Primary amyloidosis is a rare condition characterised by the deposition of free light chains in different tissues and organs (e.g. kidney, heart, liver, gastrointestinal system). The aim of the therapy in patients with primary amyloidosis is to suppress the monoclonal plasma cells that produce the amyloidogenic free light chains and to preserve the organ function. Thus, the new criteria for the haematological disease response include the measurement of serum free light chains concentrations. The case is presented on a patient diagnosed with primary amyloidosis, where the difference between bound and free serum free light chains (dFLC) was used to evaluate the haematological response to the treatment, as well as any biological progression. In contrast to dFLC, Bence Jones Protein in urine was positive but ineffective to evaluate the response to the treatment.
La amiloidosis primaria es una entidad rara caracterizada por el depósito de cadenas ligeras libres en diferentes tejidos y órganos (riñón, corazón, hígado, aparato gastrointestinal). El objetivo en la terapia de los pacientes con amiloidosis primaria consiste en suprimir las células plasmáticas monoclonales que producen las cadenas ligeras libres amiloidogénicas y preservar la función de los órganos afectados. Así, los nuevos criterios de respuesta hematológica de la enfermedad incorporan la medida de las concentraciones séricas de cadenas ligeras libres. Presentamos el caso de una paciente a quien se diagnosticó amiloidosis primaria y en la cual la diferencia de concentración en suero entre la cadena ligera libre monoclonal implicada y la no implicada (dFLC) nos permitió evaluar la respuesta hematológica al tratamiento y la presencia de progresión biológica. En contraste a la dFLC, la proteinuria de Bence Jones fue positiva pero ineficaz en la evaluación de la respuesta al tratamiento.
Primary or systemic immunoglobulin light chain amyloidosis (AL amyloidosis) is a rare entity characterised by the deposition in different tissues and organs (e.g. kidney, heart, liver, gastrointestinal system) of amyloidogenic free light chains produced by a clonal population of plasma cells.1 Importantly, the lethal consequences of AL amyloidosis are due to the toxic effect of amyloid deposits and not due to the malignant behaviour of the plasma cell clone in bone marrow. Therefore, an early diagnosis of AL amyloidosis is critical to facilitate swift access to effective chemotherapy, and consequently suppress the production of amyloidogenic free light chains before irreversible organ damage occurs. A strategy for the diagnosis and evaluation of amyloidosis includes tissue biopsy studies, investigation for paraproteinaemia and assessment for systemic disease or organ involvement.2
Recent evidence has shown that serum free light chains (sFLC) and, particularly, the difference between involved and uninvolved sFLC (dFLC) are a well-established method for monitoring the haematologic response to therapy in patients with AL amyloidosis.3 We report the case of a patient diagnosed of AL amyloidosis that highlight the importance of dFLC as useful tool in the monitoring of patients with AL amyloidosis.
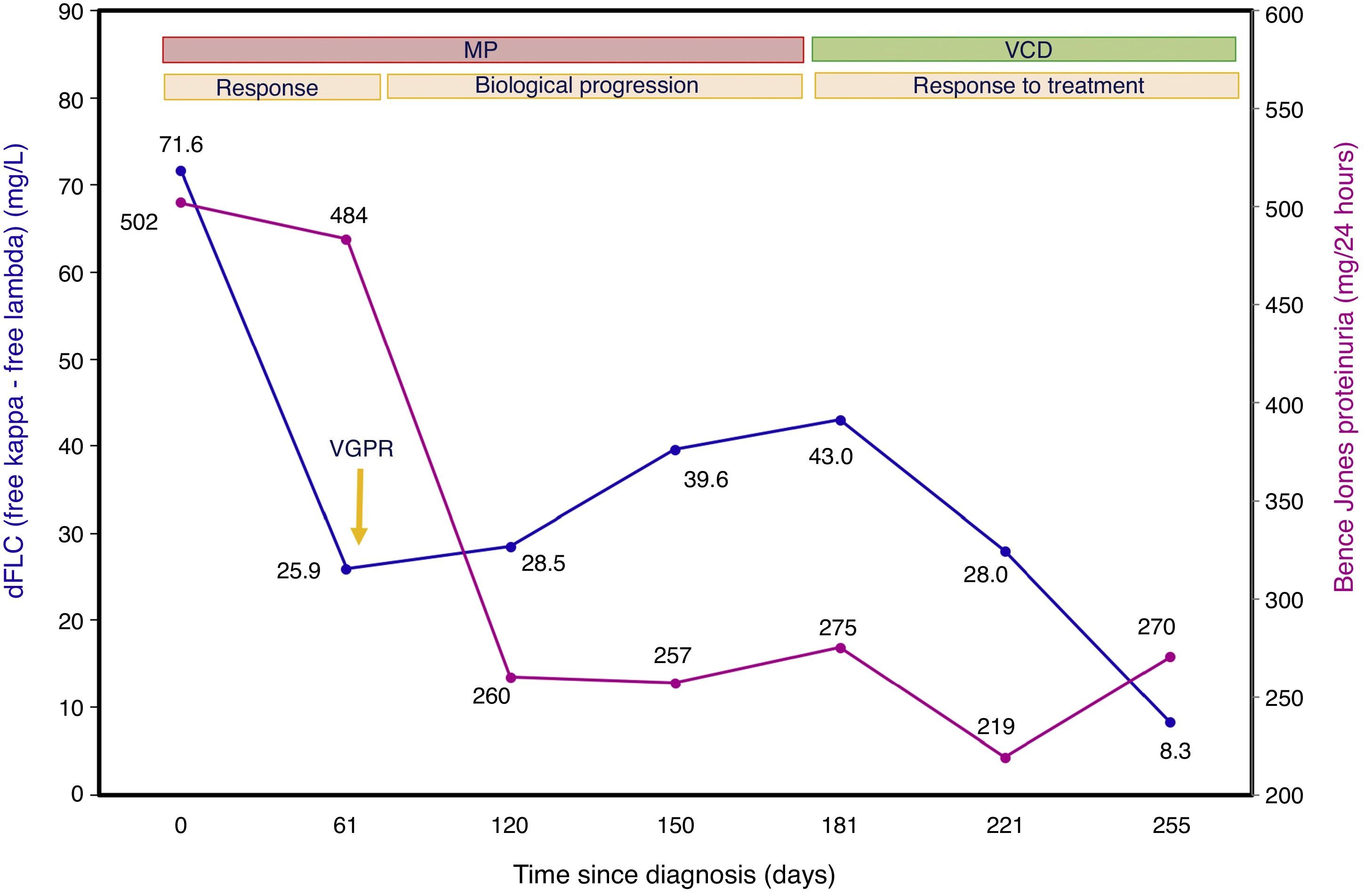
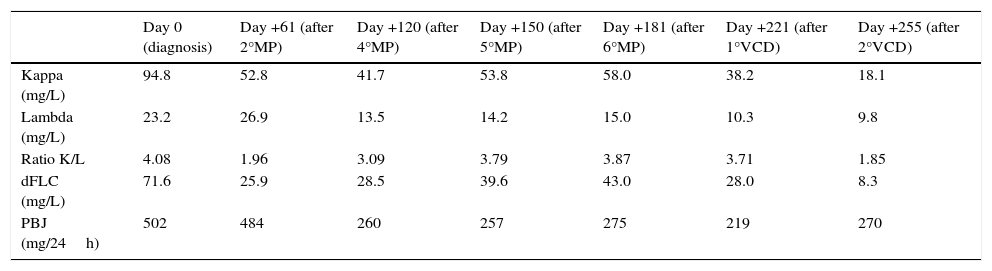
Case presentationA 46 years old woman was admitted to the Hospital with acute kidney failure presenting a creatinine of 5.9mg/L (biological reference interval (BRI): 0.6–1.2mg/L). Suspecting a possible monoclonal gammopathy, the laboratory findings showed a beta-2-microglobulin of 17.8mg/L (BRI: 0.8–2.4mg/L), albumin of 3.19g/dL (BRI: 3.0–5.2g/dL), with no monoclonal protein detected in the serum protein electrophoresis (SPE) and negative serum immunofixation (sIFE). However, the sFLC concentrations were 94.8mg/L for kappa (BRI: 3.3–19.4mg/L), 23.2mg/L for lambda (BRI: 5.7–26.3mg/L) with a ratio K/L of 4.08 (BRI: 0.26–1.65) and a dFLC of 71.6mg/L. The 24-h urine volume was 2990mL (BRI: 1000–2500mL) with a glomerular proteinuria of 5780mg/24h (BRI: <150mg/24h) described as nephrotic-range proteinuria. The 24-h urine study evidence a kappa positive Bence Jones Proteinuria (BJP) of 502mg/24h (BRI: negative). The imaging studies (X-rays and magnetic resonance imaging) did not showed evidence of lytic bone lesions and the bone marrow aspirate showed the presence of 7% of plasma cells. With these findings and the suspect of AL amyloidosis, renal biopsy and echocardiography study were done. In the renal biopsy, amyloid deposition was identified by a positive Congo-red staining and the immunohistochemical study of the amyloid material was strongly positive for kappa light chain. The echocardiography study showed septal hypertrophy and the serum concentrations of NTpro-BNP and cTnT were 479pg/mL (BRI: <100pg/mL) and 0.14ng/mL (BRI: <0.025ng/mL), respectively. The patient was finally diagnosed of AL amyloidosis in Mayo Clinic stage III and began treatment with Melphalan and Prednisone (MP). During the monitoring of the patient, the results of the sFLC were expressed as dFLC (Table 1 and Fig. 1). At diagnosis (day 0), dFLC was 71.6mg/L and PBJ was 502mg/24h. After two cycles of MP (day +61), the patient achieved a status of very good partial response (VGPR) with dFLC value of 25.9mg/L that corresponds to dFLC reduction of 64%. PBJ was 484mg/24h. After the fourth, fifth and sixth cycles of MP, the dFLC increased to 28.5mg/L at day +120; 39.6mg/L at day +150 and 43.0mg/L at day +181. PBJ values were 260, 257 and 275mg/24h, respectively. The increase of dFLC showed the existence of biological progression of the disease and the haematologist decided to change the treatment to Bortezomib, Cyclophosphamide and Dexamethasone (VCD). The patient presented good tolerance at this new treatment with a dFLC of 28.0mg/L at day +221 (after first cycle of VCD) and 8.3mg/L at day +255 (after second cycle of VCD). The BJP were 219 and 270mg/24h, respectively. SPE and sIFE were negative during the two lines of treatment of the patient.
Evolution of serum free light chains levels (sFLC) and Bence Jones Proteinuria (BJP) during the two lines of treatment of the patient. dFLC: difference between involved and uninvolved serum free light chains, MP: Melphalan and Prednisone, VCD: Bortezomib, Cyclophosphamide and Dexamethasone.
| Day 0 (diagnosis) | Day +61 (after 2°MP) | Day +120 (after 4°MP) | Day +150 (after 5°MP) | Day +181 (after 6°MP) | Day +221 (after 1°VCD) | Day +255 (after 2°VCD) | |
|---|---|---|---|---|---|---|---|
| Kappa (mg/L) | 94.8 | 52.8 | 41.7 | 53.8 | 58.0 | 38.2 | 18.1 |
| Lambda (mg/L) | 23.2 | 26.9 | 13.5 | 14.2 | 15.0 | 10.3 | 9.8 |
| Ratio K/L | 4.08 | 1.96 | 3.09 | 3.79 | 3.87 | 3.71 | 1.85 |
| dFLC (mg/L) | 71.6 | 25.9 | 28.5 | 39.6 | 43.0 | 28.0 | 8.3 |
| PBJ (mg/24h) | 502 | 484 | 260 | 257 | 275 | 219 | 270 |
Evolution of difference between involved and uninvolved serum free light chains (dFLC) and Bence Jones Proteinuria (BJP) during the treatment of the patient. MP: first line of treatment with Melphalan and Prednisone; VCD: second line of treatment with Bortezomib, Cyclophosphamide and Dexamethasone; VGPR: very good partial response.
The case presented illustrates the utility of sFLC in the diagnosis and monitoring of a patient with AL amyloidosis. At diagnosis, the International Myeloma Working Group (IMWG) guidelines recommends for the screening of monoclonal gammopathies a quick and easy protocol based on SPE, sIFE and sFLC, that enables a sensitive identification of the monoclonal component in the study of these patients. The addiction of sFLC analysis to SPE and sIFE avoids the inclusion of urine studies in the screening protocol. When the diagnosis of plasma cell disorder is made, a 24-h urine study is required for all patients. Nonetheless, in patients with a suspected AL amyloidosis, the study of 24-h urine IFE (uIFE) is essential in the protocol.4,5 In this particular patient, the abnormal sFLC ratio triggered the monoclonal gammopathy suspicion protocol and the presence of an altered sFLC ratio together with a positive BJP and the clinical findings conducted the patient study to a diagnosis of AL amyloidosis.
The measurement of sFLC concentrations had significantly changed the way that the patients with AL amyloidosis are diagnosed and monitored. The criteria for response of the haematologic disease is based on the measurement of sFLC concentrations. A measurable disease is defined as dFLC>50mg/L and covers approximately 85% of newly diagnosed patients where the elevated sFLC is the amyloid protein precursor.4,6 The described patient is a good example of measurable disease by sFLC with a dFLC of 71.6mg/L at diagnosis. AL amyloidosis patients with a dFLC<50mg/L, as described by Schonland et al. in a series of 359 newly diagnosed patients, should be considered as a distinct clinical entity with a small plasma cell clone that presents a favourable outcome.7
Due to their short serum half-life (2–6h), sFLC appears to be the most effective marker for evaluating the early effects of chemotherapy in AL amyloidosis8 and thereby, the dFLC should be used as biomarker for the evaluation of clonal response and progression.2 In the reported patient, the measurement of sFLC concentration was clearly abnormal at diagnosis and provided a measurable parameter for subsequent disease monitoring in contrast with the conventional electrophoretic assays (SPE and sIFE) where the monoclonal protein was undetectable at the diagnosis and during treatment follow-up.
Additionally, the revised prognostic staging system for AL amyloidosis incorporates the sFLC measurement to the cardiac biomarkers (NTpro-BNP and cTNT) used in the Mayo staging system for the risk in patients with AL amyloidosis. It is noteworthy that long-term outcome is dependent of the underlying plasma cell clone, so the incorporation of clonal characteristics based on dFLC may allow for better risk stratification.9,10 Importantly, the new criteria for treatment response and progression of the haematologic disease are based on sFLC data.3 Nonetheless, the revised prognostic staging system has not yet been validated.
In our patient, a rapid haematologic response was observed after two courses of treatment, with a dFLC reduction >50%, and achieving a status of VGPR with a reduction in the dFLC<40mg/L. After the fourth cycle of treatment, dFLC began to increase indicating a biological progression that lead the haematologist to change the treatment to VCD. Nevertheless, the progression could not be detected by BJP due to minimal changes in the measurement of monoclonal component in 24-h urine (Fig. 1). Currently, there are two ways of analysing free light chains; by measuring sFLC or by confirming the presence of BJP in urine. In our case, we found sFLC determinations to be a better option than BJP for monitoring the treatment of the patient. With the measurement of sFLC concentrations we avoid the additional problems associated with urine testing derived from low compliance and incorrect collection (sample not representative or non 24-h urine).11 Periodic monitoring of dFLC allows us to predict whether the patient responds correctly to chemotherapy or, conversely, has a relapse and need to change treatment. We support the findings of Kumar et al. that recommended the use of sFLC as primary marker for haematologic response assessment in patients with AL amyloidosis.12
In conclusion, the case presented highlight the importance of monitoring sFLC in patients with amyloidosis under treatment. The measurement of dFLC was the best assay that allows us the monitoring of treatment response in this patient whereas BJP was positive but ineffective to evaluate the response to the treatment. The difference between involved and uninvolved sFLC (dFLC) is an effective tool that helps the haematologists to evaluate the haematologic response to the treatment and the presence of biological progression.
Ethical disclosuresProtection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this study.
Patients’ data protectionThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
FundingNo funding received.
Conflict of interestsThe authors declare no conflict of interest.