Patients with different pathologies of the temporomandibular joint can actually benefit from total joint replacement with prosthesis; a rare complication of this procedure is infection. We present a case and its management.
Case reportA 67-year-old woman with a diagnosis of osteoarthritis of the right TMJ, who had initially been under conservative treatment with poor results. An arthroscopy was made and revealed two meniscal perforations and severe chondromalacia; the patient continued with pain. Temporomandibular joint reconstruction with total joint prosthesis was discussed, and the patient accepted this approach.
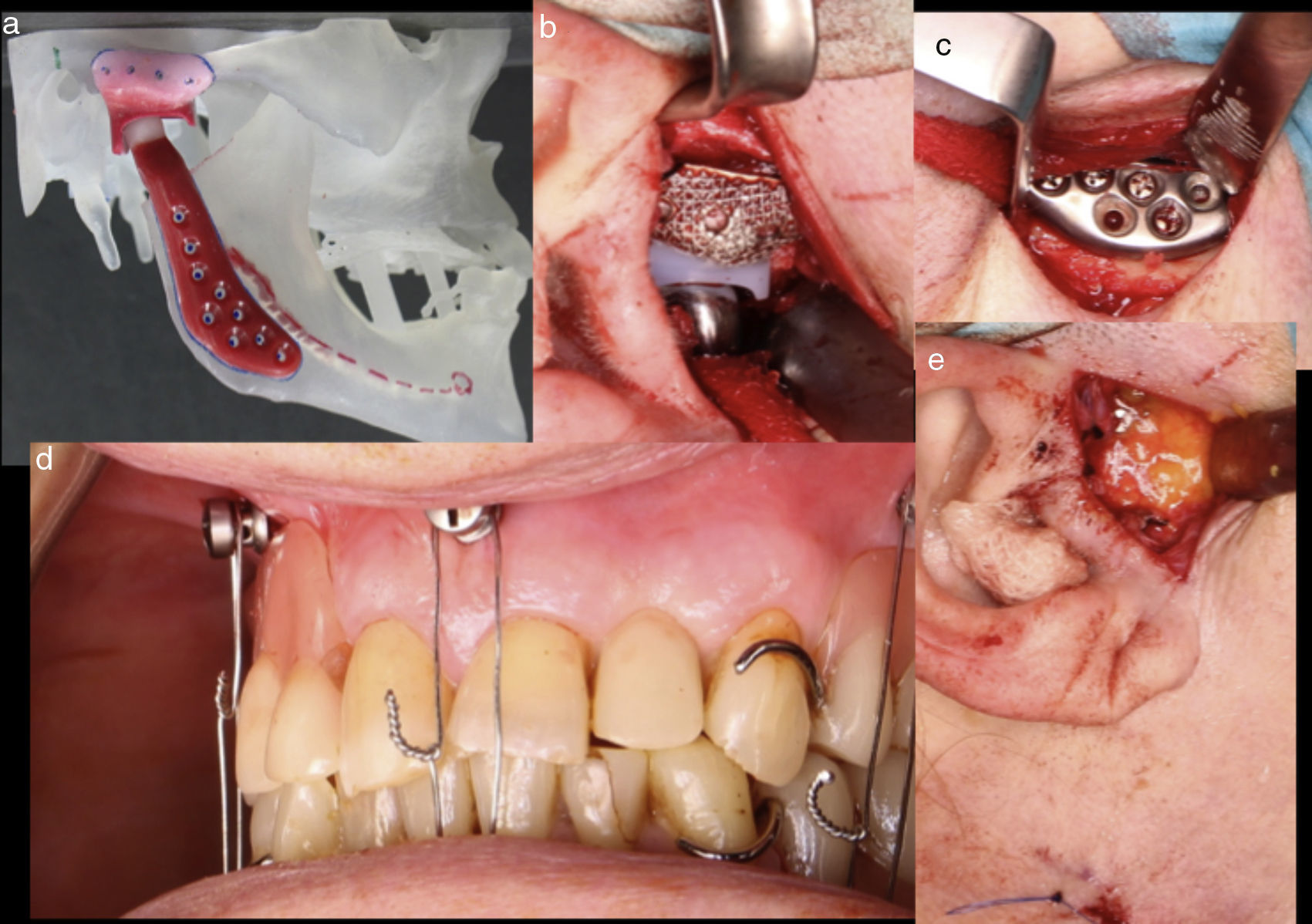
We performed a total Joint replacement according to the protocol established in our department (Fig. 1). The patient had i.v. antibiotic prophylaxis with clindamycin plus gentamicin and went under post-operative antibiotic regimen with clindamycin 300mg orally every 8h as usual when she was discharge.
The patient progressed, with minimal complaints at follow up visits for a period of two months. On the 60th postoperative day, the patient developed a right preauricular swelling, erythema and small abscess diagnosed by CT scan. She was not febrile with a normal WBC. A needle aspiration of cloudy fluid was submitted for culture and antibiotic sensitivity testing. The patient was admitted to the hospital for the administration of IV gentamicin and clindamycin because of a suspicion of surgical site infection. The original culture grew Staphylococcus epidermidis on the sixth post-admission day, and the antibiotic treatment was changed to vancomycin plus rifampicin, and after two weeks of treatment, the patient still continued with clinical signs of infection. Therefore, the patient was prepared for surgery, and we reopened the incisions, the surgical area was debrided and the biofilm was removed, material was submitted for culture and sensitivity.
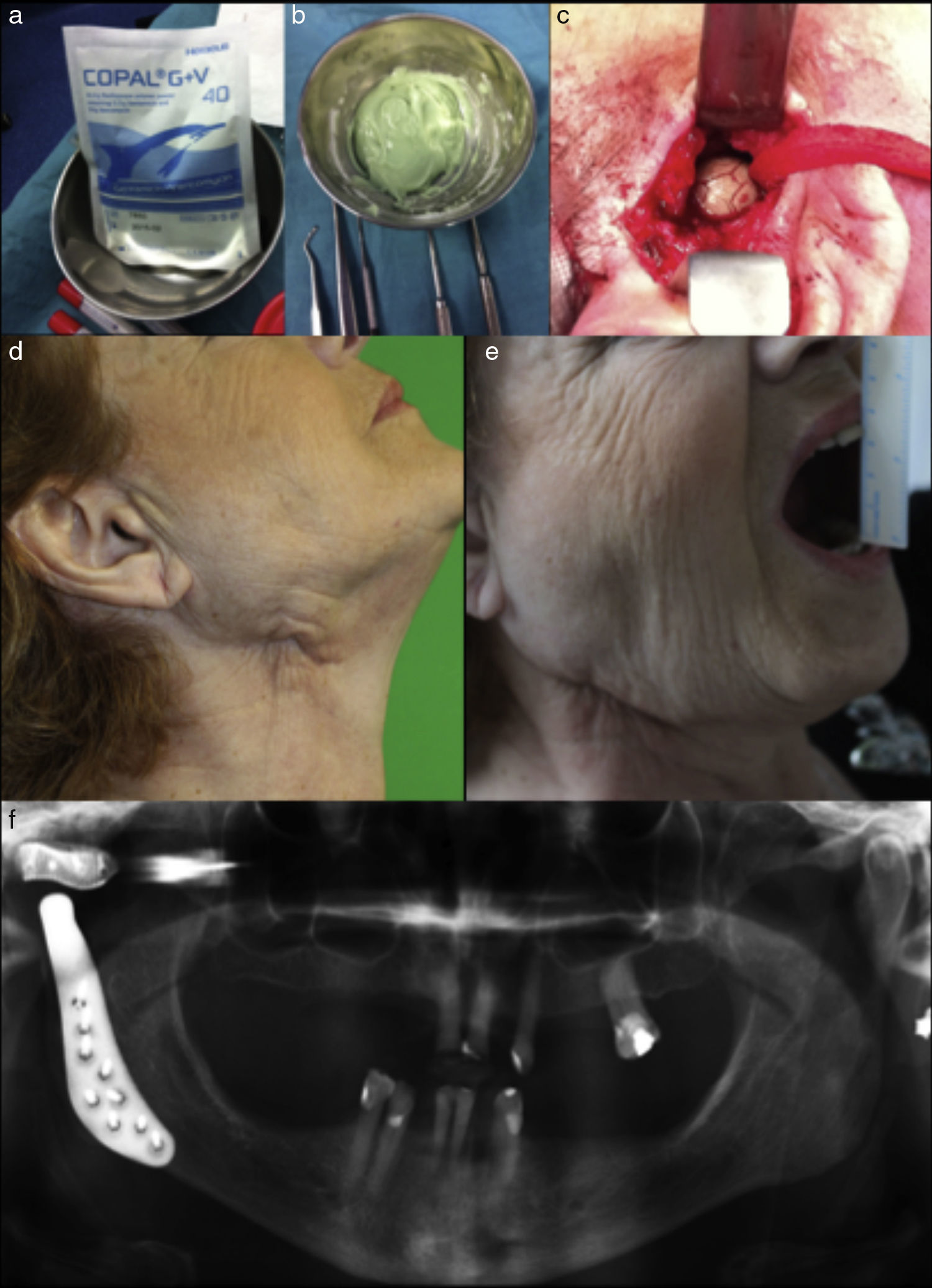
The culture of the material obtained intraoperatively showed growth of Proteus mirabilis, so we added piperacillin-tazobactam as a new antibiotic. However, even with the new antibiotic the infection continued. The patient underwent surgery to remove the infected prosthesis and debridement. Polymethyl methacrylate orthopedic cement, impregnated with 0.5g gentamicin and 2g of vancomycin (Fig. 2a and b), was placed to maintain the fossa space, eliminating the need of intermaxillary fixation (Fig. 2c).
(a) and (b) Polymethyl methacrylate orthopedic cement impregnated with antibiotic, (c) bone cement placed to maintain fossa space, (d and e) eleven months after new TMJ replacement free of symptoms with good mouth opening, and (f) radiographic control after replace a new custom made fossa and reimplant the condylar component.
She was treated for 10 more days with IV antibiotic treatment consisting of Vancomycin and piperacillin-tazobactam, and linezolid 600mg every 12h and ciprofloxacin 750mg every 12h for four more weeks. She returned to the operating room six months after finishing the antibiotic treatment to replace a new custom made fossa and reimplant the condylar component after sterilization by the manufacturing company. The patient is now symptom free eleven months after surgery (Fig. 2d–f).
DiscussionIn the human body infection can occur around any implanted device.1 Patient-related risk factors that contribute to infection susceptibility include poor soft tissue quality, number of previous surgeries, previous sepsis, diabetes mellitus, remote infection at surgery site or at a later time, sickle cell anemia, rheumatoid arthritis or other connective tissue and autoimmune diseases, concurrent neoplasm, tobacco or alcohol use, oral diseases, medications as immunosuppressant.1,2 Surgical-related risk factors include simultaneous bilateral arthroplasty, operative time longer than 160min and allogeneic blood transfusion.2
The reconstruction of the TMJ with prosthetics is not without complications and one of them is infection that has been reported between 1.3% and 1.6%.1–3
Biofilm plays an important role in prosthesis infection, because the bacteria attaches to this layer of biofilm; and micro-organisms within the biofilm are protected from host immune responses and exhibit a reduced susceptibility to antimicrobial agents as a result of the changes in metabolic processes and poor difusion.1–3 Antibiotics my affect the bacterial growth but fail to eradicate the bacteria embedded within the biofilm, large doses and longer antibiotic treatment times are needed, and often the device has to be removed to completely eradicate the infection.2,3 Extensive debridement with physical disruption of the biofilm is necessary to allow the antibiotics to penetrate and work more efficiently.1
The bacteria commonly associated with TMJ prosthesis infection are Staphylococcus aureus, S. epidermidis, Peptostreptococcus, and Pseudomonas aeruginosa.3
Polymethyl methacrylate orthopedic cement, impregnated with antibiotic is useful, to maintain fossa space, and to decrease the biofilm formation4 between the time of the infected TMJ prosthesis removal and the second stage replacement.
In conclusion, it is necessary to meet aseptic surgical protocols with the use of prophylactic antibiotic within 1h before surgical incision, intraoperative draping and operative techniques to prevent cross-contamination between the surgical sites and the oral cavity, intraoperative soaking of components and/or irrigation of the implant components with antibiotic or antibacterial solution, and post-operative antibiotic regimen.3 But, even if the TMJ prosthesis is infected, we have to make an early diagnosis with aggressive treatment, which may imply removal of the prosthesis when other measures fail.
Our patient had an infection requiring antibiotic treatment in addition to broad-spectrum aggressive removal of the prosthesis, with a favorable response, allowing for a second joint reconstruction, without complications.
The temporomandibular joint reconstruction with implants is not absent from complications and one of them is infection, which requires early diagnosis and aggressive treatment.
Ethical responsibilitiesProtection of people and animalsThe authors state that no experiments were performed on human beings or animals as part of this investigation.
Data confidentialityThe authors state they have followed the protocols of their workplace with regard to the data publication of patients.
Right to privacy and informed consentThe authors have obtained the informed consent from the patients or subjects referred to in the article. This document is in possession of the corresponding author.
Conflict of interestThe authors declare that there are no conflicts of interest.