Antiphospholipid syndrome is an autoimmune disease with antibodies against membrane phospholipids with mainly thrombotic and/or obstetric manifestations. Its treatment is generally based on indefinite anticoagulation, usually with warfarin, and which, for various factors, is not always feasible, making it necessary to use alternative therapies.
ObjectiveTo describe the experience with rivaroxaban in patients with antiphospholipid syndrome.
Materials and methodsA descriptive study was conducted on subjects that met the 2006 Sydney criteria for antiphospholipid antibodies syndrome and received anticoagulation with rivaroxaban at 20mg daily dose in 2reference hospitals in Medellin, Colombia, between January 2012 and April 2015.
ResultsThe study included 7 patients, with a mean age of 36±10.8 years (range 23–55). Four patients had venous thrombosis, 5arterial, 5were positive for anticardiolipin antibodies, 3reactive to lupus anticoagulant, 2anti-β2 glycoprotein positive subjects, and one patient had triple antiphospholipid antibody positivity. The median time of warfarin use was 15 months (RIQ 1–36). The reasons for starting rivaroxaban were: bleeding (n=2), sub-therapeutic coagulation ranges (n=2), toxicoderma, gastrointestinal intolerance, and re-thrombosis (n=1, each). The time of use was 17.9±13.4 months (range: 3–34). There were 2recurrent cases of thrombosis during follow-up, and no adverse events.
ConclusionThe use of factor Xa inhibitors in a series of patients with antiphospholipid syndrome and unable to use warfarin showed an adequate safety profile; however, 2recurrent episodes of venous thrombosis occurred.
El síndrome antifosfolípido se caracteriza por la presencia de anticuerpos contra fosfolípidos de membrana y manifestaciones clínicas, principalmente trombóticas y obstétricas. Su tratamiento se basa en la anticoagulación indefinida, generalmente con warfarina, la cual, por diversos factores, no siempre es factible por lo que es necesario el uso de terapias alternativas.
ObjetivoDescribir la experiencia con rivaroxabán en pacientes con síndrome antifosfolípido.
Materiales y métodosEstudio descriptivo en el que se evaluaron pacientes que cumplieron los criterios de Sydney de 2006 para síndrome antifosfolípido y que recibieron anticoagulación con rivaroxabán a dosis de 20mg día en 2 hospitales de referencia en Medellín (Colombia), entre enero de 2012 y abril de 2015.
ResultadosSe incluyeron 7 pacientes con una media de edad de 36.6±10.8 años (rango: 23–55). De estos, 4 individuos tenían trombosis venosa, 5 trombosis arteriales, 5 anticuerpos anticardiolipinas positivos, 3 anticoagulante lúpico positivo, 2 pacientes tenían anti-β2 glicoproteína positivo y un paciente triple positividad de anticuerpos. La mediana de utilización de la warfarina fue de 15 meses (rango: 1–36). Las razones para el inicio de rivaroxabán fueron: sangrado (n=2), rango subterapéutico de anticoagulación (n=2), toxicodermia (n=1), intolerancia gastrointestinal (n=1) y retrombosis (n=1). El tiempo de uso fue 17.9±13.4 meses (rango: 3–34) y durante el periodo de seguimiento no se presentaron eventos adversos, pero sí 2 episodios nuevos de trombosis.
ConclusiónEl uso de inhibidores del factor Xa en una serie de pacientes con síndrome antifosfolípido e imposibilidad para el uso de warfarina mostró un adecuado perfil de seguridad; no obstante, hubo 2 episodios recurrentes de trombosis.
Antiphospholipid syndrome (APS) is an autoimmune disease in which the presence of antibodies directed against membrane phospholipids generates diverse clinical manifestations, among them, mainly, obstetric morbidity and vascular thrombosis, both venous and arterial.1,2 Although currently there are no established diagnostic criteria, the classification criteria proposed in 1999 and updated in 2006 are used in clinical practice.1 In these criteria is taken into account the persistent presence of specific antibodies: anticardiolipins, lupus anticoagulant or anti-beta 2 glycoprotein 12 and the presence of at least one of the 2 most common manifestations of the disease previously mentioned.
The main objective of treatment is to avoid the recurrence of these events, for which, in the case of thrombotic manifestations, anticoagulation for an indefinite period of time is recommended.2,3 Among the different options available is warfarin, which, having more evidence and long-term follow-up, is currently the therapy of choice.3 Despite this, the chronic use of warfarin has some disadvantages, such as: the requirement of strict monitoring with the international normalized ratio (INR), as well as the multiple drug interactions that can affect its therapeutic range, which sometimes makes it difficult to achieve a stable therapeutic INR range. In addition, between 13% and 41% of patients with PSA have new episodes of thrombosis,4–6 being necessary to consider the change of antithrombotic treatment7 and, in some cases, the use of other medications such as antimalarials or immunosuppressants.8
Recently, the Food and Drug Administration approved new anticoagulants, including direct factor Xa inhibitors. These are indicated for the prevention and treatment of acute coronary syndrome, deep vein thrombosis, pulmonary thromboembolism, cerebrovascular accident and systemic embolism in the context of patients with non-valvular atrial fibrillation. So far, the information on the safety and efficacy or these new drugs in patients with APS comes, mainly, from case series.9–14 For this reason, it was intended to describe a series of patients that reflects the local experience with rivaroxaban in patients with APS and failure of warfarin, in order to increase the available knowledge.
Materials and methodsStudy designIt was conducted a descriptive, retrospective study of a series of cases of patients diagnosed with APS, according to the 2006 Sydney classification criteria.1 The patients were evaluated in the outpatient and hospitalization services of 2 reference hospitals in the city of Medellin (Colombia), between January 2012 and April 2015. Patients over 18 years of age who needed a change from warfarin to rivaroxaban, either due to an adverse event, rethrombosis or impossibility to achieve a stable INR, and who also used rivaroxaban at a dose of 20mg/day where included.
Collection processPrior approval by the ethics committees of the participating institutions, we proceeded to collect the patients’ information from the review of the medical records, using a collection format which was designed taking into account the variables of interest, and then the data were entered into a Microsoft Excel 2010 spreadsheet (Microsoft Computer Software, Redmond, Washington, USA) for further analysis.
The variables considered were: age, sex, history of arterial thrombosis, venous thrombosis, adverse obstetric outcome, positivity of lupus anticoagulant, anticardiolipin and anti-β2 glycoprotein 1 antibodies1 and coexistence of other rheumatological diseases. Concerning the pharmacological aspects, data regarding the weekly dose of warfarin, time of use and response to treatment were collected. Likewise, it was gathered information on the reasons for the change to rivaroxaban, time of use, thrombotic events during the follow-up, time in months to the thrombotic event and adverse events.
Statistical analysisThe qualitative variables were expressed in absolute or relative frequencies and the quantitative in mean±standard deviation (SD) and range, or in median and interquartile range (range), depending on the distribution of the data. Analyses were carried out using IBM SPSS 22.0.
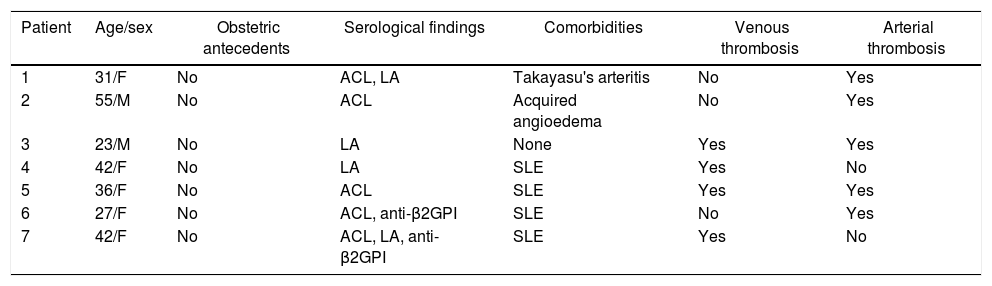
Results7 patients were included, 5 of them (71.4%) were women; the mean age was 36.6 years (range: 23–55 years). The most frequent antibodies were anticardiolipins, in 5 patients (71.4%), followed by lupus anticoagulant present in 3 cases. Five of these patients had arterial thrombosis (71%), 4 had venous thrombosis (57%), of whom 2 had also arterial thrombosis (Table 1).
Demographic and clinical characteristics of patients with APS.
| Patient | Age/sex | Obstetric antecedents | Serological findings | Comorbidities | Venous thrombosis | Arterial thrombosis |
|---|---|---|---|---|---|---|
| 1 | 31/F | No | ACL, LA | Takayasu's arteritis | No | Yes |
| 2 | 55/M | No | ACL | Acquired angioedema | No | Yes |
| 3 | 23/M | No | LA | None | Yes | Yes |
| 4 | 42/F | No | LA | SLE | Yes | No |
| 5 | 36/F | No | ACL | SLE | Yes | Yes |
| 6 | 27/F | No | ACL, anti-β2GPI | SLE | No | Yes |
| 7 | 42/F | No | ACL, LA, anti-β2GPI | SLE | Yes | No |
ACL: anticardiolipins; LA: lupus anticoagulant; anti-β2GPI: anti-beta 2 glycoprotein I; F: female; SLE: systemic lupus erithematosus; M: male.
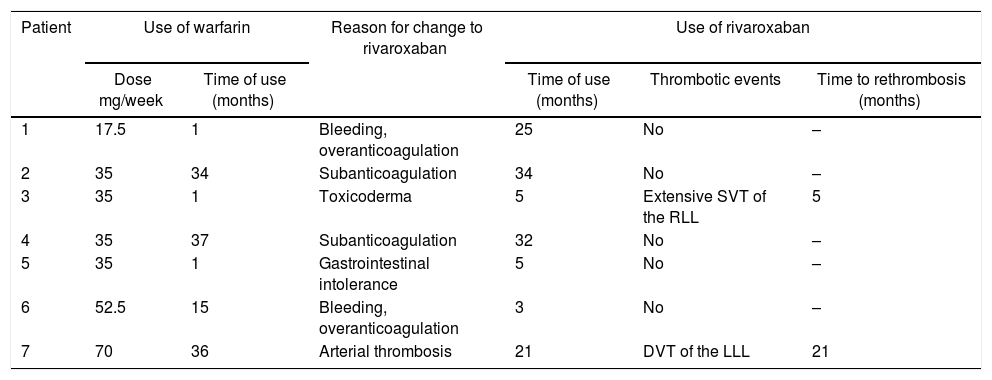
As for the use of drugs, the median time of use of warfarin was 15 months (range: 1–36 months) and the average dose was 40±16.7mg weekly with a minimum dose received of 17.5mg and a maximum dose of 70mg. Regarding the reasons for the change to rivaroxaban, 2 subjects presented bleeding associated with overanticoagulation with warfarin, in 2 individuals it was not possible to obtain an optimal range of anticoagulation and 2 patients presented adverse effects with the use of warfarin (gastrointestinal intolerance and toxicoderma); finally, one patient had episodes of recurrent thrombosis (Table 2).
Use of drugs and clinical evolution.
| Patient | Use of warfarin | Reason for change to rivaroxaban | Use of rivaroxaban | |||
|---|---|---|---|---|---|---|
| Dose mg/week | Time of use (months) | Time of use (months) | Thrombotic events | Time to rethrombosis (months) | ||
| 1 | 17.5 | 1 | Bleeding, overanticoagulation | 25 | No | – |
| 2 | 35 | 34 | Subanticoagulation | 34 | No | – |
| 3 | 35 | 1 | Toxicoderma | 5 | Extensive SVT of the RLL | 5 |
| 4 | 35 | 37 | Subanticoagulation | 32 | No | – |
| 5 | 35 | 1 | Gastrointestinal intolerance | 5 | No | – |
| 6 | 52.5 | 15 | Bleeding, overanticoagulation | 3 | No | – |
| 7 | 70 | 36 | Arterial thrombosis | 21 | DVT of the LLL | 21 |
RLL: right lower limb; LLL: left lower limb; DVT: deep venous thrombosis; SVT: superficial venous thrombosis.
The average time of use of rivaroxaban was 17.9±13.4 months (range: 3–34). During this period, one patient had extensive superficial venous thrombosis of the right saphenous vein at 5 months and another patient had deep vein thrombosis of the left popliteal and femoral veins at 21 months of treatment. During the follow-up, no patient interrupted rivaroxaban and there were no adverse reactions (Table 2).
DiscussionThe objective of anticoagulation with warfarin in patients with APS is to obtain an INR range between 2 and 3, but in certain patients, specifically in those with arterial thrombosis, a greater anticoagulant effect is sought with INR targets higher than 3.15 This fact increases the risk of bleeding and decreases the percentage of patients who achieve the goals, a difficult aspect from the beginning, as up to 27.1% of subjects with APS who are anticoagulated with warfarin will not achieve an INR higher than 2.16 In addition, a 5-year study showed that in individuals in whom the anticoagulation range was achieved, 41% and 13% will develop new thrombosis with INR between 2 and 3 and between 3 and 4, respectively,5 and that this is the most frequent cause of mortality in these subjects.17–19 Finally, in a controlled clinical study, in which follow-up was by definition more stringent and the patients were highly selected compared to real-life conditions, it was found that, at 180 days, only 55% of the time the patients had INR within therapeutic range.14
This variability of warfarin is not only due to the numerous drug interactions, but to pharmacogenetics, which plays an important role in its metabolism and affects the response to it, contributing to interindividual variability and makes necessary periodic monitoring for dose adjustment.15 Another important and specific aspect of the APS is the interaction between the antiphospholipid antibodies and the reagents to perform the INR test, which generates variability in the ranges of INR and, additionally, affects the ability of the INR to predict the anticoagulant effect in some patients.16,20 In this sense, direct oral anticoagulants, such as the factor Xa inhibitors, have the advantage of having a predictable anticoagulant effect without significant drug interactions,17,21 which makes them ideal in this subgroup of patients. Their main disadvantage is the lack of an antidote in case of bleeding or when is necessary to reverse the anticoagulant effect urgently.22
Reviewing the world literature, there are few cases reported of patients with APS anticoagulated with rivaroxaban and, in these, the results have been contradictory. Win and Rodgers10 and Schaefer et al.,14 each of them reported 2 patients with triple positivity for antiphospholipid antibodies, all with new episodes of venous thrombosis. In larger cohorts such as the one of Noel et al., 15 French individuals were reported, 4 of whom abandoned the medication due to adverse effects and only one patient due to recurrence of thrombotic microangiopathy at 8 months of use.11 In a report of 8 Brazilian patients, 5 presented rethrombosis during treatment with rivaroxaban, of whom 3 had triple positivity for antiphospholipid antibodies and 2 had a history of arterial thrombosis. Son et al. reported 12 cases in Caucasian patients: 2 had rethrombosis during treatment, both with triple positivity for antiphospholipid antibodies.12
Finally, the series with the greatest number of patients included 35 individuals, with a mean follow-up of 10 months in which no significant bleeding occurred: only 2 women had increased menstrual bleeding and no new thrombotic event was reported. It should be noted that in this series none of the patients had a history of arterial thrombosis or a requirement for anticoagulation with an INR greater than 3, which could generate a selection bias with a population with a lower thrombotic risk.13
In relation to the local literature, it was recently published a series of cases of Colombian patients, 8 women, one with triple positivity and 6 with a history of arterial thrombosis, with an average time of treatment of 19±10 months. No new thrombotic events occurred in this series despite the aforementioned antecedents, probably explained by a shorter follow-up time and the presence of only one patient with triple positivity.23
While the present series of cases shows results consistent with what was previously reported,10,13,24 it is important to emphasize that this series does not reflect the effect of rivaroxaban in a population with primary APS, since our patients presented in most cases some associated autoimmune disease (one Takayasu's arteritis, one hereditary angioedema and four systemic lupus erythematosus), which gives them an increased thrombotic risk.25,26 This phenomenon is possibly derived from a reference bias characteristic of the institutions of high complexity in which the study was conducted and it makes difficult the generalization of our results.
Regarding the effectiveness of rivaroxaban as an anticoagulant in this group of patients, 2 patients presented new episodes of thrombosis: the first, a patient with recurrent arterial and venous thrombosis despite warfarin therapy and who presented a new episode of vascular thrombosis with the use of rivaroxaban. The second patient had the coexistence of APS and systemic lupus erythematosus that initially appeared as a mixed connective tissue disease and had an associated severe Raynaud's phenomenon, in addition to triple positivity for antiphospholipid antibodies (Table 2). From the safety point of view, there were no episodes of major bleeding or significant adverse events associated with rivaroxaban during follow-up. In this regard, it is important to remember the low number of patients in our report and, although the follow-up over time is prolonged, it is heterogeneous, with 3 patients with less than 6 months of follow-up.
Finally, it is important to mention the limitations of the study: first, few patients were included; however, the series available, as already mentioned, have similar numbers; this limits the analyses – our statistical analysis is mainly descriptive – however, the results allow to raise hypothesis for future studies. In addition, this work represents, nevertheless, a group of patients managed in the usual clinical practice and, to this extent, it provides the clinician with relevant information, especially due to the prolonged follow-up period (17.9±13.4 months), similar to the other series.11,23
What has been found so far in the literature points out, then, that rivaroxaban is safe in patients with APS, especially in those with low thrombotic risk (absence of triple positivity, without a history of arterial thrombosis).27 These conclusions are supported by the findings of the RAPS study, a randomized, open-label, non-inferiority study, which compared rivaroxaban with warfarin in patients with APS with or without systemic lupus erythematosus, where the thrombin generation at day 42 was the primary outcome. It was found that in the 210-day follow-up there was no major bleeding in any of the 2 groups, nor statistically significant differences in the clinically relevant bleeding, minor bleeding or adverse events.9 The main weakness of this study is that, being a surrogate outcome, it is difficult to transfer its results to clinical practice.
A different situation occurs with patients at high risk of thrombosis (particularly with arterial thrombosis or triple positivity), in whom, with the evidence available so far, including a recent systematic review, it has not been conclusively demonstrated that oral direct anticoagulants prevent new episodes of thrombosis.28 Furthermore, and taking into account that subjects with triple positivity tend to have a higher risk of thrombosis and greater susceptibility to arterial events, it is important to determine whether each of these components behaves as an individual risk factor or if it is the triple positivity which confers the risk of arterial thrombosis and thrombotic recurrence.6
In conclusion, in a group of patients with APS associated with other autoimmune diseases, mainly lupus, with impossibility to use warfarin, the use of rivaroxaban was well tolerated and without adverse effects, although thrombotic events occurred in 2 patients. Data of studies with a higher level of evidence are required to clarify the effectiveness of these agents, especially in patients at high risk of new thrombotic effects such as those with triple positivity of antibodies or arterial thrombosis, avoiding the use of surrogate outcomes to estimate the antithrombotic effect.29
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingThe funds for the realization of this study come from the researchers’ own resources.
Conflict of interestThe authors state they don’t have any conflict of interest.
Please cite this article as: Restrepo Correa RC, Velásquez Franco CJ, Muñoz-Grajales C, Pinto Peñaranda LF, Márquez Hernández JD, Rodriguez Padilla LM, Mesa Navas MA. Uso de inhibidores directos del factor Xa en síndrome antifosfolípido: una serie de siete casos. Rev Columb Reumatol. 2018;25:16–21.