The covid-19 disease (coronavirus disease 2019) is a novel disease causing a world pandemic. Its presentation varies from an asymptomatic infection to a pneumonia with acute respiratory distress syndrome. We present a case presenting initially as a covid-19 pneumonia together with a disseminated intravascular coagulopathy consisting of arterial and venous thrombosis in different locations and a shock requiring admission in the intensive care unit. The abnormal coagulation test in covid-19 patients have been described since the first cases observed in Wuhan, China, as well as an increased incidence of venous thrombosis. On the contrary, a higher incidence of arterial thrombosis has not been described in these patients. The unusual case we present could be a manifestation of this altered tests.
La enfermedad covid-19 (coronavirus disease 2019) es una infección de reciente aparición que está causando una pandemia a nivel mundial. La forma de presentación varía desde una infección asintomática hasta una neumonía con síndrome de distrés respiratorio. Presentamos el caso de un paciente que presentó una neumonía por covid-19 junto a una coagulación intravascular diseminada con trombosis arterial y venosa en múltiples localizaciones y un estado de choque que requirió ingreso en unidad de cuidados intensivos. La alteración de las pruebas de coagulación en pacientes afectos de covid-19 se ha descrito desde los primeros casos observados en Wuhan, China, así como una mayor incidencia de trombosis venosas. Al contrario, una mayor incidencia de trombosis arterial no ha sido descrita en estos pacientes. El caso inusual que presentamos podría representar una manifestación de estas alteraciones.
In December 2019, the emergence of a new coronavirus capable of infecting the human species and causing the COVID-19 disease was reported in Wuhan, China.1 Different clinical manifestations have been described, ranging from asymptomatic infection to pneumonia with respiratory failure that can lead to death, and including mild upper respiratory tract infections.2 Critical care units have been forced to care for many patients affected by COVID-19 during the current pandemic. We report the case of a patient with COVID-19 treated in our unit presenting in the form of disseminated intravascular coagulation (DIC) with shock.
Case reportA 56-year-old man with a history of overweight (body mass index27.7kg/m2), high blood pressure under medical treatment (hydrochlorothiazide 25mg/24h and lisinopril 10mg/24h) and peripheral venous insufficiency treated with surgery. He reported a 3-day history of acute mechanical low back pain that did not improve with conservative treatment, followed by 2 episodes of diarrhoea, with no cough or fever. He presented at the emergency room complaining of general malaise, sweating, abdominal pain and a feeling of loss of strength and sensitivity in the lower extremities. Upon arrival, he was afebrile with hypotension, tachycardia, sweating, and tachypnoea, with SaO2 90% (FiO2 21%), signs of hypoperfusion with cold extremities, delayed capillary filling and acrocyanosis, left basal hypophonesis on auscultation, but no findings of note on abdominal and neurological examination.
The chest radiograph revealed bilateral interstitial pulmonary infiltrate with left pleural effusion. The electrocardiogram and bedside echocardiography showed no abnormalities. Labs showed elevated inflammatory parameters (C-reactive protein 22.76mg/dl, procalcitonin 2.74mg/dl, and leukocytes 25,930/mm3 with neutrophilia), high sensitivity troponin I of 224.7ng/mL (normal range <45.2ng/mL), creatinine 2.36mg/dl and lactate 28.8mg/dl. The initial blood gas showed mild respiratory alkalosis with a preserved pH of 7.409 and a PaO2 of 48.6mmHg (PaO2/FiO2 ratio 231). Coagulation tests were slightly impaired, with a prothrombin time (PT) of 13.7s (73.1%) and an activated partial thromboplastin time (aPTT) of 27.1s, with thrombocytopaenia of 45.000×103/mm3, elevated D-dimer (above the analytical detection limit of 10,000ng/mL) and fibrinogen of 4.5g/l. Haemoglobin was normal (17.1g/dl).
Invasive monitoring and resuscitation with fluids, oxygen, and vasoactive drugs was started. Due to suspicion of aortic dissection, an abdominal and chest computed tomography (CT) angiography was performed (Figs. 1 and 2), which ruled out this suspicion, but multiple filling defects were found in the arterial vascular system attributable to non-occlusive thrombi (aortic arch, middle portion of the splenic artery, distal portion of the inferior mesenteric artery, aorto-iliac bifurcation, left common iliac, right external iliac artery, right common femoral artery, and right deep femoral artery). Other findings were acute pulmonary embolism (PE) in the lobar and segmental branches of the middle lobe and in the basal segmental branches of the right lower lobe; portal vein and distal splenic thrombosis; multiple splenic infarcts; and haematomas of the adrenal glands. In addition, as suggested by the chest radiograph, he presented a left pleural effusion and patchy bilateral ground glass opacity with areas of consolidation and atelectasis, suggestive of an infectious process.
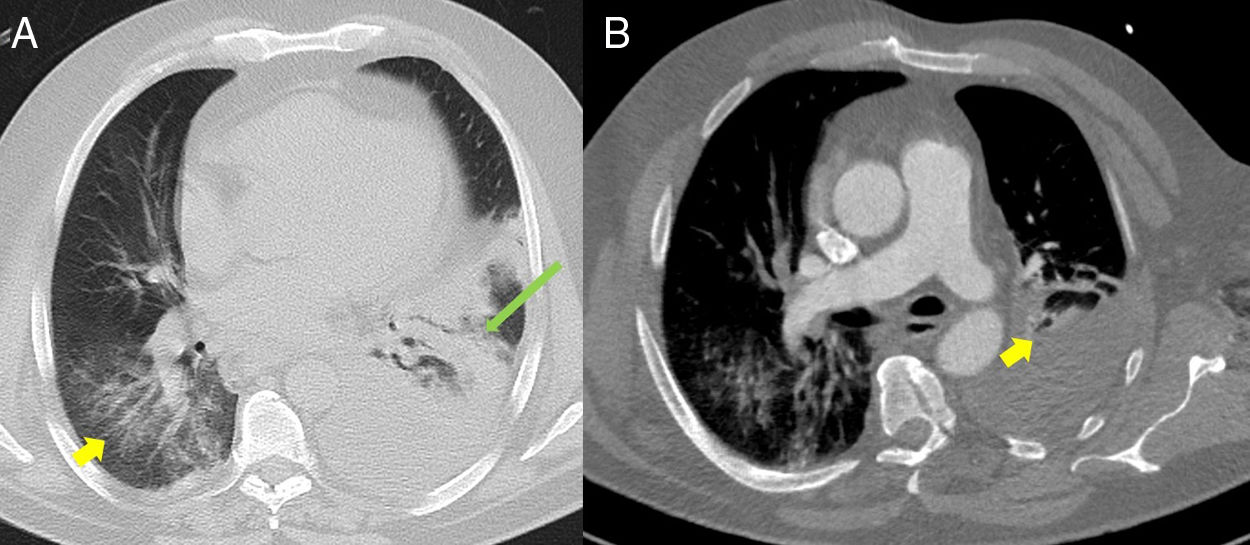
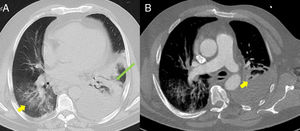
(A) Baseline computed tomography (CT) axial lung window at the level of the descending thoracic aorta, showing bilateral ground glass opacities predominantly in the lower lobes (yellow arrow), associated with an area of consolidation/atelectasis with an air bronchogram in the left lower lobe (long green arrow). Moderate to severe left pleural effusion. (B) Lung CT angiography, axial mediastinal window, showing filling defects in the lobar and segmental branches of the middle lobe of the pulmonary artery (yellow arrow) compatible with acute pulmonary thromboembolism. Moderate to severe left pleural effusion.
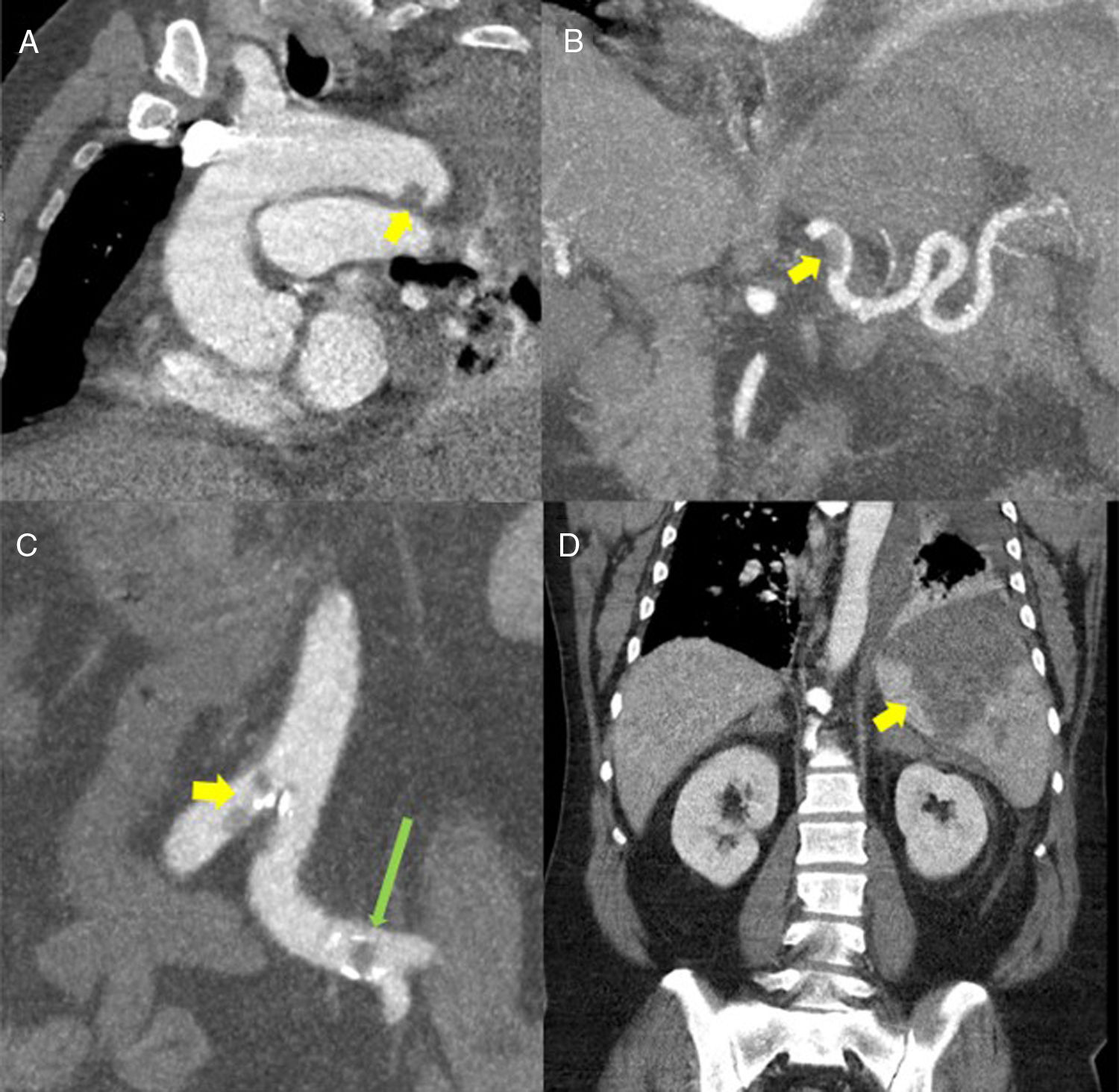
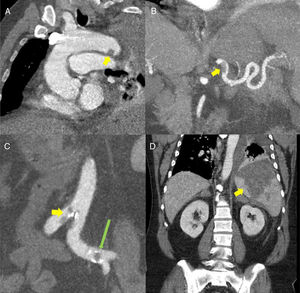
(A) Oblique multiplanar reconstruction (MPR) of contrast-enhanced chest computed tomography (CT) showing a filling defect measuring about 12mm in the distal portion of the aortic arch, suggestive of thrombosis (yellow arrow). (B) Coronal maximum intensity projection (MIP) reconstruction of contrast-enhanced abdominal CT, showing a filling defect in the proximal/middle portion of the splenic artery, suggestive of thrombosis (yellow arrow). (C) Coronal MIP reconstructions from contrast-enhanced abdominal CT showing filling defects in the aorta at the iliac bifurcation (yellow arrow) and in the left common iliac arteries (long green arrow). (D) MPR reconstruction of contrast-enhanced abdominal CT coronal slice showing a large triangular hypodense area in the spleen, consistent with splenic infarction (yellow arrow).
On the basis of these images, he was initially diagnosed with a prothrombotic process of uncertain origin, suggestive of infection by COVID-19 in the current epidemiological context. Microbiological tests were obtained, and empirical broad-spectrum antibiotic therapy, specific antiviral treatment (lopinavir/ritonavir, hydroxychloroquine, and azithromycin) and anticoagulation with sodium heparin for a target aPTT of 50–70s were started Polymerase chain reaction for COVID-19 confirmed the diagnosis. The patient was transferred to the intensive care unit (ICU).
At admission he was receiving vasoactive support (noradrenaline 0.6μg/kg/min), and ventilatory support was started with high flow nasal oxygen therapy at 50l/m with FiO2 0.5. Treatment with sodium heparin was continued, and later switched to low molecular weight heparin 1.5mg/kg/day after target levels of aPTT had not been achieved. The patient made good progress; oxygen requirements were reduced and vasoactive support was discontinued. Renal failure resolved after the initial hypoperfusion had resolved. He only presented fever on the first day of admission to the ICU, which was controlled with intravenous paracetamol. Antibiotic therapy was discontinued given the improvement in inflammatory lab parameters and negative cultures. The patient completed a 5-day course of hydroxychloroquine, but lopinavir/ritonavir was discontinued due to diarrhoea and gastric discomfort. Finally, parenteral nutrition was started due to the presence of thrombosis in the inferior mesenteric artery, following which oral nutrition was gradually re-introduced with good tolerance. Haemoglobin fell to 10g/dl. This was initially attributed to possible mesenteric ischaemia, even though no blood was observed in the stools, there were no signs of melenic stools, and the abdominal examination was normal at all times.
Regarding coagulation tests, PT reached maximum levels of 16.2s (53.7%) on the first day of admission to the ICU, and aPTT only reached 40.3s, despite heparin therapy. D-dimer was above the upper limit of detection during his stay in the ICU. Platelets recovered progressively and reached normal levels 2 days after admission. Antiphospholipid antibodies tests were negative. No other thrombophilia studies were performed given the patient’s acute thrombosis, and anticoagulation will be administered on an outpatient basis.
At the time of writing, the patient remains in the general ward.
DiscussionWe present a case of COVID-19 with an atypical presentation in the form of low back pain and DIC with non-occlusive multiple arterial thrombosis and PE associated with COVID-19 sepsis. The initial diagnosis was hampered by hypoperfusion with no fever or respiratory symptoms. Radiography and labs and the subsequent CT scan were key to the suspicion of infection, and the microbiological diagnosis was subsequently confirmed by a gene detection technique. We attributed the initial state of shock to COVID-19-induced sepsis, since the initial point-of-care echocardiogram showed preserved contractility with no segmental involvement and good biventricular function. There was also no evidence of a possible cause of hypovolaemia. The possibility of intestinal bacterial translocation due to portal and inferior mesenteric artery thrombosis cannot be ruled out. The presence of multiple arterial thrombi likely worsened the symptoms, even though they were all non-occlusive. PE probably played a minor role, as no dilation of the right ventricle was observed.
Given the patient's condition and the context of sepsis, DIC should be suspected.3 The patient met International Society of Thrombosis and Hemostasis4 criteria due to the initial platelet and D-dimer levels. The rapid recovery of platelets indicates that DIC, if present, could probably be effectively controlled with anticoagulation. Another possibility to take into account is an existing predisposition to hypercoagulability that would have been aggravated by COVID-19. Only antiphospholipid antibodies were measured during admission, and these were negative. A study of thrombophilia will probably be required after the acute symptoms, together with a follow-up imaging study.
This case was remarkable due to the extensive arterial and venous thrombosis observed. It was also interesting to observe the patient’s good progress with suboptimal anticoagulation levels during the first days of admission. Coagulopathy has been described as a frequent complication of sepsis and a contributor to a poor prognosis.3,5 In COVID-19 patients, specifically, there is some evidence that alteration of classic coagulation tests and elevation of fibrinogen degradation products indicate an increase in the severity and mortality of the disease.3,6 The mechanism behind this does not appear to differ from that of other types of sepsis, although it is not fully understood.6 Thrombocytopaenia has also been associated with a worse prognosis in these patients.7
However, it is not clear what role this coagulation alteration plays in the formation of thrombi in this disease.3 The risk, probably multifactorial, of venous thrombosis appears to increase in the context of a patient on long‐term bed rest with a significant inflammatory response.3,8 The experience of our hospital seems to confirm this propensity for venous thromboembolic disease and PE. In contrast, a similarly clear association with arterial thrombosis has not been described. As in any immobilised patient, and particularly in septic patients, antithrombotic prophylaxis with low molecular weight heparin is recommended, since its use even in patients who do not meet DIC criteria, has been shown to reduce the risk of mortality.9 If respiratory symptoms and coagulation parameters worsen, it would be prudent to suspect thrombosis and rule it out using an imaging technique.
ConclusionIn conclusion, this case raises the question of whether this new COVID-19 disease, in addition to altering coagulation tests (which predict severity and mortality), could require more aggressive prophylactic anticoagulation due to the increased risk of thrombosis, particularly venous but also arterial. Likewise, it can be useful to use additional tools to detect coagulation alterations that could go unnoticed in conventional coagulation tests in the initial stages of the disease, and the slightest suspicion of thrombosis must be ruled out by imaging.
Conflict of interestsThe authors declare no conflict of interest.
Please cite this article as: Comino-Trinidad O, Calvo A, Ojeda A, Mercadal J, Cornellas L, Ferrando C. Coagulación intravascular diseminada como forma de presentación de la enfermedad por coronavirus-19. Caso clínico. Rev Esp Anestesiol Reanim. 2021;68:41–45.