Pleural effusion is a possible complication of the thoraco-abdominal approach to the spine. It is more commonly a reactive effusion, but it also may be caused by hemothorax, empyema or, less commonly, a chylothorax. The case of a chylothorax is reported as a late onset complication of a double anterior and posterior instrumented fusion of the lumbar spine. Its management and clinical outcome, and a review of the literature are presented.
El derrame pleural es una de las posibles complicaciones del abordaje toracoabdominal de la columna. Lo más frecuente es que se trate de una efusión reactiva, pero entre sus causas posibles se encuentran el hemotórax, el empiema o, con menor frecuencia, el quilotórax. Presentamos un caso de quilotórax como complicación tardía de una artrodesis instrumentada de columna lumbar mediante doble abordaje, su manejo y evolución clínica, y una revisión de la bibliografía.
Pleural effusion is one of the possible complications in thoracoabdominal spinal approaches. It is most frequently a reactive effusion, but among its possible causes are also hemothorax, empyema and less frequently, chylothorax.
Due to the low incidence of chylothorax, the lack of comparative clinical studies prevents a consensus on its treatment, as the data come from retrospective case series.1
We present a case of chylothorax as late complication in an instrumented arthrodesis of the lumbar spine through a double approach, along with its clinical evolution and management, and a literature review.
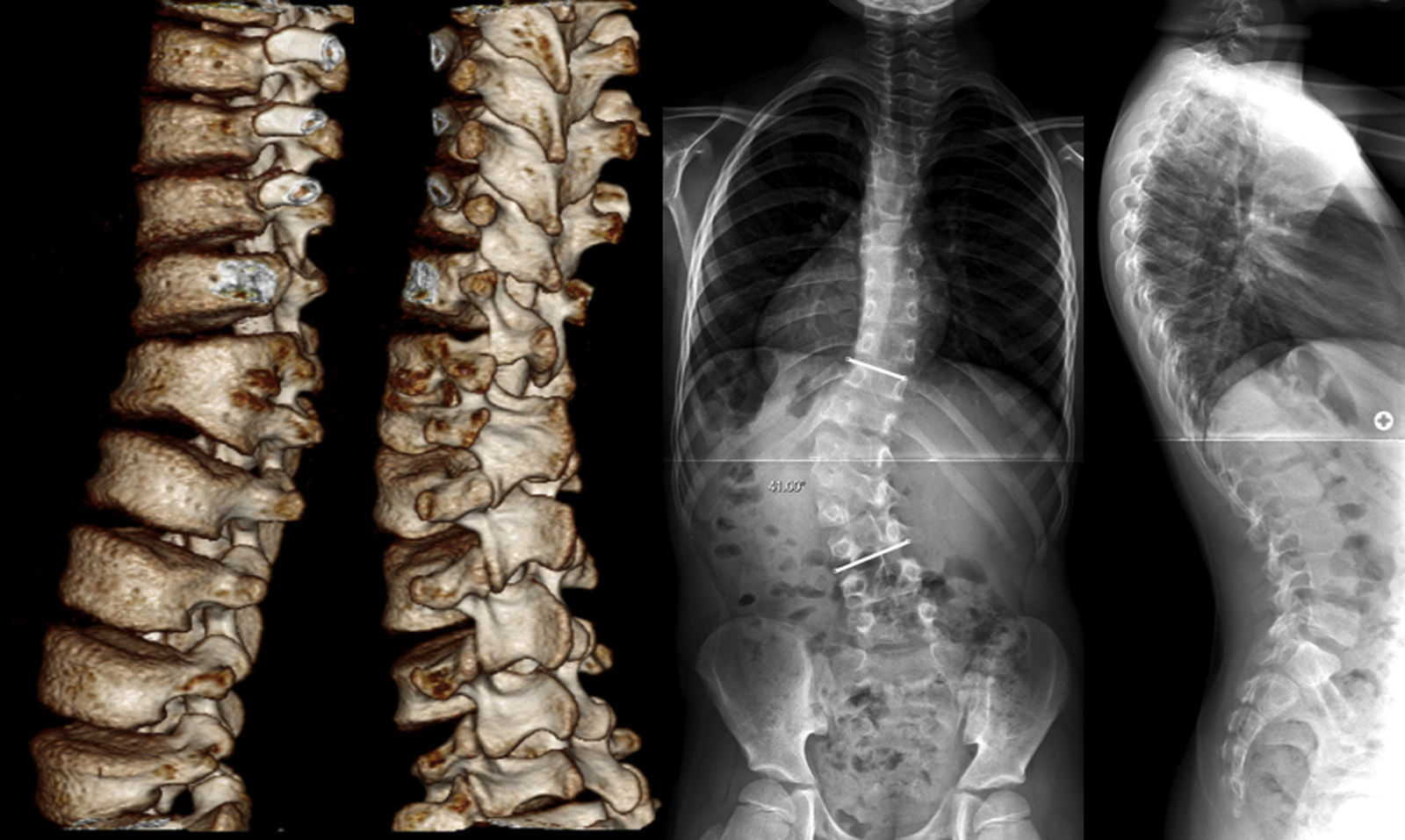
Case reportThe patient was 9 years and 3 months old, following eutocia after 41 weeks, with no pathological history of interest and currently a regional taekwondo champion. At age 4 he was diagnosed with congenital scoliosis, which was not treated due its mild degree and slow progression, without pain or neurological deficit. The patient was seen at the clinic for the first time at age 9 due to a sudden increase in progression, with a height of 138.4cm (65.4cm in sitting position) and a weight of 25.4kg. The radiographic study showed a left thoracolumbar curve of 41° from T11 to L4 (5 years earlier it was 27.6°) caused by a left semi-segmented hemivertebra L2 fixed to L1, a count of 6 lumbar vertebrae, a small compensatory thoracic curve and a coronal balance of 1cm to the right of the C7 plumb line, within a normal range. Despite the level of growth remaining (Risser 0 and open triradiate cartilages), the surgical indication was established by the recent progression of the lumbar curve (Fig. 1).
One month after attending the clinic we conducted an intervention through double approach, under control with intraoperative neuromonitoring. In a first surgical stage (anterior release), we placed the patient in right lateral position with elevation of the table to the level of the waist so as to increase lumbar curve and exposure of the discs, thus facilitating the discectomies after subcutaneous sterilization and infiltration with lidocaine and adrenaline 1/200,000. We conducted a left thoracoabdominal approach through subperiosteal resection of the 10° left costal arch, which was preserved for subsequent use as autograft in discal spaces. After accessing the pleural cavity, we entered the abdominal cavity through the diaphragm, through blunt dissection of the retroperitoneum until the psoas was located, identifying the ureter and prevertebral plexus, and accessed the left anterolateral aspect of the lumbar curve, where the discal protrusion was enhanced by the curvature and position. After radiographically assessing the level, we conducted discectomy from T12 to L3 and placed the autograft from the resected rib in palisade. Once the surface was flattened, we verified the flexibility of the curve. We then proceeded to close the wound by planes and left a thoracic drainage.
Next, during the same surgical session, we placed the patient in prone position with 30° flexion of both hips and knees. We employed a posterior approach with release of the posterior elements and instrumentation through uniplanar pedicular screws with a diameter of 4.5mm and a length of 30mm. It was possible to instrument both pedicles of T12 and L3, as well as the right pedicle of L1 (the left pedicle of L1 and that of hemivertebra L2 were extremely atrophied and their instrumentation was not possible). The fixation was established with 2 chromium-cobalt bars, and the curve was corrected in situ by compression on the left bar and distraction on the right. Once the instrumentation, reduction and fixation of segments of the lumbar curve were completed, we observed a nearly complete spontaneous correction of the compensatory thoracic curve. Lastly, we proceeded to impact the allograft, begin prophylaxis with 1g vancomycin powder and carry out closure by planes.
The intervention took place without complications, with a blood loss of approximately 200mL (30% of the estimated volemia, with postoperative hemoglobin of 9.9g/dL) and without any neurophysiological events observed in the normal motor and sensory evoked potentials up to 20min after the last reduction maneuver.
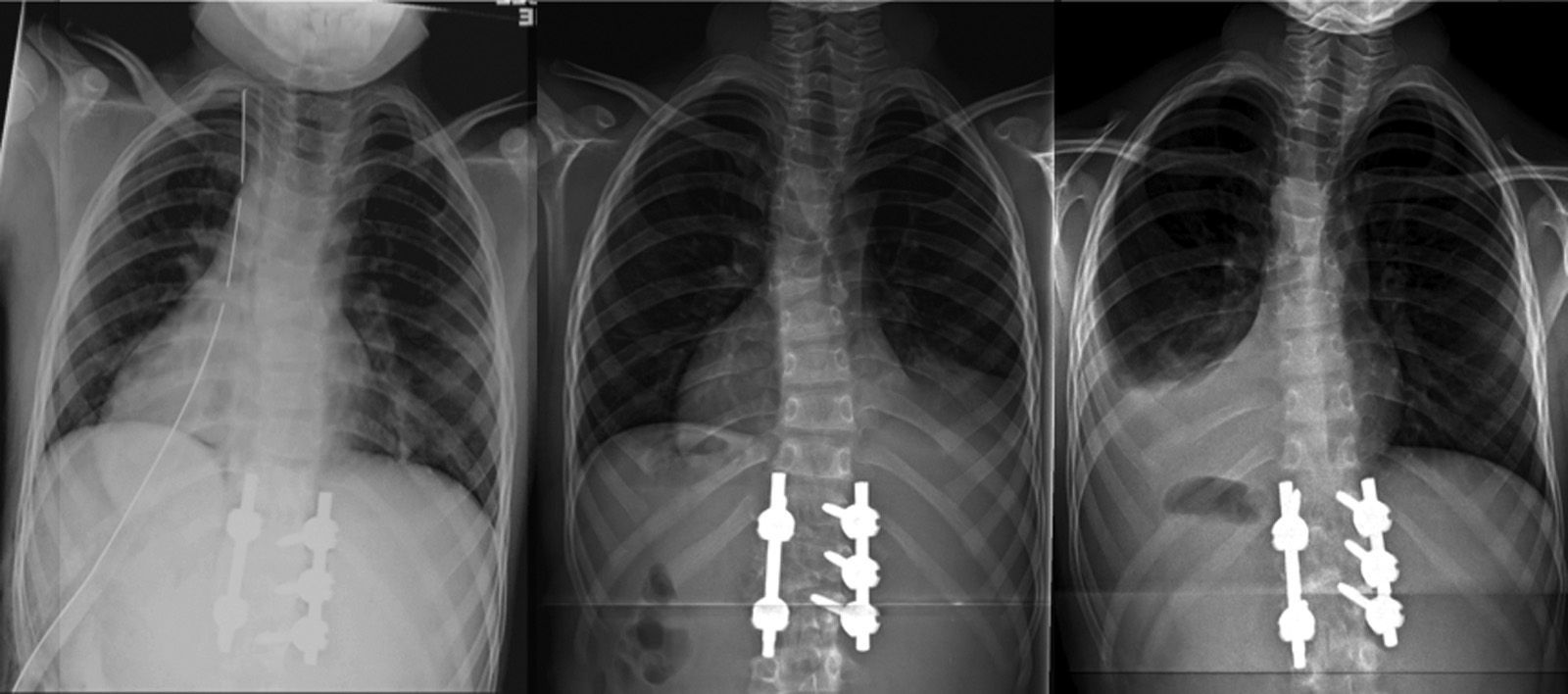
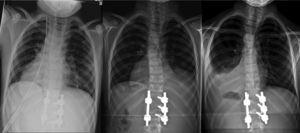
The patient was moved to the ICU, where he remained intubated for the first 24h, maintaining hemodynamic and respiratory stability. Once extubated, he was moved to the hospitalization ward, with no complications during the hospital admission and with a normal oxygen saturation breathing ambient air. The thoracic drainage was maintained for 48h and was unproductive (Fig. 2a). On the day of hospital discharge, 10 days after the intervention, we detected a minimal right pleural effusion on the postoperative standing control radiograph, which was asymptomatic and was interpreted as reactive effusion (Fig. 2b). A soft antalgic orthesis was prescribed for daytime use for the first 12 postoperative weeks.
Radiographic evolution of the thorax after the intervention: (a) immediate postoperative period, with pleural drainage, (b) at 10 days after the intervention, with the drainage removed and with minimal right reactive effusion, and (c) at 6 weeks, full resolution of the right reactive effusion and left pleural effusion with pulmonary collapse.
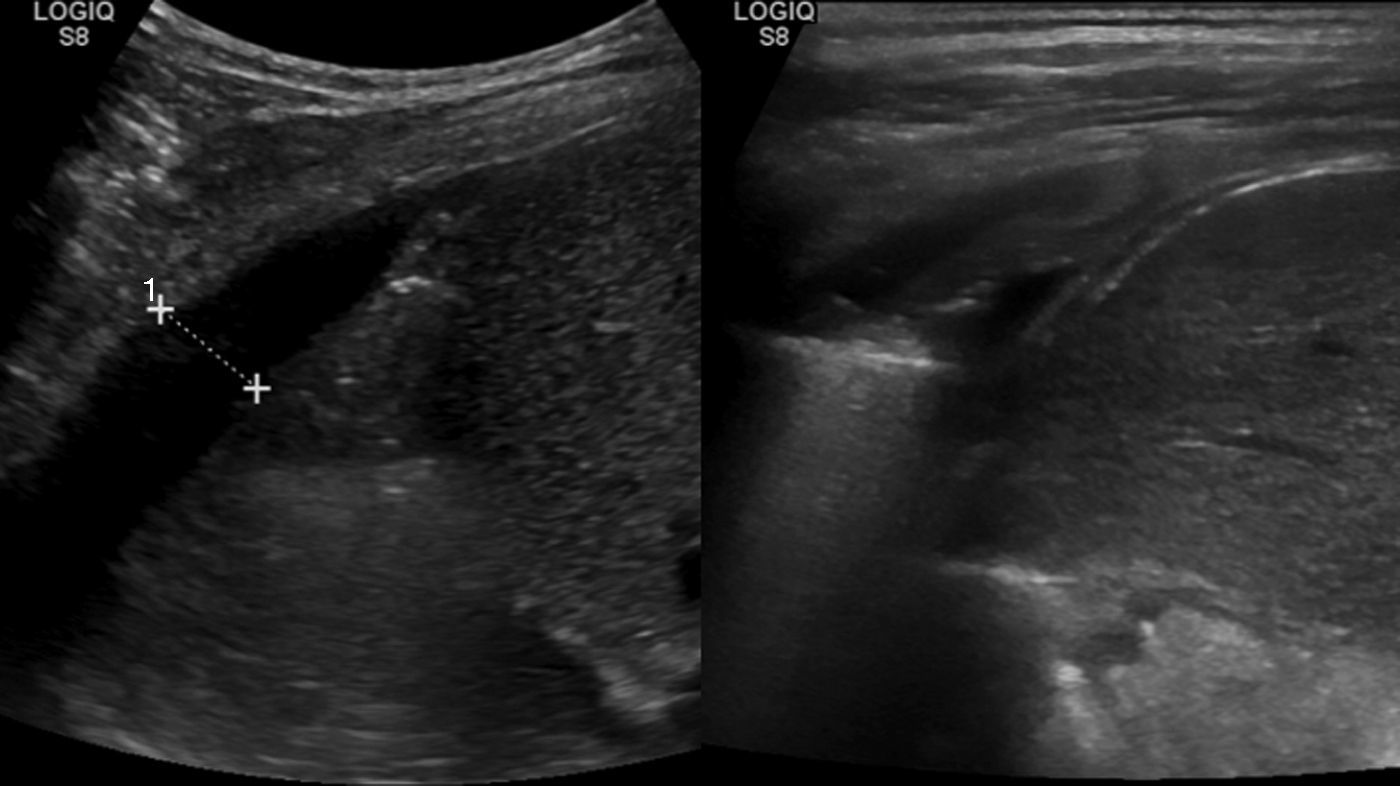
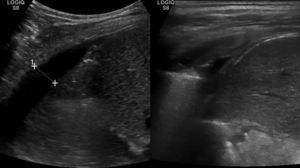
A standing radiograph during outpatient control at 6 weeks showed a full resolution of the right reactive effusion, but also showed a left unilateral pleural effusion, with lung collapse (Fig. 2c). The ultrasound showed consolidation in the left inferior lobe, with loss of volume and air bronchogram (Fig. 3a), subpulmonary fluid of about 5mm depth and pleural effusion with a maximum depth of 1.5cm.
Despite the absence of clinical repercussions (94% O2 saturation in ambient air), we decided to admit the patient to conduct ultrasound-guided thoracocentesis under general anesthesia. The result was a dense milky fluid with a macroscopic aspect of chylothorax. The analytical result supported the diagnosis of sterile chylothorax, with 20.7mmol/l (1833mg/dL) triglycerides, 73g/l total proteins and 4.6mmol/l (83mg/dL) glucose levels.
In consultation with the Radiology, Anesthesia, Respiratory and Orthopedic Surgery services, we decided to initiate treatment with a low-fat diet and maintain the thoracic drain. The patient maintained oxygen saturations of 97–100%, with no need for oxygen therapy. The initial drain was of 425mL which oscillated between 0 and 250mL in the following days, with a progressive decrease in production until the symptoms were fully resolved.
A control ultrasound scan conducted after 2 weeks was normal (Fig. 3b), and the patient was discharged after removing the thoracic drain after 18 days, with progressive reintroduction of fat into the diet and normal control at 2 months.
DiscussionThe thoracic duct is the main collector of the lymphatic system and drains ¾ of the lymph in the body to the venous system. Although it has multiple anatomical variants, in 50% of the population it originates in the Pecquet cistern (cisterna chyli), which is the confluence of retroperitoneal lymph vessels, has a diameter of about mm and a length of about 16mm and is located in the right posteromedial area of the aortic artery, at the level of the renal arteries and the L1 and L2 vertebral bodies. The thoracic duct enters the thoracic cavity through the aortic hiatus and ascends between the aorta and azygos vein. In its thoracic portion, it travels through the anterior aspect of the thoracic vertebral bodies until it drains in the union of the left internal jugular and subclavian veins,2 with a difference in diameter of 2mm in the most caudal segment and 3.6mm in the terminal segment.
The chyle is generated in the lymphatic system of the small intestine, as a product of fat digestion, and is composed of lipids, electrolytes, proteins, immunoglobulins and lymphocytes (mainly T lymphocytes).
Chylothorax is an infrequent cause of pleural effusion, mainly unilateral in 80% of cases. Unlike older studies, in which the most frequent origin was oncological disease (particularly non-Hodgkin lymphomas, which represent up to 60% of these cases), at present the most common cause is trauma (50% versus 25% described previously). The known causes of traumatic chylothorax include vertebral fractures, abrupt spinal hyperextension, penetrating trauma, increase in intraabdominal pressure in closed trauma, iatrogenic lesions during approaches to the abdominal or thoracic cavity, non-surgical invasive procedures, like placement of central catheters, and even cases of coughing and vomiting due to traction of the duct at the level of the diaphragmatic crus.2–4 The considerable anatomical variability favors accidental lesion during cervical, thoracic and lumbar surgery and, although the incidence of posttraumatic chylothorax is low (1–4%, and less than 1% of spinal interventions), its management can be complex.2,5
Infrequent lesion of the thoracic duct, cisterna chyli and retroperitoneal lymphatic trunks, which was described for the first time in 1875,6 causes a drainage of chyle toward the thoracic cavity (chylothorax) or abdominal cavity (chyloperitoneum).7 The diagnosis is confirmed by the typical milky and opaque secretion and the analytical confirmation of the fluid, with characteristics of exudate (although in non-trauma chylothorax it can be transudate in up to 1/3 of cases8) with cholesterol levels (CH)<200mg/dL and rich in triglycerides (TG). The diagnosis is confirmed by TG levels over 110mg/dL, and is ruled out under 50mg/dL, but intermediate levels require an investigation of the presence of chylomicrons and cholesterol crystals. It can also be diagnosed by a CH/TG ratio under 1.3
The differential diagnosis includes pseudochylothorax, which also has a milky aspect but contains >200mg/dL CH and <110mg/dL TG, with a CH/TG ratio higher than 1, and is associated to malnutrition.9
In low lumbar surgery with an intact diaphragm, the drainage can be attributed to a lesion of the cisterna chyli, and is exclusively confined to the retroperitoneal cavity. Intervention of levels cranial to L1 requires splitting of the diaphragm, which loses the capacity to isolate both cavities. In such cases, it is difficult to know whether chylothorax is due to a lesion of the cistern with retroperitoneal drainage and subsequent fistulization to the thoracic cavity or to a primary lesion of the duct in its ascending segment. Diaphragm splitting per se does not increase the rate of chylothorax.10
The clinical relevance of this process lies in that, without an early diagnosis, it can derive in respiratory failure, nutritional and immunological dysfunction and, ultimately, in an increase of morbidity and mortality.3
Treatment with conservative measures has been proven worse in patients with non-trauma chylothorax, among whom it only resolves a minority of cases, than among cases with a traumatic origin, half of which are cured.1
The objective of dietary treatment is to reduce the production of chyle, which is between 1500 and 2000mL per day in adults. This can be achieved by avoiding lymph circulation with parenteral feeding or else through enteric formulas with <3% long-chain TG3 with supplementation of medium-chain TG, which are absorbed directly into the portal circulation without stimulating lymphatic circulation and preserving the necessary nutritional value to allow bone consolidation.4,9 In both cases, thoracic drainage must be maintained and a support treatment must be followed, with supplementation of liposoluble vitamins and proteins. The risk of septicemia must be taken into account after 8 days of T lymphocyte depletion, and this will be greater among patients with parenteral nutrition. Chemoprophylaxis with wide-spectrum antibiotic therapy can be considered after this period.9
There have been studies of the use of octreotide, in continuous perfusion or in boluses, to decrease lymphatic flow through a reduction of gastrointestinal secretions and splanchnic blood flow, but the results have been inconsistent.3,9
Neither is there a consensus on the duration of dietary treatment, which in general should be maintained for around 2 weeks or until the resolution of chylothorax. A normal diet can be resumed subsequently. Some authors3 have suggested a trigger test through a diet with a high fat content before removing the thoracic tube in complicated cases, so as to verify a complete resolution of symptoms. We should assume a failure of conservative treatment and consider the need for surgical resolution in the following scenarios: (1) excessive drainage for over 5 days, representing >500mL/day in adults and >10mL/kg/day (or >100mL/day for each year of age) in children; (2) any production for over 14 days in adults (this can be longer in children); (3) onset of signs of metabolic complications.5,7,9
The surgical alternatives are pleurodesis, pleurectomy, thoracic duct ligation (conducted for the first time in 1948)6 or its repair, lymphovenous anastomosis (low effectiveness) and pleuro-peritoneal shunt in cases with no pulmonary re-expansion despite the evacuation of the fluid whenever a more aggressive surgery is not indicated.4 The choice between open thoracotomy and thoracoscopy depends on the preference and experience of the surgeon. Percutaneous embolization of the thoracic duct is an increasingly common alternative, with a rate of complications of 3% (chronic edema in lower limbs and chronic diarrhea) and with no fatal complications described to date.9
The response to the surgical treatment is also worse in non-trauma chylothorax, with a higher rate of recurrences and no resolution in up to 1/3 of cases.1,9
ConclusionsKnowledge of the anatomy of the thoracic duct, as well as its possible anatomical variants, is essential to avoid iatrogenic lesions during anterior approaches to the thoracolumbar spine.
The management of traumatic chylothorax should initially be based on diet control with electrolytic and nutritional support, as this is enough to resolve most cases. Complicated cases may require surgical resolution in order to avoid metabolic and immunological deterioration of patients.
Level of evidenceLevel of evidence V.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare having obtained written informed consent from patients and/or subjects referred to in the work. This document is held by the corresponding author.
Conflict of interestsThe authors declare that they have no financial or personal relationship with any persons or organizations that could give rise to a conflict of interests in relation to the present article.
Please cite this article as: Mora de Sambricio A, Garrido Stratenwerth E. Quilotórax tras abordaje anterior de columna toracolumbar. Revisión bibliográfica a propósito de un caso. Rev Esp Cir Ortop Traumatol. 2015;59:129–133.