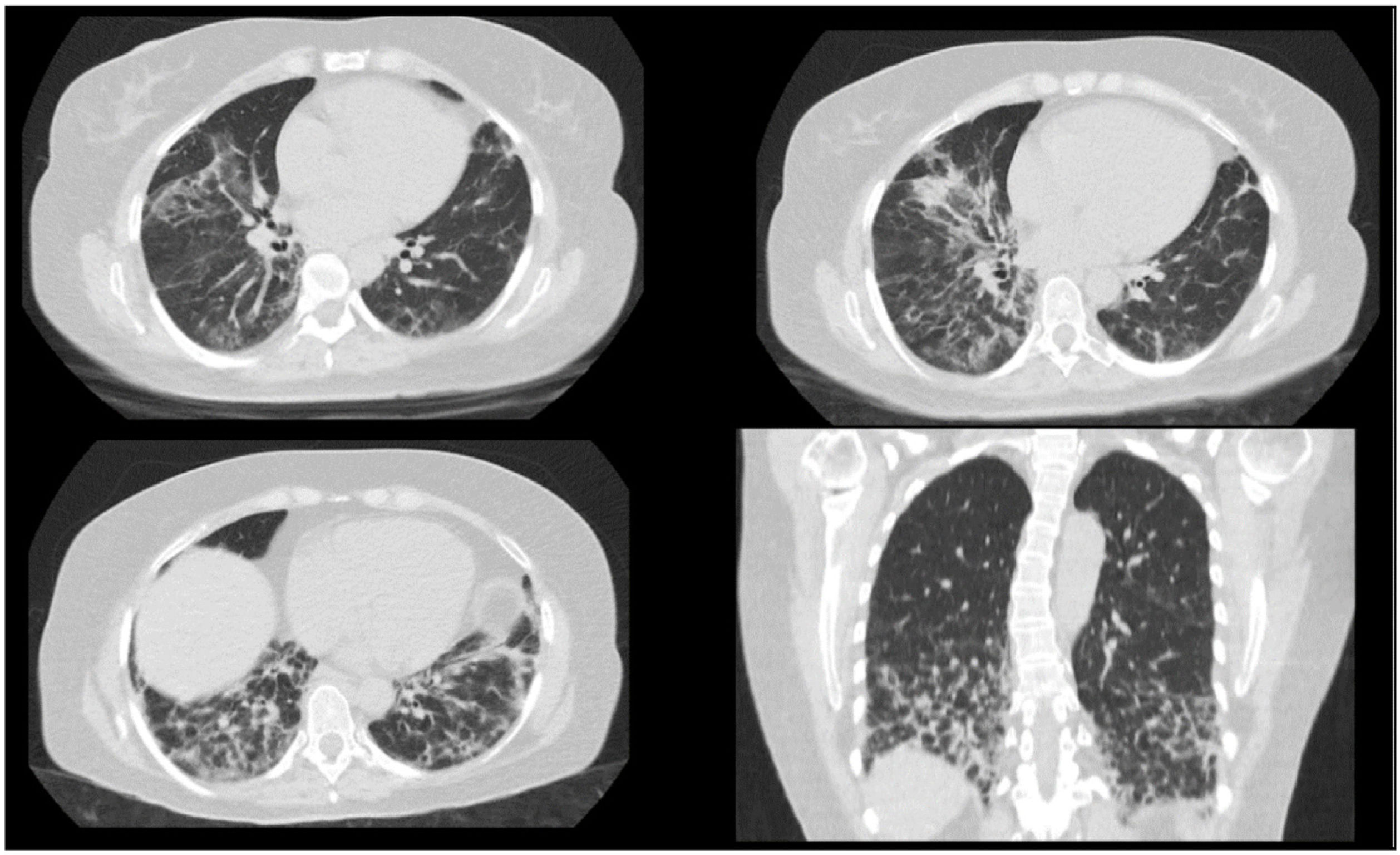
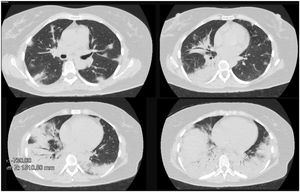
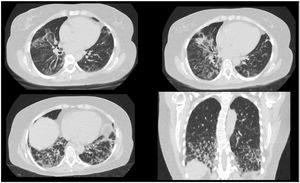
Clinical caseA 50-year-old female patient, consultation due to a medical condition of 2 months of evolution consisting of intermittent episodes of dry cough, associated with dyspnea and deterioration of its functional class, MMRC 4, she does not report fever, subjective weight loss, for which she is directed from external consultation to the emergency service. Within her antecedents she does not mention chronic pathologies, no smoking or biomass exposure, highlights hysterectomy plus pomeroy for uterine myomatosis. She enters the emergency service in regular general conditions, broken speech, blood pressure (Pa): 115/70mmHg, heart rate (h) 80 beats per minute (bpm), breathing frequency (fr) 22 breaths per minute (rpm), oxygen saturation 89–90% with ambient oxygen, the lung auscultation reveals the presence of velcro crepitus in both lung bases, paraclinical tests that show normal leukocytes, no anemia, PCR 1.67, preserved kidney function. A high-resolution chest tomography was performed (TACAR) (Fig. 1) where consolidative images were described predominantly in the lung bases, presence of air bronchogram, associated with subpleural nodules, it is initially considered a manifestation of community-acquired pneumonia, the presence of risk for Gram positive germs is also considered under the suspicion of septic seeding. Despite the antibiotic treatment, no improvement was observed in his clinical condition. It is considered that in the onset of his condition there is no presence of symptoms suggestive of an ongoing infectious process, and because it is a chronic dyspnea associated with tomographic findings, the possibility of organizing pneumonia is raised, she was taken to bronchoscopy plus biopsy, as well as percutaneous biopsy. In order to clarify the etiology of her condition, she was questioned about drug use, radiation exposure, malignancy and paraclinical tests with an autoimmunity profile were complemented (Table 1), which is normal. A percutaneous lung biopsy report was obtained with a histological pattern consistent with organizing pneumonia (Fig. 3). Management is started with methylprednisolone in boluses of 500mg iv day for 3 days, then she switched to oral corticosteroid, with which an improvement in her dyspnea and radiological improvement was observed (Fig. 2), she is discharged with corticosteroid and home oxygen management. During outpatient follow-up, pulmonary function test is performed: CVF: 60%, DLCO: 15%. The patient abandoned the treatment by her own decision, presenting worsening of her dyspnea and appearance of joint pain and Raynaud's phenomenon. Autoimmunity profile is performed again, this time the report is positive for Anti Jo and Anti RO, the patient is considered to meet Solomon criteria for which antisynthetase syndrome is diagnosed. Faced with a new episode of relapse, she is hospitalized and a cycle of intravenous cyclophosphamide is started, with which she has presented improvement in her disease pattern.
Laboratory Results.
| Paraclinical examination | First take | Second take |
|---|---|---|
| Anti SM Antibodies | 4.23 (negative<12U/mL–positive>18U/mL) | Negative |
| Anti RNP antibody | 2.78 (negative<12U/mL–positive>18U/mL) | Negative |
| Anti RO/SSA antibody | 3.89 (negative<16Eu/mL) | 63 (moderate positive>25Eu/mL) |
| Anti LA antibody | 3.21 (<20Eu/mL) | Negative |
| Ancas, P ANCA, C ANCA | Negative | Negative |
| Antinuclear antibodies | Negative | Negative |
| Complement C3 | 123 (90–180mg/dl) | 111 (88–201) |
| Complement C4 | 36 (10–40mg/dl) | 27 (10–40mg/dl) |
| Rheumatoid factor | Negative (<30ui/mL) | |
| Anti Jo | – | 125 (>25EU/mL) |
| Anti SCL70 | – | Negative |
Lung biopsy report. Lung biopsy report: Pulmonary parenchyma with lymphoplasmacytic-type inflammatory infiltrate in alveolar septa. Alveolar spaces lined by pneumocytes with reactive changes, there are also macrophages, multinucleated giant cells and the presence of fibroblast polyps. Non-necrotizing granulomas are also observed, poorly formed with giant cells that include cholesterol crystals, the colorations of ZN and PAS D, are negative for microorganisms. The histological picture is consistent with a pattern of organizing pneumonia.
Organizational pneumonia is an entity that does not present a pathognomonic clinic, in fact, it is a slightly later onset entity, even after a pneumonic process. Differential diagnoses of syndromes compatible with community-acquired pneumonia or with pulmonary consolidation syndromes have therefore been proposed, understanding that not all consolidations are due to an infectious process, etiologies such as lung edema, lung cancer, pulmonary infarction, organizing pneumonia, eosinophilic pneumonia, sarcoidosis, vasculitis, drug and radiation toxicity, can present as differential diagnoses.6 In our case, it was a patient with chronic dyspnea, without a clear clinic of an infectious process, which in the chest image showed large consolidations with the presence of an air bronchogram, which initially suggested by the emergency group that it was pneumonia, a diagnosis that is ruled out as there was no evidence of improvement despite 5 days of antibiotic treatment. Time when organizing pneumonia is questioned.
Characteristic radiographic findings within organizational pneumonia consist of ground glass opacities (88%), consolidation (83%), peribronchovascular opacities (52%), reticular infiltrates (38%) bronchiectasis (33%), interstitial nodules (27%), reverse halo sign (17%). Being the compromise predominantly in the lower lobes and subpleural distribution.7,8 One of the important characteristics regarding radiological evolution is that the findings have a changing pattern over time. As can be seen in Figs. 1 and 2, a pattern of pulmonary consolidation is presented with greater involvement of the pulmonary bases, with presence of subpleural nodules and small areas of ground glass.
The diagnosis of cryptogenic organizing pneumonia is based on 3 pillars. (1) The clinical and radiological presentation compatible with the disease. (2) The demonstration of a histopathological pattern consistent with the disease. (3) The exclusion of a secondary pathology that explains the current process.9 Although biopsy is required to define the disease, in some cases it may not be necessary, treatment could even be started when the diagnostic images and the clinical picture are very telling. In the case of our patient, treatment was started empirically, later obtaining the result of the pathology. As for the best way to obtain the biopsy, it could be done by bronchoscope, guided by CT or by thoracoscopy, the latter having better performance due to the size of the biopsy that can be obtained.9,10
Once the diagnosis of organizing pneumonia has been established, the next step is to determine if it is a cryptogenic condition or a secondary etiology. The drug consumption was questioned, there were no signs of malignant disease and the autoimmunity profile was requested, which was negative. Initially, a cryptogenic organizing pneumonia was considered, for which corticosteroid treatment was started, observing clinical improvement, for which hospital discharge is indicated. The patient decides to suspend the treatment, presenting on this occasion poly-articular pain that compromises hands, knees, as well as Raynaud's phenomenon, an immunological profile that reports anti-Jo positive and anti-RO positive was requested again. With which it is evaluated that it meets Solomon criteria for antisynthetase syndrome. It stands out in the patient that, although the antisynthetase syndrome corresponds to an inflammatory myopathic disease has started with predominantly respiratory symptoms, this is consistent with other reports where it has been mentioned that up to 16–30% of patients present pulmonary symptoms as first manifestations of the illness. Highlighting that pulmonary involvement is the predominant extra-articular symptom, found in up to 78–100% of cases.11,12
Eight (8) antisynthetase antibodies have been described: anti-JO-1 (anti-histidyl), anti-PL12 (anti-alanyl), anti-PL7 (anti-threonine), anti-OJ (anti-isoleucyl), anti-EJ (anti-glycine), anti-KS (anti asparaginyl), anti-YRS/Ha (anti-tyrosyl), and anti-Zo (anti-phenylalanyl), the most frequently documented being anti JO-1 in up to 60% of cases.13 It should be noted that anti Jo and anti RO antibodies are not only necessary for diagnosis, but have also been involved in the prognosis of patients, in one cohort, it was found that those patients with positive anti JO-1 had a cumulative survival at 5 and 10 years of 90% and 70% while those with non-JO-1 antisynthetase antibodies was 74% and 47% respectively.13 On the other hand, it has been documented that when the two antibodies (anti JO-1 and Anti RO) coexist, more severe forms of interstitial lung disease can develop.14
The treatment of antisynthetase syndrome has not had strong evidence, currently there are no clinical trials, a clinical trial is registered in a clinical trial, in which it is intended to evaluate cyclophosphamide plus azathioprine VS tracrolimos for interstitial lung disease. Current treatment is based on case reports or expert recommendations. Regarding interstitial lung disease, it is recommended to start with corticosteroid cycles, continuing them for maintenance, using corticosteroid sparing agents such as azathioprine, cyclophosphamide or mycophenolate.15 In case of non-response to first-line drugs or in case of relapse, intravenous cyclophosphamide can be used, even in some cases rituximab has been used with satisfactory responses in patients resistant to corticosteroids.16 See Table 2.
Pharmacological treatment.
| Drug | Initial dose | Clinical use | Tracing | Adverse effects |
|---|---|---|---|---|
| CorticoidMethylprednisolone | 1mg/kg/day1g IV/3 days. | First line | Glucose, weight, blood pressure | Hypertension, weight gain, hyperglycemia |
| Azathioprine | 1mg/kg/day | Second line (most commonly used). Useful in patients with active arthritis. | Blood count, kidney function, liver function | Leukopenia, liver injury, pancreatitis. |
| Mycophenolate mofetil | 500mg, every 12h.Holder 1000–1500 twice a day. | Add to the first line. | Hemogram | Cytopenias, diarrhea |
| Tacrolimus | 1mg every 12h (0.075mg/kg/day).Titrate to a minimum target level of 5–8ng/mL. | Add to the first line. | Kidney function, blood pressure, electrolytes, blood count, drug levels. | Kidney injury, hypertension, hyperglycemia. |
| Rituximab | 375mg/total body area | Rescue therapy, add to standard therapy. | Hemogram | Infection, neutropenia, reaction during infusion. |
| Cyclophosphamide | 1–2mg/kg/day for a month or 500–1000mg IV every 4 weeks. Consult rheumatology. | Rescue therapy. Serious or refractory disease. | Hemogram, urinalysis, kidney function. | Malignancy, cytopenias, hemorrhagic cystitis, increased infections. |
Ethics committee approved the research. Patient signed the informed consent form and authorized the preparation of the clinical case and its potential publication without exposing the patient's personal data.
Conflict of interestNone.
FundingThere is no source of institutional funding and/or sponsors.