Homocysteine (Hcy) is a nonessential amino acid which links the methionine and the folate cycles. Hcy levels are increased in genetic disorders, such as classic homocystinuria and methylmalonic aciduria combined with homocystinuria. Other monogenic, multifactorial diseases and physiological conditions are also associated with high Hcy levels. The aim of this study is to validate a method to quantify Hcy in plasma.
Material and methodsA method to quantify Hcy in plasma by HPLC was validated, by determining the following parameters: specificity, linearity, precision, accuracy, and detection and quantification limits according ICH and EMEA guidelines. Homocysteine was measured in 43 healthy individuals, 2 patients with high levels of methylmalonic acid, and a previously diagnosed patient with classic homocystinuria.
ResultsThe method was able to identify and quantify Hcy without interferences. A linear behavior was observed in a range of 6–100μM with r2=0.9967. The precision and accuracy studies showed variation coefficients under 6%. The limits of detection and quantification were 3.12μM and 6.25μM, respectively. The 43 healthy individuals studied had normal Hcy levels. The patient with elevated urinary methylmalonic acid levels and the homocystinuria patient showed high Hcy levels.
ConclusionThe method is valid for the quantification of Hcy in plasma as it fulfills the requirements for validation of analytical methods. Its introduction into diagnostic and follow up algorithms is important in genetic diseases where this amino acid is increased.
La homocisteína (Hcy) es un aminoácido no esencial que enlaza el ciclo de la metionina con el ciclo del folato. Los niveles de Hcy se incrementan en trastornos genéticos como la homocistinuria clásica y la aciduria metilmalónica combinada con homocistinuria. Otras enfermedades monogénicas, multifactoriales y condiciones fisiológicas han sido asociadas con altos niveles de Hcy. El objetivo de este estudio es validar un método para la cuantificación de Hcy en plasma.
Material y métodosSe validó un método para la cuantificación de Hcy en plasma por HPLC. Se determinaron los parámetros de validación: especificidad, linealidad, precisión, exactitud, límite de detección y cuantificación según las guías ICH y EMEA. El método se aplicó en 43 individuos sanos, 2 con ácido metilmalónico elevado y uno con diagnóstico previo de homocistinuria clásica.
ResultadosEl método permitió la identificación y la cuantificación de la Hcy sin interferencias. Se observó un comportamiento lineal en un rango de 6-100μM, con un r2=0,9967. En el estudio de precisión y exactitud se obtuvieron coeficientes de variación inferiores al 6%. Los límites de detección y cuantificación fueron de 3,12 y 6,25μM, respectivamente. Los 43 individuos sanos mostraron niveles normales de Hcy. Uno de los pacientes con ácido metilmalónico elevado presentó niveles aumentados, igual que el paciente con homocistinuria.
ConclusiónEl método validado para la cuantificación de Hcy en plasma cumple con los criterios para la validación de métodos analíticos. Su introducción en los algoritmos de diagnóstico y seguimiento es importante en las enfermedades genéticas que cursan con incremento de este aminoácido.
Homocysteine (Hcy) is an intermediary in the metabolism of amino acids with sulfhydryl groups, and links the methionine cycle with the folate cycle. Hcy is synthesized from methionine, by transference of methyl groups. The metabolism of this nonessential amino acid depends on the levels of B vitamins, including folate, cobalamin (B12), pyridoxine (B6) and riboflavin (B2). An imbalance between these cycles influences in Hcy concentration.1
Classic homocystinuria (OMIM 236200) is an autosomal recessive disorder of the metabolism of sulfur amino acids. It is caused by a deficiency in cystathionine β synthase (CBS), with a birth prevalence of 0.3/100000.2 This disease is characterized by myopia, lens dislocation, mental retardation, Marfanoid features and thromboembolic events, these symptoms usually appear in the first or second decade of life. Increased Hcy and methionine levels are present in the metabolic profile of these patients. From a phenotypic point of view, there are two variants: a severe form, unresponsive to pyridoxine treatment, and a milder form responsive to this treatment.3
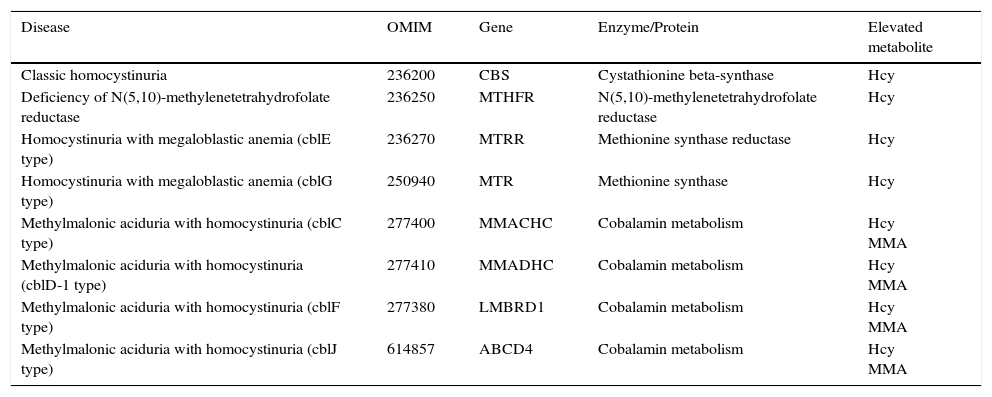
High Hcy levels are related to other genetic diseases,3 for example the N(5,10)-methyl tetrahydrofolate deficiency, the homocystinuria with megaloblastic anemia and other cobalamin defects that result in combined methylmalonic aciduria and homocystinuria (Table 1). In the last years, Hcy has been associated with other conditions, such as cardiovascular diseases,4 renal failure,5 pregnancy complications,6 osteoporosis,7 psychiatric diseases,8 neurology diseases9 and cancer.10
Genetic diseases associated to elevated levels of homocysteine.
| Disease | OMIM | Gene | Enzyme/Protein | Elevated metabolite |
|---|---|---|---|---|
| Classic homocystinuria | 236200 | CBS | Cystathionine beta-synthase | Hcy |
| Deficiency of N(5,10)-methylenetetrahydrofolate reductase | 236250 | MTHFR | N(5,10)-methylenetetrahydrofolate reductase | Hcy |
| Homocystinuria with megaloblastic anemia (cblE type) | 236270 | MTRR | Methionine synthase reductase | Hcy |
| Homocystinuria with megaloblastic anemia (cblG type) | 250940 | MTR | Methionine synthase | Hcy |
| Methylmalonic aciduria with homocystinuria (cblC type) | 277400 | MMACHC | Cobalamin metabolism | Hcy MMA |
| Methylmalonic aciduria with homocystinuria (cblD-1 type) | 277410 | MMADHC | Cobalamin metabolism | Hcy MMA |
| Methylmalonic aciduria with homocystinuria (cblF type) | 277380 | LMBRD1 | Cobalamin metabolism | Hcy MMA |
| Methylmalonic aciduria with homocystinuria (cblJ type) | 614857 | ABCD4 | Cobalamin metabolism | Hcy MMA |
Hcy: homocysteine; MMA: methylmalonic acid.
In Cuba, classic homocystinuria is diagnosed by a qualitative test using silver nitroprusside, which is specific but insensitive. This method does not detect the less severe forms of classic homocystinuria, or other diseases with high Hcy levels. The aim of this study is to validate a chromatographic method to quantify Hcy in plasma. This method allows introducing the Hcy quantification in the diagnosis and follow up of diseases associated with high levels of this amino acid. Also, it will be used in future studies to estimate reference intervals of the Cuban population.
Materials and methodsThe validation and application of the method for Hcy quantification were performed in the Laboratory of Biochemical Genetics at the National Center of Medical Genetics of Cuba in a year period.
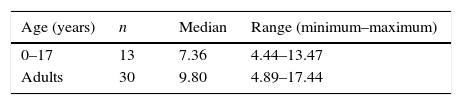
ControlsHealthy infants and children from the William Soler Pediatric Center (Havana, Cuba) were selected under the same inclusion criteria: healthy infants, weight between 10th and 90th percentile for boys and girls. Infants were excluded if they had malformations; congenital coronary or vascular, gastrointestinal, renal, hepatic, metabolic or neurologic diseases; evidently pathological biochemical markers; and hemolytic plasma. A total of 13 children from 4 months to 17 years old (7 female and 6 male) were included.
Adult negative controls were healthy volunteers tested at the National Center of Medical Genetics during routine care. The subjects were excluded if they referred smoking habit, regular intake of alcohol, diseases such as renal failure, severe psoriasis, cancer, acute lymphoblastic leukemia, lupus erythematosus, diabetes mellitus, organ transplantation, obesity, acute inflammatory response, anemia, vitamin B12 deficiency or showed symptoms of malnutrition. Also, individuals under consumption of drugs such as methotrexate, phenytoin, carbamazepine, oral contraceptives or other hormone, nicotinic acid and cyclosporine were excluded. A total of 30 adults (21 female and 9 male) were included.
PatientsWe analyzed samples from two masculine patients (5 and 6 months of age), where the urine organic acid profile showed elevated methylmalonic acid (performed by Gas Chromatography coupled to Mass Spectrometry in our laboratory), and a 24 years old masculine patient with classic homocystinuria under treatment. This patient was diagnosed previously by the silver nitroprusside test and its clinical features.
In all cases we required the informed consent from the subjects participating as negative controls, patients or their legal tutors.
Collection of blood samplesBlood samples from patients and controls were collected after more than 6h fasting by venipuncture into EDTA tubes (Vacutainer, Portugal), and were kept in ice bath immediately. Plasma was separated by centrifugation at 35000g (Kukusan H-103N) for 10min and stored at −20°C until analysis.
ReagentsThe standard of dl-homocysteine, ethylenediaminetetraacetic acid (EDTA), albumin, potassium dihydrogen phosphate (KH2PO4) and dithiothreitol (DTT) were supplied by Sigma–Aldrich (USA). Acetonitrile (Acn), hydrochloric acid (HCl), 7-fluorbenzo-2-oxa-1,3-diazole-4-sulfonic acid (SBD-F), orthophosphoric acid, trichloroacetic acid (TCA), HPLC water, disodium-tetraborate anhydrous (Na2B4O7), sodium hydroxide (NaOH), were provided by Merck (Germany).
Standard solutionsHcy solution (2mM) and albumin solution (40g/L) were prepared in distilled water. Both solutions were stored at −4°C.
Sample preparationA modification of the method published by Sawula et al. was used.11 Briefly: 100μL of plasma samples were added to 20μL of DTT (500mM) and incubated at room temperature for 30min. Deproteinization was achieved by the addition of 100μL of TCA 10% with EDTA (1mM). Precipitated proteins were removed by centrifugation 10min at 10000g (Eppendorf 5415D). The supernatant (100μL) was mixed with 20μL of NaOH (1.55M), 240μL of borate buffer solution (0.125M, pH=9.5) in 4mM EDTA and 20μL of SBD-F (1mg/mL in borate buffer). Samples were incubated in the darkness at 60°C for 60min. After derivatization, the samples were acidified with 20μL of HCl (6N) and kept refrigerated until HPLC injection.
Chromatographic conditionsThe analysis was performed on a Shimadzu LC consisting of a LC-20AT quaternary pump, a RF-10AXL fluorescence detector, a SIL-20A sample autoinjector and a CTO-20AC oven. LC Solution software (ver 1.25 SP 3 2011 Shimadzu, Japan) was used for data acquisition and processing of signals. Chromatographic separation was carried out on a LiChrospher RP-18×250mm (Merck) column with 5μm of particle size. The HPLC analysis was performed isocratically in reverse phase with KH2PO4 (20mM, pH=2.1): ACN (5%). The flow rate was 1mL/min, the column temperature was 30°C, the injection volume was 50μL and the total analysis time was 20min. The fluorescence detector was set at excitation/emission of 385/515nm.
Method validationThe method was validated according to the documents of the International Conference on Harmonisation (ICH) and European Medicines Agency (EMEA) for the validation of analytical methods.12,13 Different analytical validation parameters such as selectivity, linearity, precision, accuracy and limit of detection and quantification were analyzed.
SelectivityThe selectivity was determined by the analysis of blank samples (plasma and albumin) and samples (plasma and albumin, n=9) prepared by adding 100μM of Hcy. The chromatograms of both matrixes were compared to discount the interferences. The Hcy retention time (tr) was established.
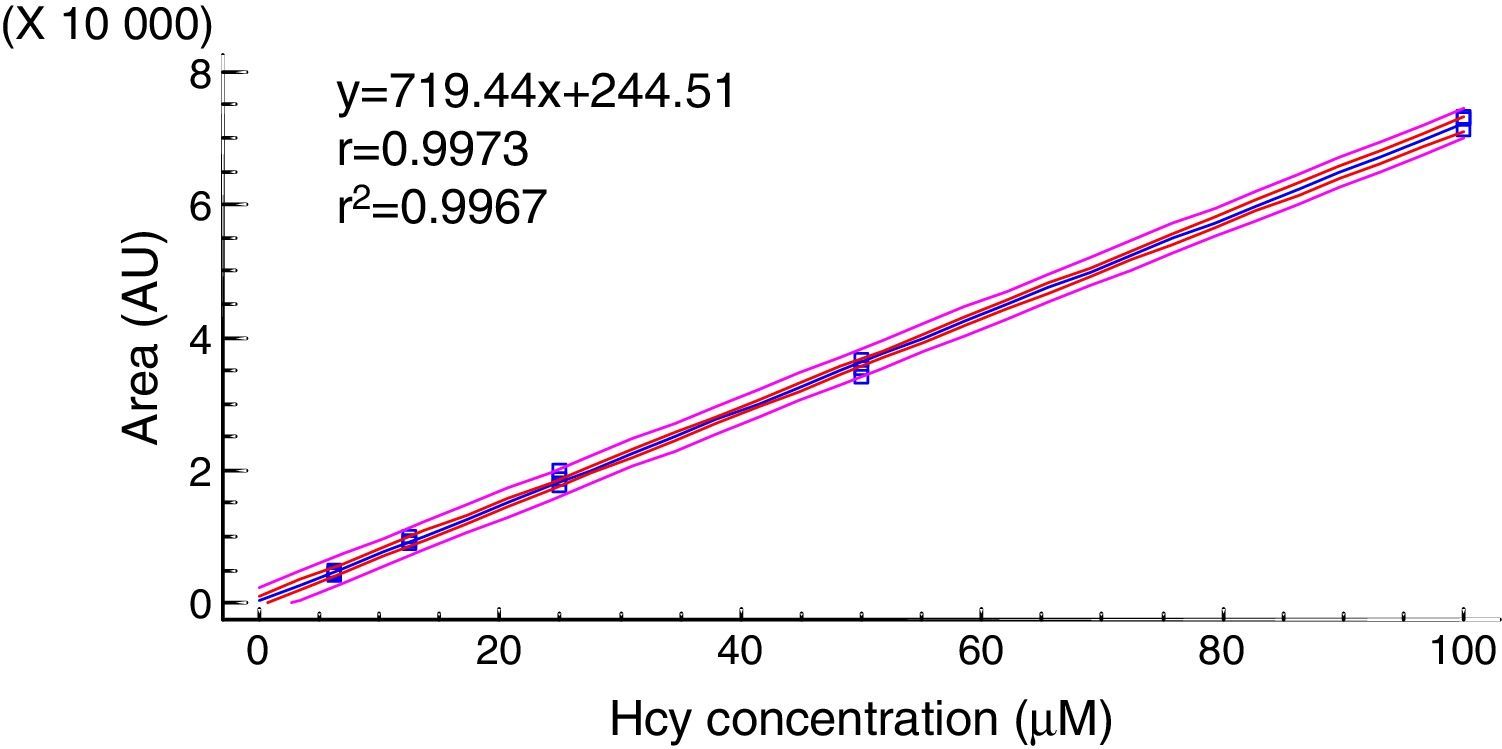
LinearityThe linearity was studied in a range of concentrations to 6–100μM (6, 12, 25, 50 and 100μM) in albumin (40g/L), from the Hcy standard solution (2mM). Each sample was analyzed in triplicate. The calibration curves were obtained by the analysis of least squares linear regression of the Hcy peak area vs. the Hcy concentration. The mean, standard deviation (SD) and coefficient of variation (CV) were calculated for each concentration. Besides, we estimated the values of the slope and intercept, correlation and determination coefficients. The slope of the calibration curve was used to calculate Hcy concentration. The values under 15μM were considered normal.1,14
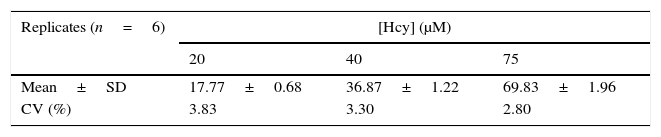
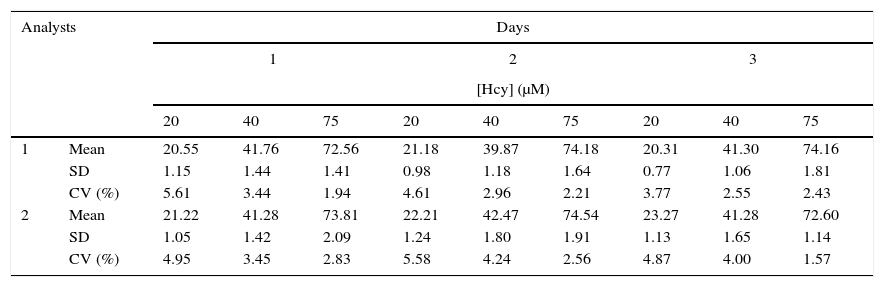
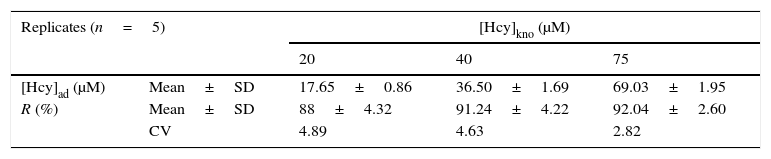
Precision and accuracyThe intra-assay (within day) precision included the analysis of 5 determinations of three known concentrations (20, 40 and 75μM). While in the inter-assay (between days) precision, we determined the variation of the results by considering two analysts on three different days. The determination of these parameters was assessed by the CV of the Hcy peaks. Accuracy was estimated from recovery factors (R) obtained from the analysis of 5 determinations of three known concentrations (20, 40 and 75μM). The mean, SD and CV of R were determined.
Matrix effect and carry overTo measure the matrix effect, the matrix factor was evaluated by comparing the presence and absence of Hcy in the albumin and plasma at three concentration levels. The carry over analysis was assessed by injecting blank sample after a high Hcy concentration sample and before the analysis of the next sample.
Detection and quantification limitsThe limit of detection (LOD) and quantification (LOQ) were determined from the analysis of serial dilutions of the lower concentration aliquot prepared during the linearity study. Both limits were estimated from the signal/noise ratio (S/R).
Dilution effectDilution effect was analyzed by spiking blank albumin solution with Hcy solution (2000μM) at dilution factors of 2 in six replicates.
StabilityThe Hcy stability in the albumin matrix was conducted at the two concentration levels (12 and 50μM, n=3) in two storage conditions.
The freeze and thaw stability was evaluated by analyzing Hcy samples after three freeze–thaw (−4°C temperature) cycles on three consecutive days. The stability of the Hcy fluorogenic derivative compound was studied by analyzing prepared samples at 25°C for 48h.
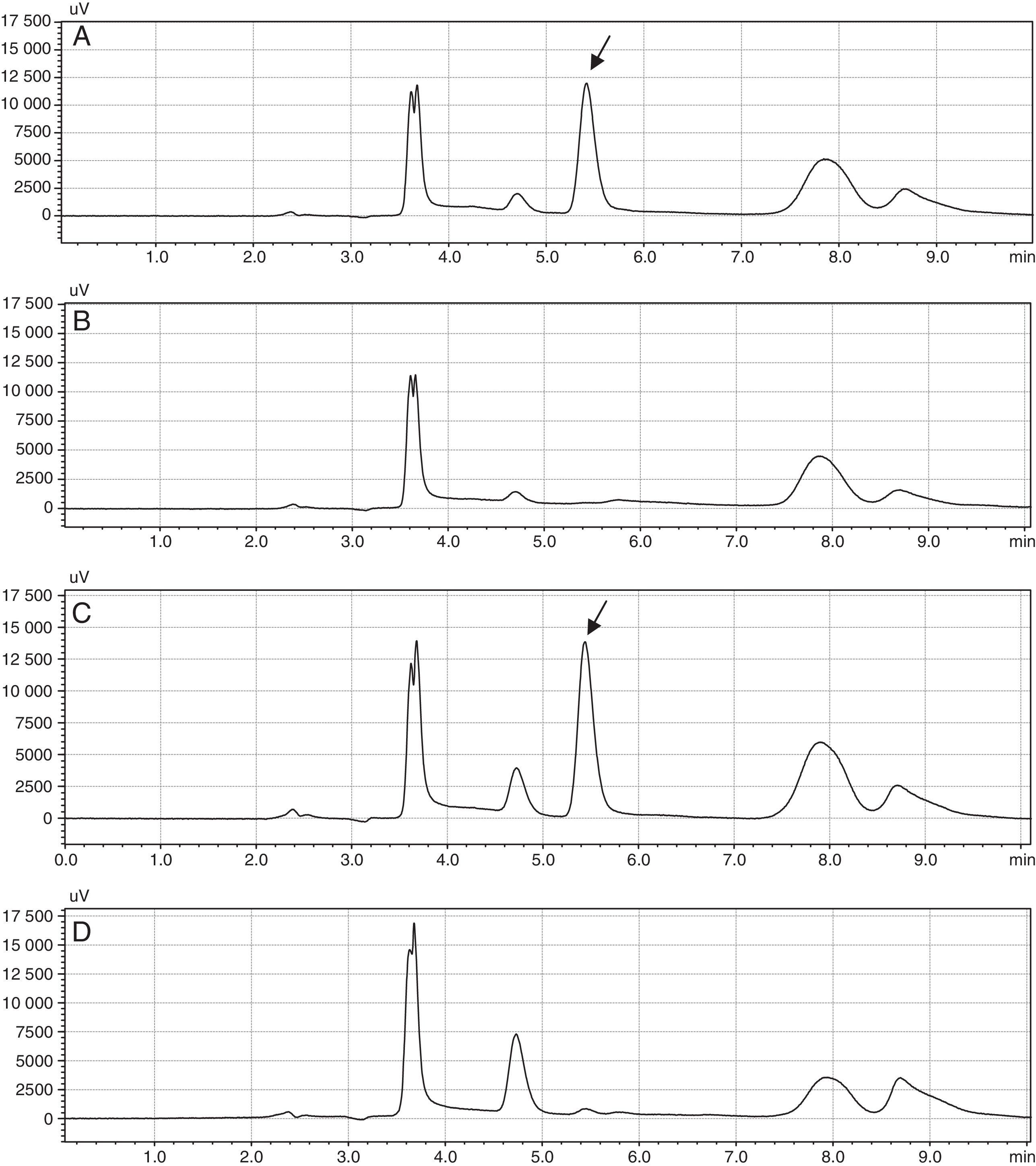
ResultsIn the specificity study, we analyzed nine chromatograms of samples of plasma and albumin with Hcy and without Hcy. The Hcy was identified with a retention time of 5.4min (Fig. 1), while the DTT was observed at 4.7min.
The linearity was determined graphically from the areas corresponding to peak of Hcy and their concentrations (Fig. 2). Both variables showed a linear relationship.
In the precision study, the intra-assay precision showed CV values under 5% (Table 2). The inter-assay precision showed CV values lower than 6% (Table 3). Accuracy was estimated from the obtained R (Table 4). The R was greater than 85% and the CVs were under 5%. The limits of detection and quantification were 3.62μM and 6.25μM, respectively.
Inter-assay variance in three homocysteine concentrations.
| Analysts | Days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||||
| [Hcy] (μM) | ||||||||||
| 20 | 40 | 75 | 20 | 40 | 75 | 20 | 40 | 75 | ||
| 1 | Mean | 20.55 | 41.76 | 72.56 | 21.18 | 39.87 | 74.18 | 20.31 | 41.30 | 74.16 |
| SD | 1.15 | 1.44 | 1.41 | 0.98 | 1.18 | 1.64 | 0.77 | 1.06 | 1.81 | |
| CV (%) | 5.61 | 3.44 | 1.94 | 4.61 | 2.96 | 2.21 | 3.77 | 2.55 | 2.43 | |
| 2 | Mean | 21.22 | 41.28 | 73.81 | 22.21 | 42.47 | 74.54 | 23.27 | 41.28 | 72.60 |
| SD | 1.05 | 1.42 | 2.09 | 1.24 | 1.80 | 1.91 | 1.13 | 1.65 | 1.14 | |
| CV (%) | 4.95 | 3.45 | 2.83 | 5.58 | 4.24 | 2.56 | 4.87 | 4.00 | 1.57 | |
CV: coefficient of variation, [Hcy]: homocysteine concentration, SD: standard deviation.
Analytical recovery of homocysteine in albumin.
| Replicates (n=5) | [Hcy]kno (μM) | |||
|---|---|---|---|---|
| 20 | 40 | 75 | ||
| [Hcy]ad (μM) | Mean±SD | 17.65±0.86 | 36.50±1.69 | 69.03±1.95 |
| R (%) | Mean±SD | 88±4.32 | 91.24±4.22 | 92.04±2.60 |
| CV | 4.89 | 4.63 | 2.82 | |
CV: coefficient of variation, [Hcy]kno: known homocysteine concentration, [Hcy]ad: added homocysteine concentration, R: recovery factor, SD: standard deviation.
The matrix factors in plasma and albumin were under 10%. No carry over was observed in blanks injected after a high Hcy concentration control. Diluted Hcy samples with six replicates were determined after dilution to the concentration of 2000μM, and the results of the tested samples were under 8%.
Stability study indicated that the Hcy solution in albumin is stable, after undergoing three freeze–thaw cycles. The CVs obtained of two levels were 0.82 (12μM) and 2.78 (50μM). Besides, we observed a lost response of the Hcy derivative compound when samples were stored at 25°C for 48h.
Table 5 shows the Hcy values obtained in samples from healthy infants, children and adults. One patient with high methylmalonic acid levels showed also high homocysteine levels (22.81μM) while the other had normal Hcy values (7.12μM). The patient with classic homocystinuria had a plasmatic Hcy level of 41.37μM.
DiscussionSeveral methods have been described for quantification of Hcy in the laboratory. HPLC is one of the most used technologies, coupled with UV/Visible detection,15 fluorescence,11 electrochemical16 and mass spectrometry (MS).17 Also, the gas chromatography coupled to MS and tandem mass spectrometry have been utilized.18 The selection of a particular method depends on the required sensitivity and equipment available in the laboratory.
Each of these methods has limitations in terms of equipment cost, complexity, processing time per sample and number of metabolites simultaneously analyzed. Ultraviolet detection presents low sensitivity and specificity, while the loss of selectivity is one of the disadvantages of electrochemical detection.19 Furthermore MS detection is expensive and it has a good precision but a superior technical complexity. MS/MS requires deuterated patterns, while immunoenzymatic methods do not allow multiple simultaneous determination of thiols and generally there are commercial kits with spectrophotometric detection.20 In the other hand, HPLC with fluorescence detection is very used in the quantification of thiols by its high sensitivity, relative simplicity, easy automation and high performance.11,21–23 The developed method allows the determination of Hcy fulfilling with the above criteria.
Fowler (2008) presents a protocol with similar conditions of sample treatment and analysis, but with a different reducing agent. Other methods employ gradient separation with similar chromatographic run time (20–25min).11,24 Hcy is unstable in biological matrixes due to oxidation or conjugation and therefore it exists in multiple forms: the most abundant is linked by disulfide bonds to albumin, while the rest circulates freely, or linked to cysteine or to another molecule of Hcy. Therefore Hcy determinations require the application of reducing agents to obtain the free amino acid.25 There are various reducing agents, the most useful for the quantification of Hcy are phosphines,26,27 although some authors demonstrated the use of DTT with equal end.15,28 The developed method in this work uses this reducing agent, which allows the release of Hcy for their sulfhydryl groups, enabling their quantification. Furthermore, there is not interference because it elutes with a different retention time to Hcy (Fig. 1).
Derivatization agents allow the detection of compounds and enhance separation efficiency. Within agents with benzofurazan structures (2,1,3-benzoxadiazol), the SBD-F is the most used in the determination of Hcy and other thiols. It is a compound of small molecular size but their reactivity with the compounds of interest is high. Also it generates derivatives which are moderately hydrophobic, facilitating their separation in reverse phase column. This derivatization helps to avoid interference from biological matrixes because the wavelengths of excitation and emission of the formed derivatives are long.29 In the chromatograms obtained, the peak corresponding to Hcy was observed with an appropriate resolution (Fig. 1).
The comparative analysis between plasma and albumin, matrix used for preparing the calibration curve, did not showed differences; therefore its use in Hcy quantification is acceptable.
The calibration curve obtained showed a linear relationship between the Hcy concentration and the fluorescence signal. The coefficients of regression and determination showed a good correlation between the peak areas and Hcy concentration.
In the study of precision, the CVs obtained in the intra-assay and inter-assay precision were under 6% and the upper limit accepted by international guides (15%). Besides the R presented values over 85%, values accepted when working with biological fluids. These results indicate that the method is precise and accurate for the quantitation of Hcy.
The matrix factor obtained was under 15%, acceptable criteria of validation guidance.13 Therefore, the matrix components do not alter of the Hcy response.
In addition, LOQ and LOD obtained were lower than the cutoff points described in the literature (<15μM normal value and >50μM pathological value for classic homocystinuria), so the method can be used to quantify Hcy if a classic homocystinuria is suspected.30
Samples from healthy children and adults, quantified through this method, showed values bellow the upper normal limit described by other authors. However, reference intervals of the Cuban population are unknown, and the amount of subjects analyzed is not enough to establish them.
Also, the patient with classic homocystinuria presented values over the upper normal limit (15μM).1,14,30 This value is under 100μM (typical value of an untreated patient with classic homocystinuria) because the patient is under medical treatment and he is responsive to pyridoxine.
In one patient with increased methylmalonic acid, an increment in Hcy level was observed, while in the other, Hcy values were normal. The observed increment in the first case can be related to a homocystinuria combined with methylmalonic aciduria or other pathological condition such as vitamin B12 deficiency, folate deficiency or a renal failure.14 The isolated increment of methylmalonic acid in the second patient can be related to an isolated methylmalonic aciduria. In both cases, further tests are necessary to discard an inborn error of metabolism.
The use of this Hcy quantification method in patients and healthy subjects showed that its application is useful for diagnosing and following-up of Hcy related genetic disorders.
In conclusion, the proposed chromatographic method for the quantification of homocysteine by HPLC in plasma samples fulfills with the criteria established for validation of analytical methods. This method can be used to establish reference intervals in the Cuban population and should be included in the diagnosis and follow-up algorithms of classic homocystinuria and other multifactorial diseases.
FundingNational Center of Medical Genetics.
Conflicts of interestsThe authors do not have conflicts of interests.