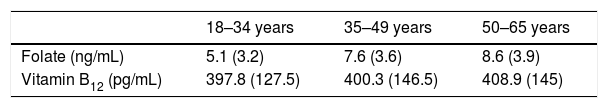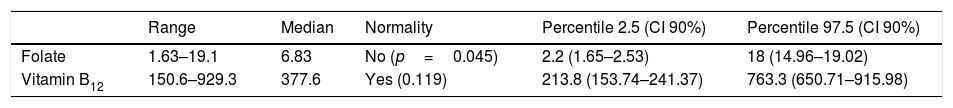Despite being the most widely used medical decision-making tool, reference intervals are not usually determined by clinical laboratories, due to the highly demanding activities and costly process it involves. However, scientific societies encourage individual clinical laboratories to establish their own reference values. This is especially important in the cases of folate and vitamin B12, due to strong differences in vitamin status among different populations.
ObjectiveOur aim is to establish reference intervals for folate and vitamin B12 levels in a healthy blood donor population using an electrochemiluminiscent method (ROCHE DIAGNOSTICS).
MethodFolate and vitamin B12 levels were measured in 141 healthy blood donors aged between 18 and 65 years. Biochemical analyses were performed using a Modular E170 analyzer (ROCHE DIAGNOSTICS) and an electrochemiluminiscent method. Reference intervals were calculated with a non-parametric percentile method following the CLSI guidelines.
ResultsThere were not significant differences in folate or cobalamin levels between age or sex subgroups. The limits of the reference interval for folate were 2.2 and 18ng/mL (5–40.7nmol/L), and 213.8 and 763.3pg/mL (158.2–564.8pmol/L) for vitamin B12. These intervals differed from those claimed by the manufacturer.
ConclusionsOur results emphasize the convenience of building reference values based on the population served by the laboratory, in order to unequivocally rule out deficiencies of folate or vitamin B12.
A pesar de ser una de las herramientas más usadas en la toma de decisiones médicas, los intervalos de referencia son raramente determinados en los laboratorios, debido a que es una labor compleja en términos de tiempo, esfuerzo y coste. Sin embargo las sociedades científicas recomiendan que los laboratorios establezcan sus propios valores de referencia. Esto es especialmente importante para el folato y la vitamina B12, debido a las grandes diferencias entre poblaciones.
ObjetivoNuestro propósito es establecer los valores de referencia en una población de donantes sanos, mediante un método electroquimioluminiscente (Roche Diagnostics).
MétodoLos niveles de folato y vitamina B12 se midieron en una muestra integrada por 141 donantes sanos de una edad comprendida entre 18 y 65 años. Los análisis bioquímicos se realizaron en un analizador Modular E170 (Roche Diagnostics). Los intervalos de referencia se calcularon siguiendo el método no paramétrico propuesto por las guías del CLSI.
ResultadosNo hubo diferencias en los niveles de folato ni vitamina B12 entre sexos ni grupos de edad. Los límites de referencia para el folato fueron 2,2 y 18ng/ml (5-40,7nmol/l), y 213,8 y 763,3pg/ml (158,2-564,8 pmol/l) para la vitamina B12. Estos intervalos difirieron de los propuestos por el fabricante.
ConclusionesNuestros resultados subrayan la importancia de obtener valores de referencia en la población a la que da servicio el laboratorio para excluir inequívocamente las deficiencias de folato y vitamina B12.
Folate and vitamin B12 are water soluble vitamins involved in one carbon transfer (methylation) reactions necessary for the production of monoamine neurotransmitters, phospholipids and the nucleotides used in DNA synthesis.1–5 Vitamin B12 or folate depletion both increase homocysteine levels, being high circulating levels of homocysteine often consequence of an inadequate status of these vitamins.6 Hyperhomocysteinaemia has been associated with cardiovascular, haematological and neurological diseases.6,7
Deficiency of folate or of cobalamin may cause different types of disorders, mainly haematological disorders7 such as anaemia and macrocytosis, but also others as memory disturbances, delirium, mood disorders and psychoses.8,9 Folate and vitamin B12 deficiencies are still important problems worldwide. The difficulty to establish consensus levels is due to the fact that serum concentrations of these vitamins differ according to age, gender and other variables.6 Vitamin B12 levels decrease with age, being clinical and subclinical deficiency more prevalent in the elderly.10 Epidemiological data suggest an estimated prevalence of at least 10% of individuals over 60 years.5,11 Age influence is not so clear-cut in the case of folate levels.12 In addition diet, habits, and physiological and pathological factors influence cobalamin and folate status. For instance, low vitamin B12 levels are more frequent in vegetarians,10,13 in patients with gastrointestinal diseases,14 and in individuals with high alcohol consumption or with renal failure.10,14 Folate status is also negatively influenced by dietary, pathological (alcoholism, depression) and pharmacological (anticonvulsants) factors.15 Variability is further enhanced by the compulsory fortification of flour with folate and vitamin B12 dictated by some government agencies.5,15 As a consequence, prevalence rates for folate and vitamin B12 deficiencies vary considerably across different studies.12 Besides the aforementioned factors that promote variability, it is important to take also into account methodological differences, especially when immunoassays are used.
The aim of this work is to define the reference values for serum folate and vitamin B12 in our population. According to Horn and Pesce,16 reference intervals are the most widely used medical decision-making tools. Ideally, all clinical laboratories should determine their own reference values. However, most of them do not, due to the fact that the establishment of reliable reference intervals is a highly demanding activity,17 and therefore the usual practice is to adopt the intervals provided in the manufacturer's inserts. We have calculated reference intervals for a population from Central Spain using an electrochemiluminiscent method (Roche Diagnostics) in a Modular E-170 analyzer.
Materials and methodsPatientsOne hundred and forty one healthy volunteers aged 18–65 years were selected among blood donors of our hospital. Written informed consent was obtained from all of them. Abuse drug consumers, smokers and alcohol drinkers were excluded from the study. The individuals did not have noticeable previous disease, and were not being treated with prescription medications or vitamin supplements at the moment of inclusion. Table 1 shows the exclusion criteria used for patient selection. Blood was collected in serum separator tubes and serum samples obtained after centrifugation at 1000×g during 10min.
Exclusion criteria applied for selection of individuals.
| • Recent disease |
| • Recent surgery with hospital admission |
| • Hypertension |
| • Genetic diseases |
| • Pregnancy |
| • Breast feeding |
| • Alcohol intake |
| • Tobacco smoking |
| • Drug abuse |
| • Treatment with therapeutic drugs |
| • Use of vitamin supplements |
| • Obesity |
| • Special diets |
Vitamin B12 and folate levels were measured using a competitive electrochemiluminiscent immunoassay in a Modular E170 analyzer (ROCHE DIAGNOSTICS®, Manheim, Germany). In this method the sample is first pretreated to release vitamin B12 and folate from their carrier proteins. The sample is then incubated with a ruthenium-labelled protein (intrinsic factor in the vitamin B12 assay and folate binding protein in the folate assay), giving rise to an analyte-protein complex whose concentration depends on the concentration of analyte in the sample. Subsequently, streptavidin-covered microparticles and biotin-labelled analyte are added. The latter occupies the remaining free binding sites on the ruthenium-labelled protein, and the resulting complex binds to the solid phase due to the interaction between biotin and streptavidin. The microparticles attach then to the surface of an electrode due to magnetic forces, non-bound components are washed away and, finally, an electric current is applied, which induces a chemiluminiscent reaction whose intensity is proportional to vitamin B12 or folate concentration.
Statistical analysisData analysis was performed using the software package Medcalc 11.3 (Ostend, Belgium). Reference intervals were calculated using the non-parametric percentile method, following the recommendations of the Clinical Laboratory Standards Institute document CLSI C28 A3.18 The presence of outliers was assessed using the Dixon's test.16 Kolmogorov–Smirnov's test was used to evaluate the Gaussian distribution.
Additionally, we examined if it was possible to partition reference values according to sex or age. The sample was split in three age categories: (I) 18–34 years; (II) 25–49 years and (III) 50–65 years. Assessment of differences between these groups was done using the Harris and Boyd criterion,19 according to which separate intervals must be established if the ratio between the subgroup standard deviations is greater than 1.5.
ResultsThe sample representing our population was composed of 141 healthy volunteers aged 18–65 years, 87 of them male (61.7%) and 54 female (38.3%). The age arithmetic mean was 41.9 years (median 43, standard deviation 12.1). The descriptive characteristics of the study group are shown on Table 2.
Descriptive characteristics of the sample.
| Male (87) | Female (54) | Total (141) | |
|---|---|---|---|
| Age mean (years) | 43.03 (11.1) | 40 (13.3) | 41.87 (12.1) |
| Folate (ng/mL) | 7.79 (3.8) | 6.5 (3.7) | 7.3 (3.8) |
| Vitamin B12 (pg/mL) | 396 (125.5) | 412.2 (159.91) | 402.2 (139.34) |
Values represent arithmetic means. Standard deviations are shown in brackets. Concentrations are expressed in the conventional units used in our laboratory. Conversion factors into international units are 2.26 for folate and 0.74 for vitamin B12.
Table 3 shows the results when the sample was split in age groups.
Concentrations of folate and vitamin B12 in different age groups.
| 18–34 years | 35–49 years | 50–65 years | |
|---|---|---|---|
| Folate (ng/mL) | 5.1 (3.2) | 7.6 (3.6) | 8.6 (3.9) |
| Vitamin B12 (pg/mL) | 397.8 (127.5) | 400.3 (146.5) | 408.9 (145) |
Values represent arithmetic means. Standard deviations are shown in brackets. Concentrations are expressed in the conventional units used in our laboratory. Conversion factors into international units are 2.26 for folate and 0.74 for vitamin B12.
Kolmogorov–Smirnov's test showed a Gaussian distribution for vitamin B12 but not for folate levels (Table 4). The reference interval for folate ranged from 2.2 to 18ng/mL (5–40.7nmol/L), and in the case of vitamin B12 from 213.8 to 763.3pg/mL (158.2–564.8pmol/L). These reference intervals are shown on Table 4, where the numbers in brackets represent the 90% confidence intervals.
Calculated reference intervals for folate and vitamin B12.
| Range | Median | Normality | Percentile 2.5 (CI 90%) | Percentile 97.5 (CI 90%) | |
|---|---|---|---|---|---|
| Folate | 1.63–19.1 | 6.83 | No (p=0.045) | 2.2 (1.65–2.53) | 18 (14.96–19.02) |
| Vitamin B12 | 150.6–929.3 | 377.6 | Yes (0.119) | 213.8 (153.74–241.37) | 763.3 (650.71–915.98) |
Calculated reference intervals. Concentrations are expressed in the conventional units used in our laboratory. Conversion factors into international units are 2.26 for folate and 0.74 for vitamin B12.
Application of the Harris and Boyd's method leads us to conclude that there are no significant differences for folate or vitamin B12 concentration among different sex or age groups, as standard deviation ratios are under 1.5 in every case. Given that folate values did not show a Gaussian distribution logarithmic normal transformation was carried out for this variable before applying the Harris and Boyd's approximation.
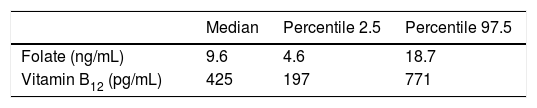
Reference intervals claimed by the manufacturer are shown in Table 5.
Reference intervals claimed by the manufacturer.
| Median | Percentile 2.5 | Percentile 97.5 | |
|---|---|---|---|
| Folate (ng/mL) | 9.6 | 4.6 | 18.7 |
| Vitamin B12 (pg/mL) | 425 | 197 | 771 |
Reference values claimed by the manufacturer. Concentrations are expressed in the conventional units used in our laboratory. Conversion factors into international units are 2.26 for folate and 0.74 for vitamin B12.
One of the critical aspects of the practice of clinical laboratory medicine is the assessment of test results to determine whether patients are healthy or, on the contrary, subject to some pathological condition. Thus, the inclusion of reference intervals in clinical laboratory reports is mandatory.17 Each laboratory should produce its own reference values and estimate the corresponding reference intervals.20,21 However, this recommendation seems to have had limited impact in most clinical laboratories,17 the usual practice being to adopt values included in the reagent inserts. This is due to the fact that the calculation of an adequate reference interval is a highly demanding and difficult activity.16,17 The Clinical Laboratory Standard Institute (CLSI) and the International Federation of Clinical Chemistry (IFCC) recommend the inclusion of at least 120 individuals in the calculation of a reference interval16,18,20; this recommendation is widely fulfilled in our sample (n=141). Establishing reference intervals has always been a challenging task, as significant differences may exist between ethnic, gender or age groups, and as they may also vary depending on specimen collection techniques, test assays, and other factors. This variability is even greater in the cases of folate and vitamin B12 which, apart from the aforementioned factors, can also be influenced by diet, drug therapies, and policies of grain fortification. This reinforces the need of elaborating reference intervals for this analytes by every individual clinical laboratory.
In our case, the manufacturer's change of the composition of folate and vitamin B12 reagents21 (folate III, vitamin B12 II) has led to lower results than the ones we used to obtain with previous versions (particularly for folate). In spite of this fact, the reference intervals stated in the vendor's insert are similar to the previous ones. This has prompted us to produce our own reference values.
As we expected, in the case of folate we obtained a 2.5 percentile considerably lower than the one claimed by the vendor (2.2 vs. 4.6ng/mL, or 5 vs. 10.2nmol/L), although upper limits were similar. The difference was not so marked in the case of vitamin B12, but our 2.5 percentile was also lower than that claimed by the manufacturer (197 vs. 213.8pg/mL, or 145.8 vs. 158.2pmol/L). This fact reinforces the need of each clinical laboratory to build its own reference intervals. When we applied Harris and Boyd method19 to our data we found no age- or sex-related differences between serum vitamin levels. Although the influence of age in vitamin B12 status is well known,10–13 our sample did not include elderly people, a fact that explains the lack of difference between subclasses. Sex differences are not so clear cut in previously published studies,6,12 and accordingly we found no relation between reference intervals and sex.
A limitation of our study is that we did not measure other metabolites such as homocysteine, methyl malonic acid or red blood content of folate. Although this would have exceeded the scope of our study, it would have been interesting in order to define more precisely the cobalamin and folate status in our population. Unfortunately, we have no available methods for the routine determination of these analytes in our laboratory.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentPatient data are not shown in this article.
Conflict of interestThe Authors declare that they do not have any conflict of interest with the company which provides reagents and instrumentations.
The authors are in debt with ROCHE DIAGNOSTICS™ for the gift of reagent kits, which supported the expenditure of the study.