Microscopic polyangiitis is a systemic anti-neutrophil cytoplasmic antibody-associated vasculitis, characterized by necrotizing involvement of the small caliber vessels. Its clinical manifestations are varied, and the most severe clinical forms manifest with rapidly progressive glomerulonephritis and pulmonary capillaritis. The nervous involvement mainly affects the peripheral system. Central compromise is rare, and clinical findings of psychosis and behavioral disfunction are quite infrequent, in the context of these autoimmune diseases.
La poliangitis microscópica es una vasculitis sistémica, asociada a la positividad de anticuerpos anticitoplasma de neutrófilos, caracterizada por el compromiso necrosante de los vasos de pequeño caliber. Las manifestaciones clínicas de la poliangitis microscópica son variadas y las formas más severas se manifiestan con glomerulonefritis rápidamente progresiva y capilaritis pulmonar. El compromiso nervioso afecta principalmente el sistema periférico. La afectación a nivel central no es común, y hallazgos clínicos de psicosis y alteración del comportamiento son bastante infrecuentes en el contexto de estas enfermedades autoinmunes.
Although there is the believe that microscopic polyangiitis (MPA) may compromise any organ or tissue, a significant involvement of the central nervous system is infrequent.1 It is yet more infrequent a finding of sensoperceptual impairment as a key clinical trait. Case reports that have associated MPA with alterations such as psychotic outbreak have linked the event with the administration of high doses of steroids.2
In the case of vasculitis associated with positive antineutrophil cytoplasmic antibodies (ANCA positive) the nervous involvement most frequently compromises the peripheral system, giving rise to conditions such as sensitive or motor peripheral polyneuropathy and multiple mononeuritis.3 Additional cases of cerebral vasculitis associated with ischemia and hemorrhage have been documented in the literature.3 Necropsies of a large percentage of patients with ANCA positive vasculitis evidence ante mortem ischemic areas with fibrotic changes in the white matter of the brain4; consequently, its unusual presentation does not imply absence of risk or the non-existence of subclinical processes.
Clinical case35-Year old female patient, with unremarkable history, who early in 2017 was admitted to the emergency department because of a clinical condition of 2 weeks of evolution, presenting with dyspnea with minimal exertion, generalized edema, adynamia and asthenia. The physical examination identified signs of respiratory distress, quieter vesicular breath sounds of the bases of the lungs and anasarca. There were findings of elevated nitrogen compounds and hyperkalemia, with associated EKG changes. Upon documenting the dialysis emergency, renal replacement therapy was required. It was then believed that the kidney failure etiology could be autoimmune, hence steroid pulses (methylprednisolone 1000mg/day for 3 doses) were administered. The immunology profile showed positive MPO/ANCA, with no additional findings (Table 1). A kidney biopsy was obtained showing pauci-immune necrotizing glomerulonephritis in a sclerosing phase, with 58% of global glomerular sclerosis and 10% extra capillary proliferation. The ANCA-associated small vessel vasculitis diagnosis was confirmed, with no granulomatous manifestations, leading to the suspicion of MPA. Based on this diagnosis, cyclophosphamide therapy (15mg/kg, 3 doses per week, and then 3 pulses every 3 weeks) and oral steroid (50mg/day) was initiated. Intrahospital induction was indicated and the patient was discharged with a prescription for the following doses. The patient discontinued the oral steroid and failed to receive the next doses of cytostatic agent. In January 2018 the patient was readmitted to the emergency department with a 12-hour evolution of a condition compatible with an acute psychotic episode: psychomotor agitation, disorientation, disorganized language and auditory hallucinations. At the time of admission, the person accompanying the patient claimed that she was not taking any medication and had not been exposed to hallucinogenic substances. In the emergency department the patient received benzodiazepines, with resolution of the psychomotor agitation. Notwithstanding the medication, the patient persisted with hallucinations and delirium. The admission paraclinical tests were conducted, including axial head CT with no abnormal findings, high nitrogen levels (TFG: 21ml/min/1.73m2 according to Cockroft-Gault formula); CBC, liver enzymes, ant the toxicological profile were normal, serology and ELISA for HIV negative (Table 2). The patient was assessed by a psychiatrist who found maniform and abrupt onset psychotic symptoms, with no personal or familial psychiatric history. The neurology assessment initially suspected steroid-related psychosis; however, the patient had stopped receiving psychotics for several months. A plain head MRI was ordered, since the patient's kidney disease was a contraindication for IV contrast media. The head MRI showed non-specific supratentorial subcortical hyperintensities, with no other related findings. A lumbar puncture produced a specimen with no macroscopic alterations, normal biochemistry, negative cultures for bacteria, mycobacteria and fungi; nonreactive VDRL, film array with no positive reports. These tests were complemented with video telemetry with no evidence of epileptiform activity. Following the administration of nephroprotection protocols, the patient underwent a brain magnetic resonance angiography which showed changes suggestive of vasculitis or vasoconstriction phenomena (Figs. 1 and 2). The kidney function deteriorated and hence a brain angiography was contraindicated. The patient received steroid pulses (500mg/day for 3 doses) and the cyclophosphamide protocol was reinitiated (at the previously described doses), subsequently evidencing resolution of symptoms and complete recovery of the neurological status. One year after this episode, the patient does not show any symptoms of mental illness, and her neurological status is optimal. No anomalies have been identified during the psychiatric follow-up visits and there have been no further psychotic episodes. Furthermore, her kidney function has improved significantly, and the current filtration rate is 35.4ml/min.
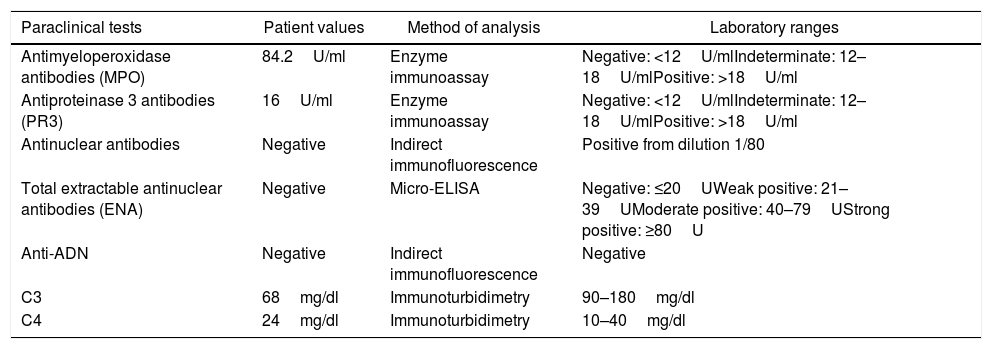
Immunological profile of the patient at her first hospitalization.
| Paraclinical tests | Patient values | Method of analysis | Laboratory ranges |
|---|---|---|---|
| Antimyeloperoxidase antibodies (MPO) | 84.2U/ml | Enzyme immunoassay | Negative: <12U/mlIndeterminate: 12–18U/mlPositive: >18U/ml |
| Antiproteinase 3 antibodies (PR3) | 16U/ml | Enzyme immunoassay | Negative: <12U/mlIndeterminate: 12–18U/mlPositive: >18U/ml |
| Antinuclear antibodies | Negative | Indirect immunofluorescence | Positive from dilution 1/80 |
| Total extractable antinuclear antibodies (ENA) | Negative | Micro-ELISA | Negative: ≤20UWeak positive: 21–39UModerate positive: 40–79UStrong positive: ≥80U |
| Anti-ADN | Negative | Indirect immunofluorescence | Negative |
| C3 | 68mg/dl | Immunoturbidimetry | 90–180mg/dl |
| C4 | 24mg/dl | Immunoturbidimetry | 10–40mg/dl |
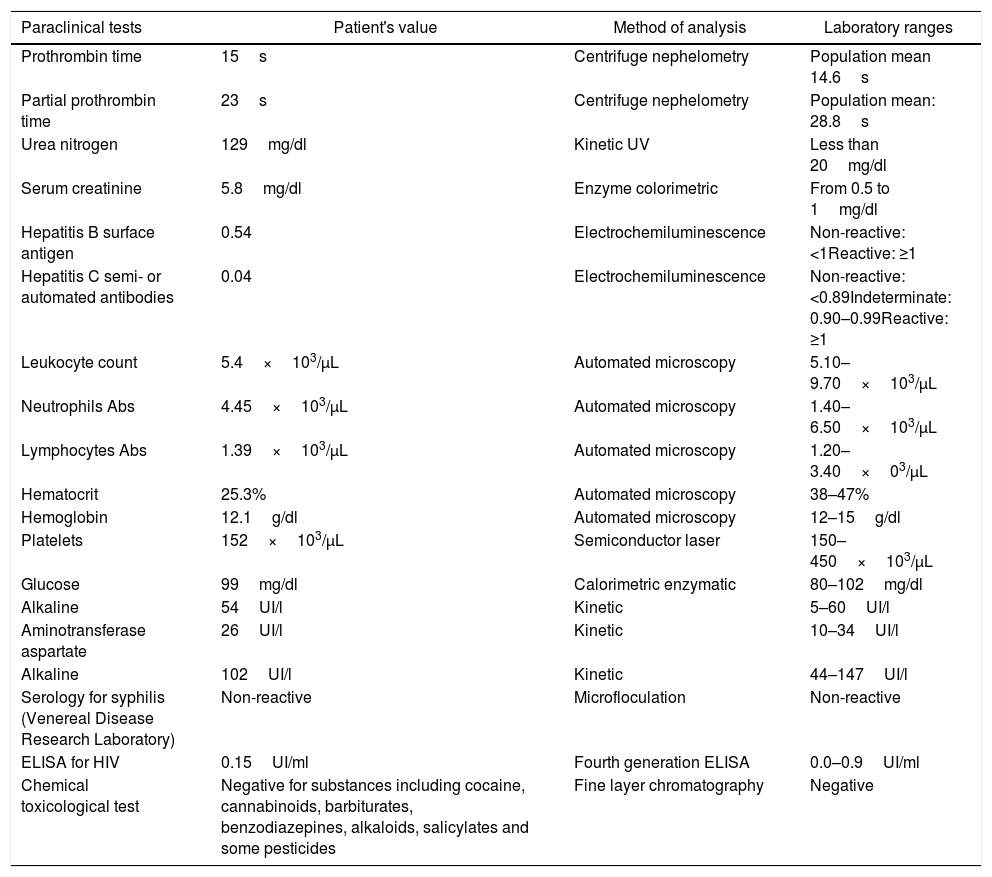
Paraclinical information of the patient at her second hospitalization.
| Paraclinical tests | Patient's value | Method of analysis | Laboratory ranges |
|---|---|---|---|
| Prothrombin time | 15s | Centrifuge nephelometry | Population mean 14.6s |
| Partial prothrombin time | 23s | Centrifuge nephelometry | Population mean: 28.8s |
| Urea nitrogen | 129mg/dl | Kinetic UV | Less than 20mg/dl |
| Serum creatinine | 5.8mg/dl | Enzyme colorimetric | From 0.5 to 1mg/dl |
| Hepatitis B surface antigen | 0.54 | Electrochemiluminescence | Non-reactive: <1Reactive: ≥1 |
| Hepatitis C semi- or automated antibodies | 0.04 | Electrochemiluminescence | Non-reactive: <0.89Indeterminate: 0.90–0.99Reactive: ≥1 |
| Leukocyte count | 5.4×103/μL | Automated microscopy | 5.10–9.70×103/μL |
| Neutrophils Abs | 4.45×103/μL | Automated microscopy | 1.40–6.50×103/μL |
| Lymphocytes Abs | 1.39×103/μL | Automated microscopy | 1.20–3.40×03/μL |
| Hematocrit | 25.3% | Automated microscopy | 38–47% |
| Hemoglobin | 12.1g/dl | Automated microscopy | 12–15g/dl |
| Platelets | 152×103/μL | Semiconductor laser | 150–450×103/μL |
| Glucose | 99mg/dl | Calorimetric enzymatic | 80–102mg/dl |
| Alkaline | 54UI/l | Kinetic | 5–60UI/l |
| Aminotransferase aspartate | 26UI/l | Kinetic | 10–34UI/l |
| Alkaline | 102UI/l | Kinetic | 44–147UI/l |
| Serology for syphilis (Venereal Disease Research Laboratory) | Non-reactive | Microfloculation | Non-reactive |
| ELISA for HIV | 0.15UI/ml | Fourth generation ELISA | 0.0–0.9UI/ml |
| Chemical toxicological test | Negative for substances including cocaine, cannabinoids, barbiturates, benzodiazepines, alkaloids, salicylates and some pesticides | Fine layer chromatography | Negative |
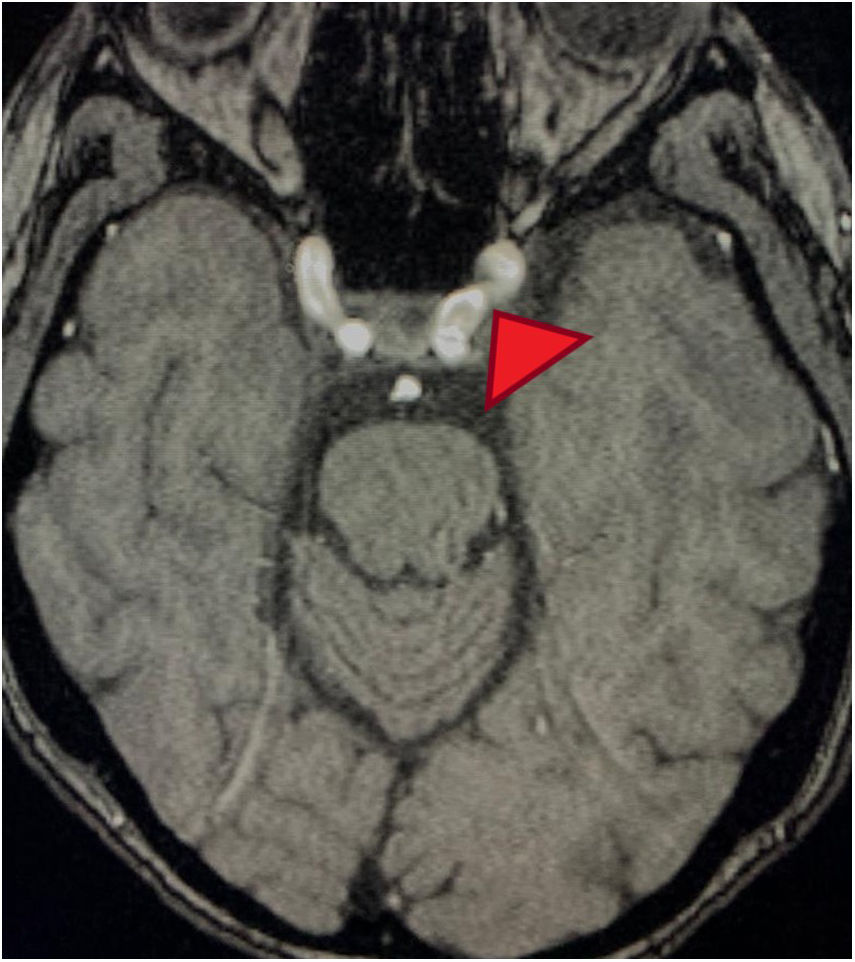
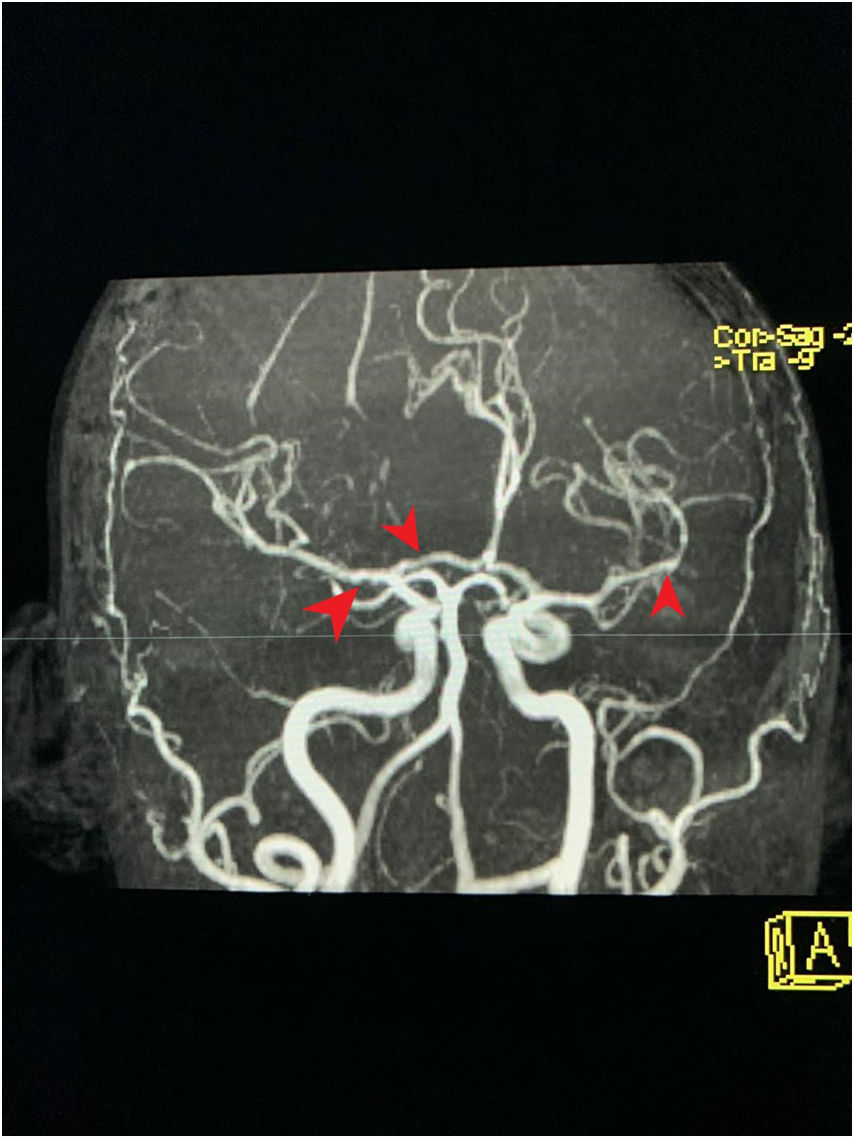
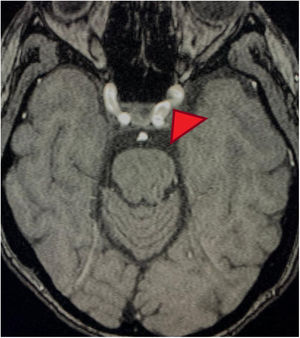
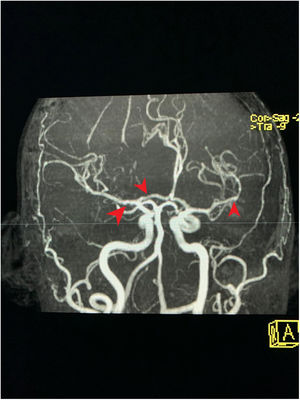
MRA cross-section of the brain. Magnetic resonance angiography of the patient depicting morphological alterations in the vessel walls. Arterial sections, areas of stenosis and irregularities can be observed. Loss of definition and tortuosity of the vessel walls with blurred margins in structures of varying calibers and segment alterations.
Just as any other small vessel ANCA positive vasculitis, MPA is a severe disease with variable evolution; it may involve a clinical spectrum ranging from insidious manifestations of the disease over many years of evolution, up to fulminant rapid onset conditions.5,6 The pathophysiological development of small vessel vasculitis is not fully understood yet, and similarly to most rheumatological diseases, it seems to be the result of the interaction of genetic factors with environmental exposure. This combination gives rise to an immune response generated by the deregulation of T-lymphocytes, and the production of antibodies capable of neutrophil activation, leading to endothelial damage.7 This is the reason why during the Chapel Hill consensus the condition was defined as a necrotizing vasculitis with deposition of a few immune complexes.8
It has been suggested that ANCAs are only temporarily present during the immune response associated to infections. Its subsequent conversion in the immunological substrate of the disease is related to a process of molecular mimicry.9 It has been described in infected patients and with ANCA-positive findings, without vasculitis, in response to antibiotic therapy that lowers the antibody levels.10 The correlation between Staphylococcus aureus infections and vasculopathies such as granulomatous polyangiitis is striking.11
The research shows that the anti-cytoplasmic antibodies may stimulate neutrophils through antigen binding, such as proteinase 3.12 This interaction promotes an inflammatory setting in which the activation of the alternate complement pathway amplifies and perpetuates the response through a stimulus on the lymphocytes and monocytes.13 In this context, the neutrophil undergoes morphological changes that facilitates its interaction with the endothelium for the release of enzymes causing fibrinoid necrosis and small vessel destruction.14 Exposure of the basal membranes stimulates platelet aggregation.14 An increase in transmigration has been evidenced in capillary flow models with ANCA-exposed neutrophils.15 In combination, these phenomena of adhesion and transmigration perpetuate with the release of chemotactic agents that attract a larger number of white blood cells.16 Mice infused with IgG PR3-ANCA rapidly develop progressive glomerulonephritis and alveolar hemorrhage.17 The interaction between ANCA and neutrophils, triggers intracellular signaling pathways giving rise to activation of the p21RAS molecule: an essential protein for the metabolic activity of this cell.18,19
The nervous system involvement with MPA is predominantly at the peripheral level, whilst the central involvement is less frequent. The involvement of the central nervous system in vasculitis is usually associated with a more severe clinical presentation of the disease, refractory management, and ensuing complications.20 A compromised nervous system is not the major contributing factor to the mortality caused by these diseases and most fatalities are associated with renal and pulmonary decline.21 In the case series studied in 2009 by the department of rheumatology of the Peking Union Medical College Hospital, in Beijing, 36% of the patients with systemic vasculitis studied, had nervous system involvement. Peripheral neuropathy was the neurological condition more strongly associated with MPA, with significant prevalence of multiple mononeuritis and symmetrical distal polyneuropathy.22,23 In terms of the central nervous system, arachnoid hemorrhage and ischemic events were the most relevant clinical conditions.24 Research conducted over the past few years has been able to prove that any disorder of a particular system or organ radically changes based on demographic characteristics. When comparing the North American populations against the Asian countries (where most of the studies have been made), there is a significant variation in the percentage of manifestations depending on the tissue studied. In a study conducted in Korea, a high prevalence of nervous system manifestations was identified (43.6%).22
Three primary areas of central involvement are described in MPA: the pituitary, the pachymeninges and the vasculature.3,21 Some cases of long-lasting hormonal disorders due to pituitary deficit have been reported, even in patients who have responded to management with corticoids and achieved almost complete clinical resolution of symptoms.1,3,21 Meningeal hypertrophy may be evidenced in systemic manifestations of the disease, in association with anti-PR3 or in limited forms in relationship with anti-MPO.3,21
Cerebral and spinal vasculitis are a rare condition that may encompass various syndromic aspects and manifests with either a focal or generalized anomaly.1,22,23 It has been suggested that ischemic events are caused by necrotizing angiitis and hemorrhages are caused by rupture of the necrotized vessels.1,21,22 The ischemic complications are the most frequent and may cause encephalopathy, motor or sensitive deficit, ischemic myelopathy, and seizures, inter alia.3,22 The findings in the central nervous system usually develop as a late manifestation of the disease, following the onset of systemic symptoms. However, there are reports in the literature in which MPA began with manifestations such as headache, seizures, and ischemic events with other systemic symptoms.25 Long term sequelae are more frequent when the central involvement is due to ischemic lesions and spinal pachymeningitis.25,26
Although the central nervous system involvement is associated with a poor prognosis, there are some case series in which a percentage of patients respond to the combination of steroids with cytostatic agents in regimens similar to those used in different settings such as lung-kidney syndrome.26,27 Likewise, there are case reports of patients with subarachnoid hemorrhage that have partially responded to combined steroid and cyclophosphamide treatment.27 In general, the nervous condition presents concomitantly with potentially lethal manifestations such as alveolar hemorrhage, active glomerulonephritis or gastrointestinal hemorrhage. This is why the treatment protocol established with steroids, cytostatic agents and even biologics, is the indicated approach.
The relationship between psychosis and MPA is not fully established. We were unable to find in the literature any case reports including psychosis as a clinical manifestation of this vasculitis. The only setting in which we found a relationship between these two conditions is in the reports of mental disorder secondary to the use of steroids (which does not seem to be the case previously discussed, since the patient had discontinued the steroids a few months before). However, the relationship between psychotic events and vasculopathy has been clearly established. The altered or distorted perception of reality secondary to vasculitis in the case of our patient with MPA, could be accounted for by pathophysiological mechanisms similar to those present in other clinical conditions that share some similarities. There was a complete resolution of the condition following the administration of methylprednisolone and the protocol with cytostatic agents. The MRA is not the ideal method to diagnose cerebral vasculitis, although the kidney function and the patient's status prevented us from conducting an angiography. The suggestive findings, ruling out other causes of dementia, and the resolution of the situation with the treatment described, are the basis to support our clinical experience in this case.
FinancingNo funding was received to prepare this paper.
Conflict of interestsThe authors have no conflict of interest to disclose.
Please cite this article as: Vanegas Duarte E, Puentes Suarez G, Villarreal Hernández S, Chaparro Beltrán J. Psicosis y alteraciones en la sensopercepción como manifestaciones atípicas de la poliangitis microscópica. Rev Colomb Reumatol. 2020;27:135–140.