An increased urinary oxalate and reduced urinary citrate are considered major risk factors in the formation of calcium oxalate kidney stones. In this work, an HPLC–MS method is presented for the simultaneous measurement of oxalate and citrate in urine.
MethodsSample preparation was carried out using a liquid–liquid extraction with ethyl acetate. Chromatographic separation was performed in a C18 column by gradient elution with methanol and 1M formate buffer at 35°C. Citrate and oxalate were monitored on a single-quadrupole MS system.
ResultsThe method was linear in the concentration range of 0.5mg/L to 450mg/L for oxalate and from 2.5mg/L to 950mg/L for citrate. The Lower Limit of Measurement was 0.56mg/L for oxalate and 2.5mg/L for citrate. The within-day imprecision was 6% for oxalate and 3% for citrate, and the between day imprecision was lower than 15% for both analytes. LC–MS method was compared with capillary electrophoresis and it was shown that both methods were interchangeable to measure oxalate, but not citrate.
ConclusionsHPLC–MS method is a good approach to measure oxalate and citrate in 24-hour urine, and it is applicable in clinical routine for patients with recurrent stone formation.
La hiperoxaluria e hipocitraturia están consideradas el principal factor de riesgo en la formación de cálculos renales de oxalato cálcico. En este trabajo se ha desarrollado un método de HPLC-MS para la medida simultánea de oxalato y citrato en orina.
MétodosLa preparación de muestras se realizó por una extracción líquido-líquido con etilacetato. La separación cromatográfica se llevó a cabo en una columna C-18 a 35°C con un gradiente de elución con metanol y ácido fórmico 1M. El citrato y el oxalato se monitorizaron mediante espectrometría de masas con un cuadrupolo simple.
ResultadosEl método fue lineal para el oxalato en el rango de concentración de 0,5 a 450mg/l y para el citrato de 2,5 a 950mg/l. El límite menor de intervalo de medida fue de 0,56mg/l para el oxalato y de 2,5mg/l para el citrato. La imprecisión intradía fue del 6% para el oxalato y del 3% para el citrato, y la interdía fue inferior al 15% para ambos analitos. El método de LC-MS desarrollado se comparó con un método de electroforesis capilar y se demostró que ambos eran intercambiables para el oxalato, pero no para el citrato.
ConclusionesEl método de HPLC-MS desarrollado constituye una buena aproximación para medir oxalato y citrato en orina de 24 horas y es aplicable en la rutina clínica para pacientes con riesgo de formación de cálculos renales.
Urolithiasis is a frequent disease in developing countries. Hypocitraturia is considered the major risk factor for calcium stone formation and hyperoxaluria is related to the formation of oxalate kidney stone.
Enteric hyperoxaluria has been reported after gastric bypass surgery and bowel resection1–3 and hypocitraturia is associated with body mass index,4 diets rich in animal protein and certain therapeutic drugs like acetazolamide, topiramate, ACE inhibitors and thiazides.5 Thus, urine oxalate and citrate test request has been increased in last years to evaluate patients in different clinical settings.
Current methodologies for analysis of oxalate and citrate include enzymatic techniques,6 capillary electrophoresis (CE) and liquid chromatography–tandem mass spectrometry (HPLC–MS/MS). HPLC–MS/MS methods have been reported recently for the quantification of oxalate in urine using anion exchange chromatography7 and for citrate with a C18 column.8 Both reported methods showed good concordance with enzymatic method. Quantification of oxalic and citric acids have been described by an HPLC–MS method after derivatization based on Fischer esterification in soil- and root-related samples.9 However, simultaneous determination of citrate and oxalate in underivatizated urine samples only has been reported before using CE.10,11
In this work, a method was developed to detect and quantify simultaneously citrate and oxalate in urine by liquid chromatography simple mass spectrometry (HPLC–MS). This method was compared with the CE method. The HPLC–MS method has an easy sample preparation without derivatization, separation with a C18 column and a simple performance to obtain fast and reliable results to implement in clinical routine.
Material and methodsChemicals and reagentsStandards of oxalic acid and citric acid were purchased from Sigma–Aldrich®. Methanol, hydrochloric acid (37%) and sodium hydroxide from MerckKraal (Darmstadt, Germany). Sodium chloride, ethyl acetate and formic acid were obtained from PanreacQuimica (Barcelona, Spain).
Instruments and conditionsAnalytes were separated using a Supelco (Sigma) reverse phase column C18 (150mm×4.6mm×5μm) at 35°C and detected in a Single Quadrupole LC-MS 2020 system (Shimadzu Corporation, Kyoto Japan). HPLC gradient was generated by mixing an aqueous formic acid solution (1N) with methanol. Optimized chromatographic method started with 20% methanol for 2min and changed linearly to 65% over 8min, hold for 1min and then decreasing to 20% methanol over 1min. The total method run time was 14min and the flow rate was 0.3mL/min. Oxalate and citrate were ionized by electrospray ionization (ESI) in negative-ion mode (spray voltage: −4.5kV; probe heater: 350°C). Nebulizing gas (1.5L/min) and drying gas (10L/min) was a source of N2 generated by a Nitrogen Generator (Peak Scientific). Desolvation temperature was 250°C. Oxalic acid and citric acid were monitored by Selected Ion Monitoring (SIM) mode at m/z 89.0 and m/z 191.0, respectively.
Capillary electrophoresis was performed in a ProteomeLab PA800 (Beckman Coulter, Pasadena, USA) equipped with an ultraviolet detector set at 200nm. Electrophoretic separation was carried out using a neutral coated capillary with a detector window at 30cm from the inlet (internal diameter of 50μm). Separation buffer consisted of 0.2M phosphoric acid with 10% methanol (v/v) adjusted to pH 6.08 with NaOH 1M. Analyses were run at 25°C using reverse polarity (−10kV) for 13min. Pressure for samples injection was 0.5 p.s.i. during 5seg.
Sample preparationOxalate and citrate measures in urine were requested in different clinical settings in the hospital scope. Twenty-four hour urine samples were collected from patients who have been assessed for risk stone formation and aliquots were stored frozen at −40°C until analysis. Both analytes were measured with the routine method (CE) and HPLC–MS for optimization and validation of the proposed method.
Urine samples were centrifuged for 20min at 12500rpm. Supernatants were extracted by liquid–liquid extraction: 200μL of urine was acidified to pH≤1 with 100μL of HCl 6N, saturated with 100mg of NaCl and extracted with 600μL of ethyl-acetate. Ten microliters of sample were injected into the HPLC–MS system.
Urine samples were centrifuged for 30min at 30000rpm and supernatants were injected directly in the CE system for capillary electrophoresis analysis.
Method validationLinearity, Lower Limit of the Measurement Interval (LLMI), recovery, imprecision, matrix effect and method comparison were evaluated for method validation. The new Clinical and Laboratory Standards Institute (CLSI) C62-A guidelines12 were followed for assay verification.
Oxalate and citrate were measured in hundred 24-hour urine samples by HPLC–MS and CE. Method comparison was performed using Passing–Bablock regression and Bland–Altmant graphs. MedCalc statistical program was used to perform data analysis.
ResultsLinearityLinearity was tested with nine levels of concentration for both analytes with two replicates each. The regression equations were linear over the range of 0.5–450mg/L for oxalate (R2≥0.9979, CV=12%) and 2.5–950mg/L for citrate (R2≥0.9977, CV=12%).
Lower Limit of the Measurement Interval (LLMI)Lower Limit of the Measurement Interval was estimated as the lowest amount of the analyte that can be reliable detected. The LLMI (S/N=10:1) for oxalate and citrate were 0.56mg/L (CV=7%) and 2.5mg/L (CV=6%), respectively.
RecoveryThe recovery study was performed by adding known amounts of oxalate (11.25 and 45mg/L) and citrate (60 and 240mg/L) in a standard solution of known concentration and in a urine pool.
The recovery range was between 90–100% for oxalate and 95–115% for citrate.
ImprecisionPrecision studies were conducted to evaluate repeatability and reproducibility imprecision. Intra-day and inter-day imprecision include the entire testing process in standards solutions and urine samples. Concentrations span the analytical measurement interval and also include samples within +/−25% of the medical decision point.
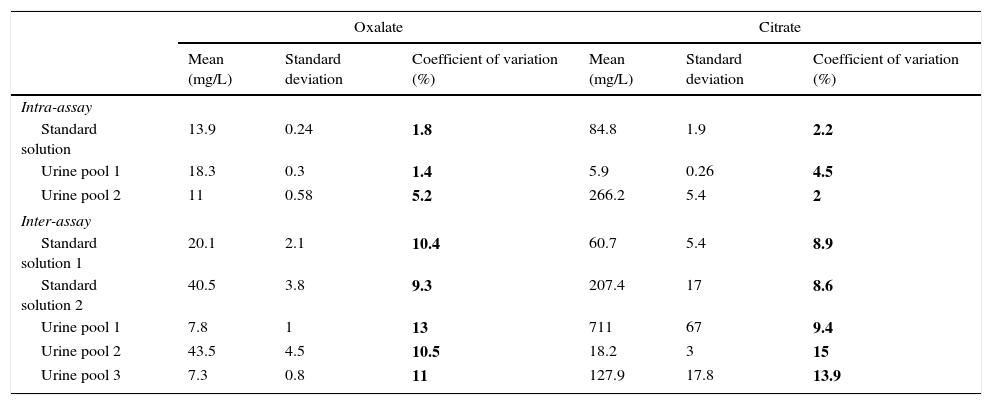
Method precision was evaluated as shown in Table 1. The intra-day imprecision was less than 6% in oxalate and less than 3% in citrate. Inter-day imprecision was less than 15% in oxalate and citrate.
Imprecision values.
| Oxalate | Citrate | |||||
|---|---|---|---|---|---|---|
| Mean (mg/L) | Standard deviation | Coefficient of variation (%) | Mean (mg/L) | Standard deviation | Coefficient of variation (%) | |
| Intra-assay | ||||||
| Standard solution | 13.9 | 0.24 | 1.8 | 84.8 | 1.9 | 2.2 |
| Urine pool 1 | 18.3 | 0.3 | 1.4 | 5.9 | 0.26 | 4.5 |
| Urine pool 2 | 11 | 0.58 | 5.2 | 266.2 | 5.4 | 2 |
| Inter-assay | ||||||
| Standard solution 1 | 20.1 | 2.1 | 10.4 | 60.7 | 5.4 | 8.9 |
| Standard solution 2 | 40.5 | 3.8 | 9.3 | 207.4 | 17 | 8.6 |
| Urine pool 1 | 7.8 | 1 | 13 | 711 | 67 | 9.4 |
| Urine pool 2 | 43.5 | 4.5 | 10.5 | 18.2 | 3 | 15 |
| Urine pool 3 | 7.3 | 0.8 | 11 | 127.9 | 17.8 | 13.9 |
Matrix effect was evaluated comparing the linear regression slope between calibration standard curve and addition standard curve.
The slopes of the standard solution curves (7.2±0.2 for oxalate and 4.2±0.9 for citrate) were similar to the standard addition curves (6.8±1.4 for oxalate and 4.2±1.0 for citrate).
Method comparisonBland–Altman plot and Passing–Bablock regression were used for method comparison.
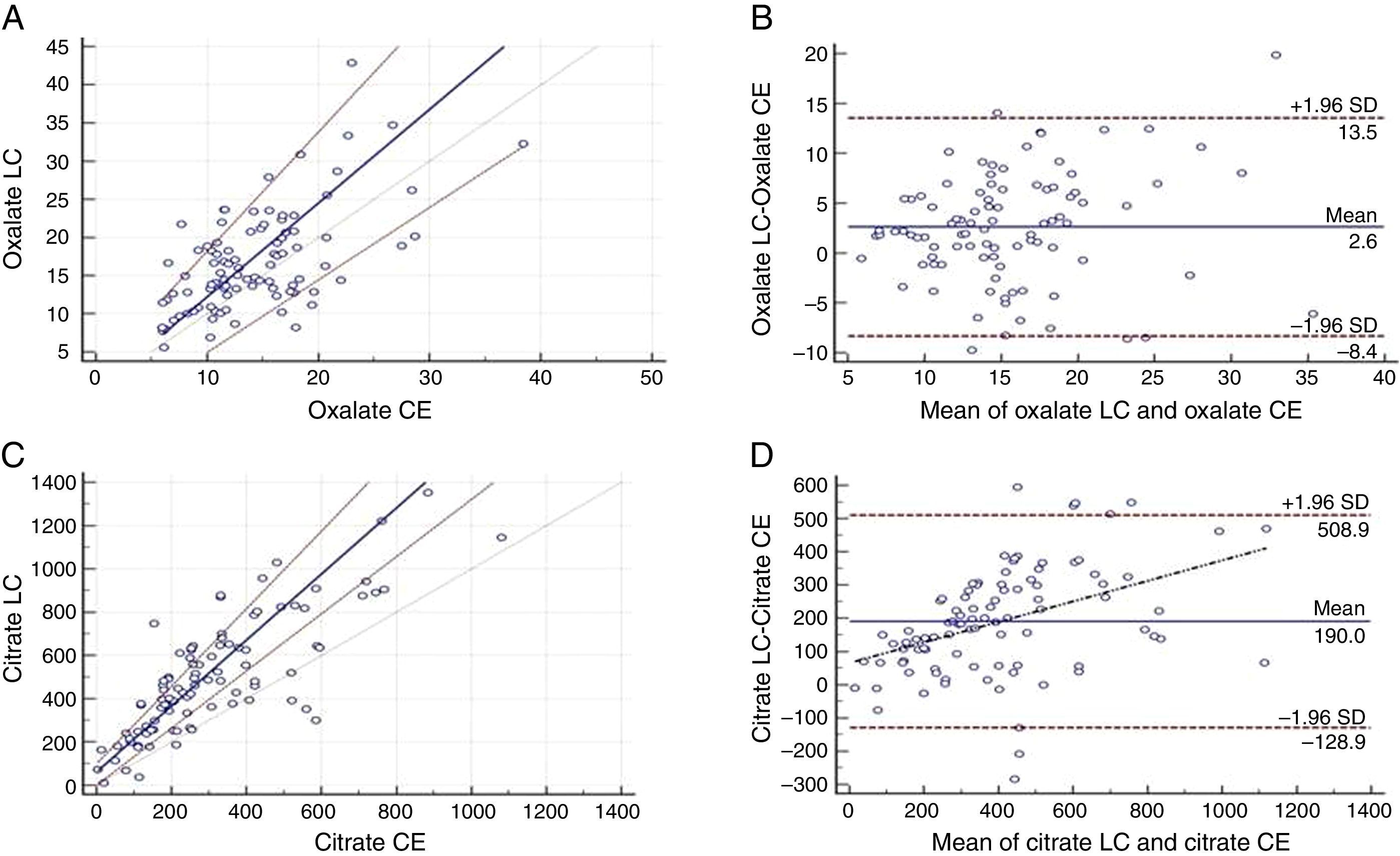
Passing–Bablock equation indicated no significant differences between the two methods for oxalate (Fig. 1A). Regression line equation was y=−0.052 (95% CI −4.6 to 2.6)+1.2x (95% CI=0.9 to 1.6). Bland–Altman plot showed a good relationship between the differences and the averages of the two methods (Fig. 1B).
Passing–Bablock (P–B) and Bland–Almant (B–A) analysis have been used for method comparison. (A) P–B between CE and LC–MS method for oxalate indicates no significant differences. (B) B–A analysis for CE and LC–MS method for oxalate indicates a good relationship between the differences and the averages of the two techniques. (C) P–B between CE and LC–MS method for citrate indicates a proportional error. (D) B–A analysis for CE and LC–MS method for citrate indicates LC–MS overestimate CE values.
Passing–Bablock equation obtained for citrate was y=49.26 (95% CI=−8.6 to 102)+1.51x (95% CI=1.3 to 1.8). These results indicated proportional differences between the two methods (Fig. 1C). Bland–Altman graph showed a proportional error between differences and the magnitude of the measurements and an absolute systematic error of a mean value of 190mg/L (Fig. 1D).
Discussion and conclusionsClinical oxalate and citrate measurement in 24-hour urine collection samples is used to monitor patients with stone formation risk factors and patients in treatment with citrate salts. In this work, we developed an HPLC–MS method with an easy sample preparation to quantify oxalate and citrate simultaneously.
The method has been validated over a wide range that includes normal and pathologic concentrations. The CV values indicated an acceptable method imprecision (10–15%), including sample collection and storage, extraction and analysis. In recovery experiments, we obtained good accuracy results for oxalate and citrate and no matrix effects have been observed.
Usually, manual and automatic enzymatic methods were used in the determination of these organic acids. However, these methods have a high cost per test and both CE and HPLC–MS could be a suitable option.
Mass spectrometry is a relatively expensive technique because of set-up costs, but usually has very low running costs. Several methods have been described for HPLC–MS/MS in the measurement of oxalate and citrate in urine.7,8 It has been reported a sensitive method for quantification of these organic acids in soil extracts, but requires derivatization.9 However, urine samples have higher concentrations of oxalate that allow avoiding derivatization. An LC–MS method without sample derivatization to measure both organic acids simultaneously is an interesting approach for clinical purposes. An alternative to quantify these organic acids is CE.
Both methods, CE and HPLC–MS, were interchangeably to measure oxalate. However, HPLC–MS method overestimated citrate measures in relation to CE, especially for high concentrations. For this reason, new reference values of urine citrate will be require in our population for clinical use of this HPLC–MS method.
In conclusion, we have developed a method for oxalate and citrate measurement in urine that is easy to use and applicable in clinical routine.
Ethical disclosers statementThis study was revised and approved by the Ethics Committee of the Clínico San Carlos Hospital of Madrid and was conducted in accordance with the Declaration of Helsinki.
Conflict of interestThere is no conflict of interest between the authors.
This research work was supported by the Fundación para la Investigación Biomédica of Hospital Clínico San Carlos.