To compare the in vivo bone formation capacity of of biomaterials designed as bone substitutes with respect to iliac crest autograft, one based on carbonate hydroxiapatite and the other one on bioactive mesoporous glass.
Materials and methodsExperimental study consisting on 14 adult female New Zeland rabbits where a critical defect was made in the rabbit radius bone. The sample was divided into four groups: defect without material, with iliac crest autograft, with carbonatehydroxyapatite scaffold, and with bioactive mesoporous glass scaffold. Serial X-ray studies were carried out at 2, 4, 6 and 12 weeks and a microCT study at euthanasia at 6 and 12 weeks.
ResultsIn the X-ray study, autograft group showed the highest bone formation scores. Both groups of biomaterials presented bone formation similar and greater than the defect without material, but always less than in the autograft group. The results of the microCT study showed the largest bone volume in the study area in the autograft group. The groups with bone substitutes presented greater bone volume than the group without material but always less than the autograft group.
ConclusionBoth scaffolds seem to promote bone formation but are not capable of reproducing the characteristics of autograft. Due to their different macroscopic characteristics, each one could be suitable for a different type of defect.
Comparar in vivo la capacidad de formación ósea de 2 tipos de biomateriales diseñados como sustitutivos óseos respecto a autoinjerto de cresta ilíaca, uno basado en carbonatohidroxiapatita y otro en vidrio mesoporoso bioactivo.
Material y métodoEstudio experimental compuesto por 14 conejos hembras adultas de Nueva Zelanda donde se realizó un defecto crítico en hueso radio. La muestra fue dividida en 4 grupos: defecto sin material, con autoinjerto de cresta ilíaca, con soporte de carbonatohidroxiapatita y con soporte de vidrio mesoporoso bioactivo. Se realizaron estudios seriados de radiología simple a las 2, 4, 6 y 12 semanas y estudio de micro-TC a eutanasia a las 6 y 12 semanas.
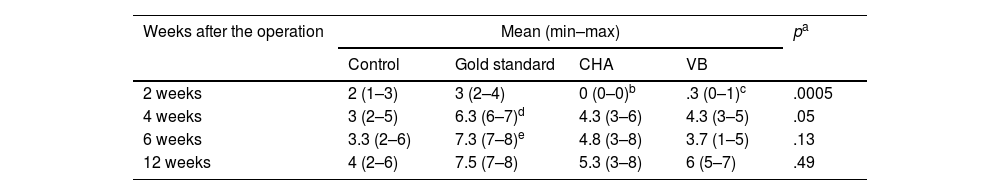
ResultadosEn el estudio de radiología simple, el grupo de autoinjerto mostró las mayores puntuaciones de formación ósea (7,5 puntos). Ambos grupos de biomateriales presentaron formación ósea similar (5,3 y 6 puntos, respectivamente) y mayor al defecto sin material (4 puntos), pero siempre menor que el grupo de autoinjerto. Los resultados del estudio de micro-TC mostraron el mayor volumen de hueso en el área de estudio en el grupo de autoinjerto. Los grupos con sustitutivos óseos presentaron mayor volumen de hueso que el grupo sin material, pero siempre menor que en el grupo de autoinjerto.
ConclusionesAmbos soportes parecen favorecer la formación ósea, pero no son capaces de reproducir las características del autoinjerto. Por sus diferentes características macroscópicas cada uno podría ser adecuado para un tipo diferente de defecto.
The treatment of bone defects is one of the most demanding challenges in the field of orthopaedic surgery. In this context, bone autograft is the best solution to treat bone defects and stimulate new bone formation and is therefore considered the gold standard,1 given its osteoconductive, osteoinductive and osteogenic capacity. However, its use is limited by factors such as increased intraoperative time, morbidity in the donor site and the limited amount of autograft that can be obtained. Within this framework, the need arises within the field of tissue engineering to create constructs capable of carrying out the function of the autograft without the limitations derived from its use.
In the field of bone substitutes research there are two main families of biomaterials, those formed by apatites and those formed by mesoporous bioactive glasses (MBG). Both types of materials have been shown to be biocompatible, bioactive and resorbable to varying degrees,2,3 and they have shown to integrate with the recipient tissue without fibrous tissue interposition in in vivo studies.4,5
The purpose of this study was to compare the behaviour of nano-carbonate hydroxyapatite (nCHA) ceramic-based constructs with those based on MBG under the same in vivo conditions and to analyse their bone-forming capacity compared with iliac crest autograft and graftless defect.
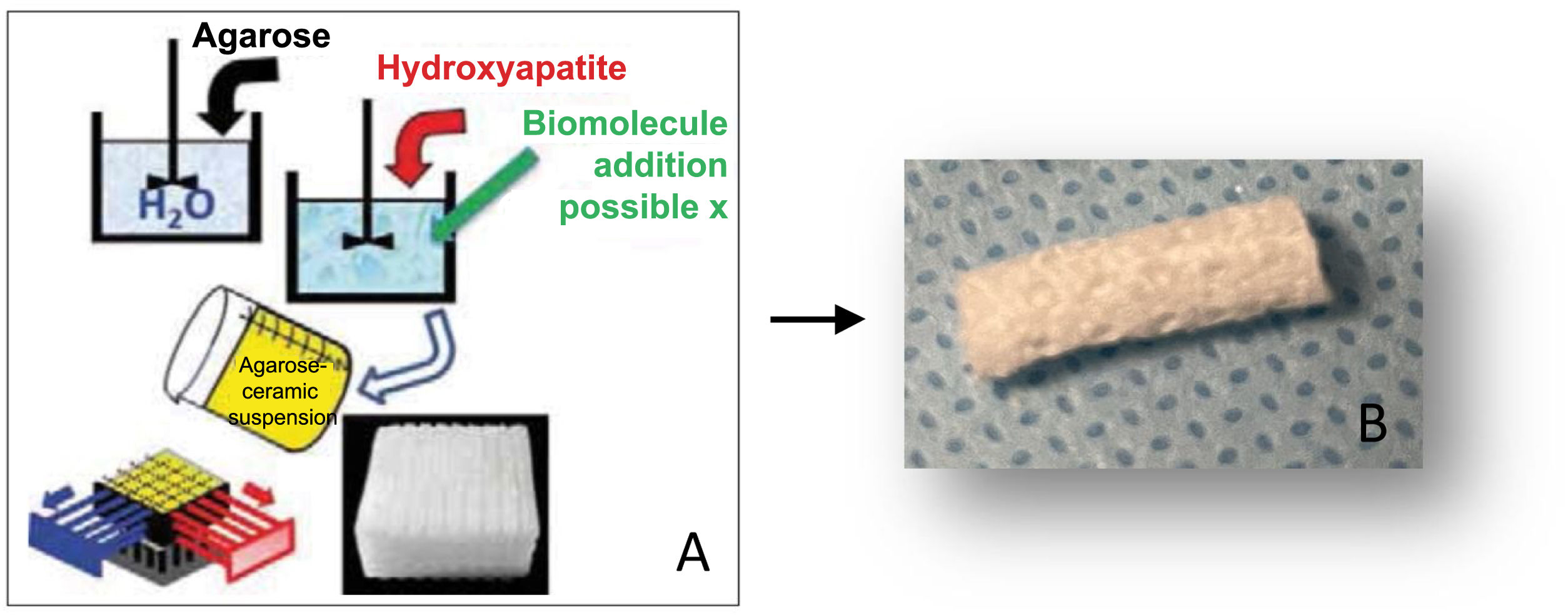
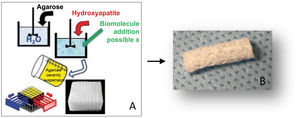
Material and methodPreparation of materialsCarbonate hydroxyapatite (CHA) scaffolds were made by the GELPOR3D method6,7 by preparing an aqueous solution of agarose (3.5% w/v) which was heated to 80–90°C, Subsequently, lowering the temperature to 40°C, at which temperature the gelatine and the corresponding CHA were added (agarose/gelatine/CHA ratio: 45/10/45). Once a homogeneous suspension was obtained, the mixture was poured into a previously designed three-dimensional mould, consisting of rigid stainless-steel filaments of the desired diameter (300–900μm), which determines the size of the pores in the three dimensions of the space. Once complete gelation was obtained, the mould was removed to obtain the piece, which was subsequently dried by freeze-drying for sterilisation using UV light (Fig. 1A and B).
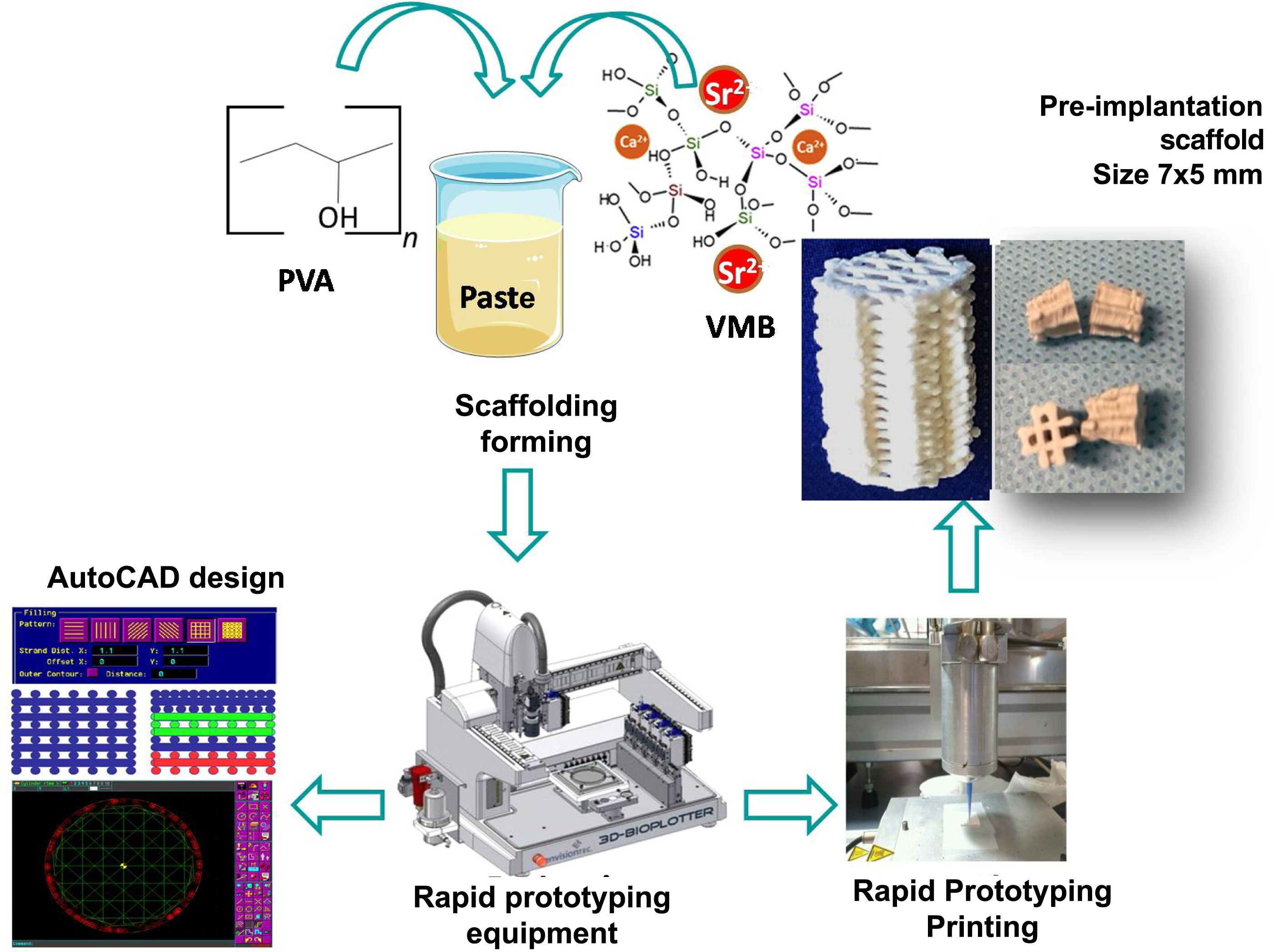
MBG scaffolds were made following the procedure previously described by Jiménez-Holguín et al.8 To fabricate the scaffolds, a 15% PVA suspension (Sigma Aldrich, St. Luois, MO, USA) was prepared by mixing 1.25g of PVA in 10mL of milliQ H2O at 90°C under magnetic stirring and mixed with the MBG generating a PVA/VM paste for subsequent extrusion in the rapid prototyping equipment of the 3D printer (Regemat 3D S.L., Spain). The dimensions of the cylindrical scaffolds obtained were 6mm in diameter and 7.5mm in length. The scaffolds were dried at 100°C for 2h and sterilised with ultraviolet light for 2h (Fig. 2).
Animal modelFourteen adult female New Zealand rabbits weighing 4±.5kg were used. In compliance with RD 1090/2015, the study was evaluated and approved by the Ethics Committee for Research with Experimental Animals of the I+12 Research Institute, the Ethics Committee/Animal Experimentation Subcommittee of the Autonomous University of Madrid and by the Directorate General for Agriculture, Livestock and Food of the Community of Madrid. This experimental animal study adheres to the ARRIVE guidelines and has been conducted in accordance with the UK Animals (Scientific Procedures) Act 1986 and the related recommendations of the EU Directive 2010/63/EU for animal experiments.
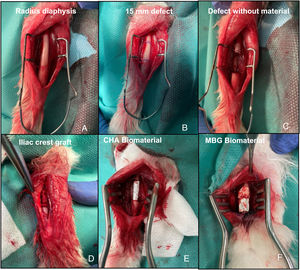
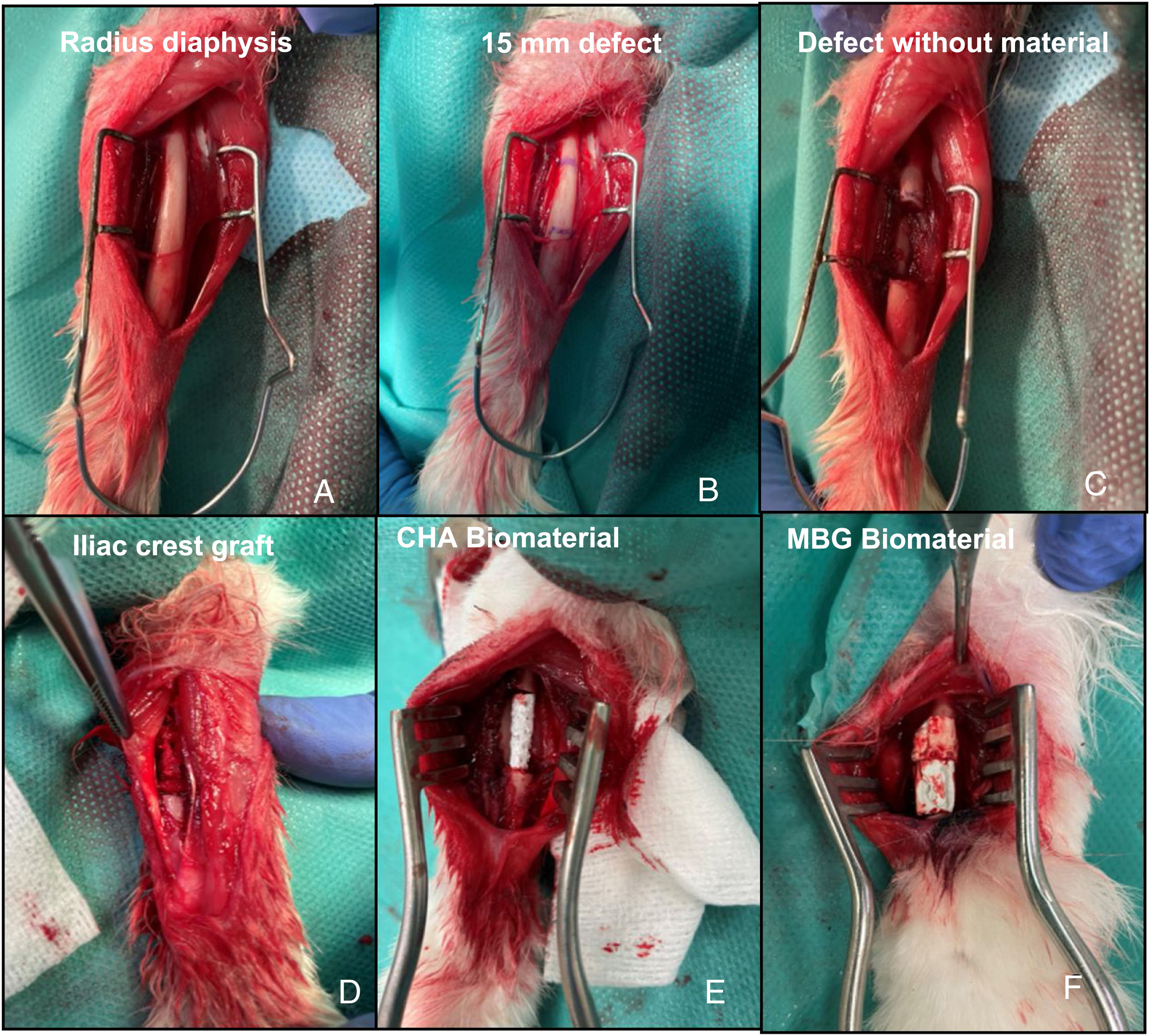
Surgical interventionPre-anaesthetic sedation was performed with ketamine (40mg/kg, IM) and xylazine (5mg/kg, IM). Once the animal was sedated, a venous catheter was placed in the marginal vein of the ear. Antibiotic prophylaxis was then administered with cefazolin (50mg/kg IV) and the forearm and iliac crest were shaved according to the study group, and the skin was aseptically prepared with polyvidone iodine. Anaesthesia was maintained by inhalation with 4% sevoflurane, with continuous monitoring of heart rate and oxygen saturation. A longitudinal incision was made over the dorsal diaphysis of the radius, which was exposed after careful dissection of the adjacent musculature. Subsequently, a 15mm bone segment with surrounding periosteum was removed to achieve a critical,9 with no potential for self-repair, in this bone. The 14 rabbits were divided into four study groups. In the first group (G1) of three rabbits, the defect was not filled to study the behaviour of the defect; in the second group (G2) of three rabbits, the defect was filled with the animal's own iliac crest autograft; in the third group (G3) of four rabbits, the CHA scaffold was implanted; and in the fourth group (G4) of four rabbits, the MBG scaffold was implanted (Fig. 3). Subsequent plane closure was performed by suturing the forearm fascia with 4/0 braided resorbable thread and the skin with 5/0 non-resorbable monofilament. Immediate postoperative loading of the operated limb was allowed. In each group, one rabbit was sacrificed at six weeks and the rest at 12 weeks. Euthanasia was performed using the previously described sedation protocol followed by intravenous administration of 0.5g sodium thiopental.
Intraoperative images of the rabbit radius procedure. (A) Exposure of the radial bone diaphysis. (B) Planning of the 15mm defect to be created. (C) Exposure of the defect without material (G1). (D) Filled defect with autograft of the animal's own iliac crest (G2). (E) Filled defect with CHA scaffold (G3). (F) Filled defect with MBG scaffold (G4).
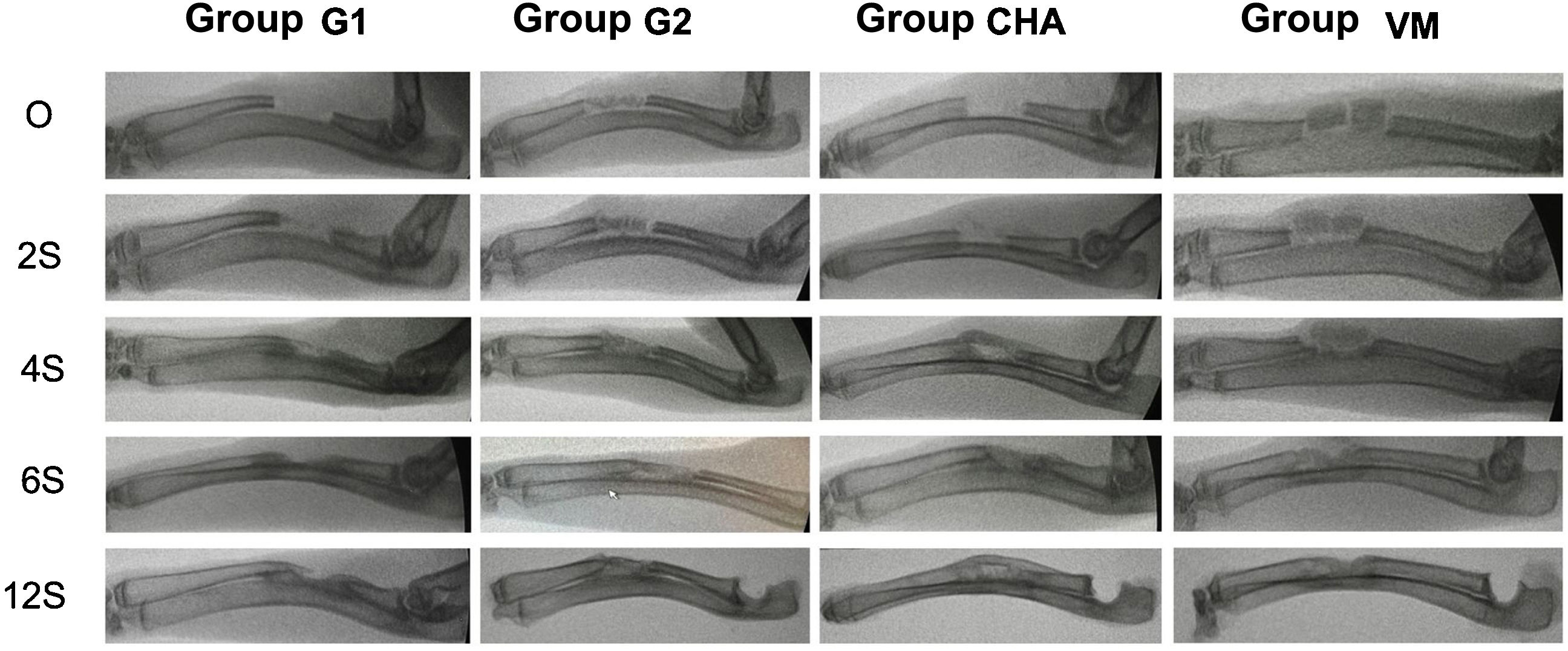
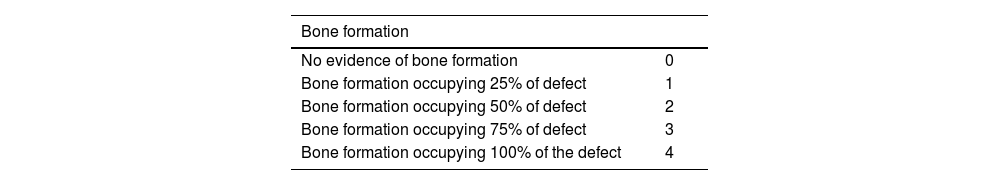
Radiological controls of the defect were performed using a Xiscan 4400 fluoroscope in the immediate postoperative period and at two, four, six and 12 weeks after surgery depending on the study group to assess bone formation, union and remodelling of the defect. The results were scored using the modified Lane and Sandhu10 scale as shown in Table 1.
Modified Lane and Sandhu radiological scoring scale.
| Bone formation | |
|---|---|
| No evidence of bone formation | 0 |
| Bone formation occupying 25% of defect | 1 |
| Bone formation occupying 50% of defect | 2 |
| Bone formation occupying 75% of defect | 3 |
| Bone formation occupying 100% of the defect | 4 |
| Union (proximal and distal evaluated separately) | |
|---|---|
| No union | 0 |
| Union possible | 1 |
| Radiograpahic union | 2 |
| Total points possible by category | |
|---|---|
| Bone formation | 4 |
| Proximal union | 2 |
| Distal union | 2 |
| Remodelling | 2 |
| Maximum score | 10 |
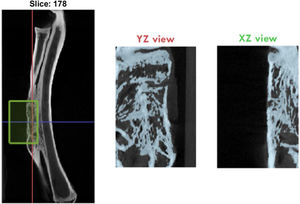
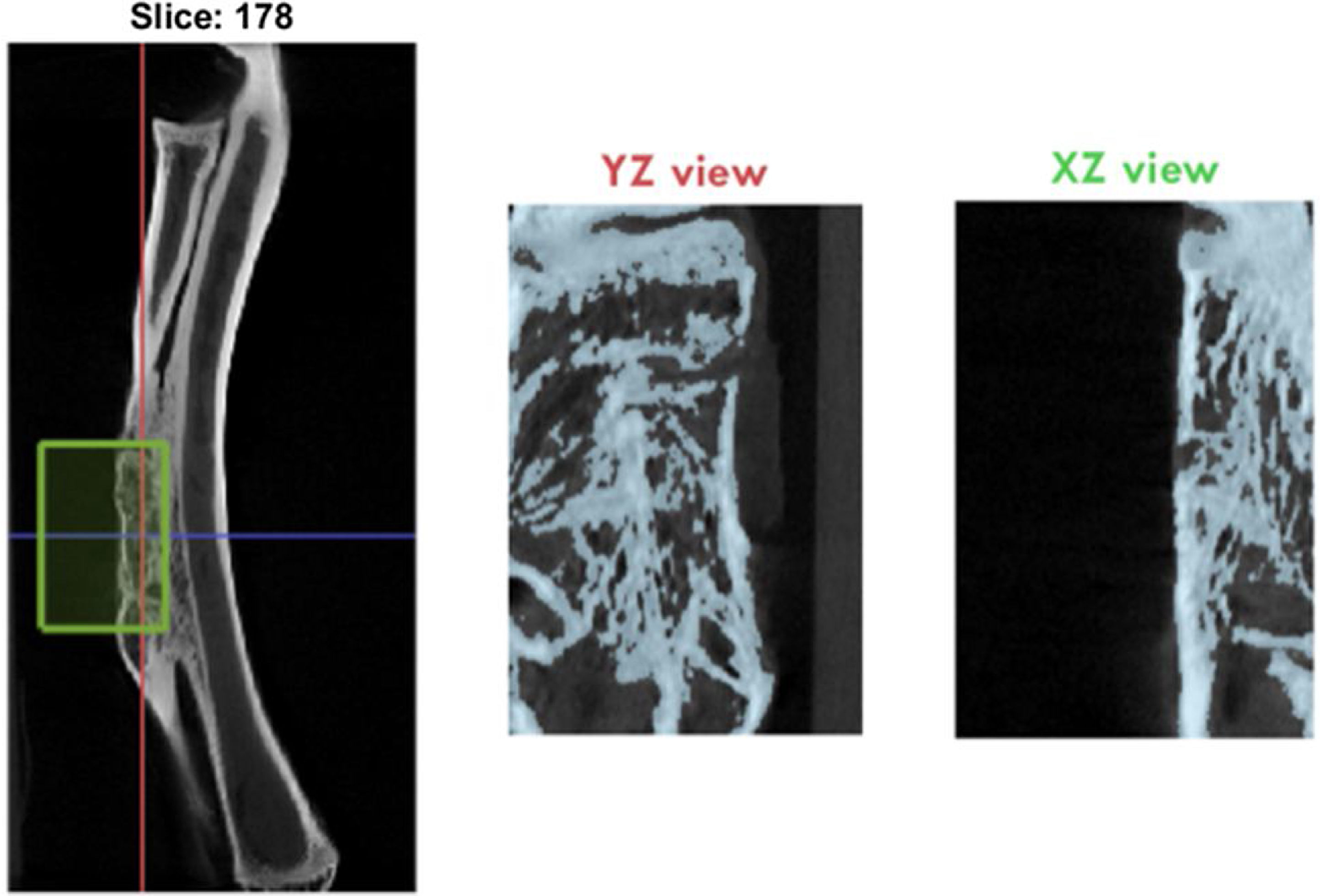
Image analysis and three-dimensional micro-CT reconstruction was performed at the end of the study where the following variables of newly formed bone were determined: BV/TV (bone volume/total volume, %), Tt.Ar (total cancellous bone, mm2), Ct.Ar (total cortical bone, mm2), and Ct.Th (cortical thickness, mm) in the defect area (Fig. 4).
Statistical analysisPlain radiology and micro-CT results were compared by mean difference using the non-parametric Kruskal–Wallis test, and when p-values were <0.05, pairwise comparisons were performed using the Mann–Whitney U-test. A p value<0.05 was considered statistically significant. The statistical package SPSS Statistics for Windows, version 24.0, was used. Armonk, NY: IBM corp.
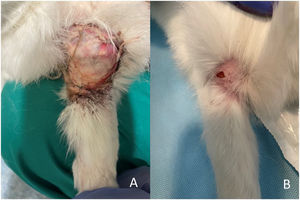
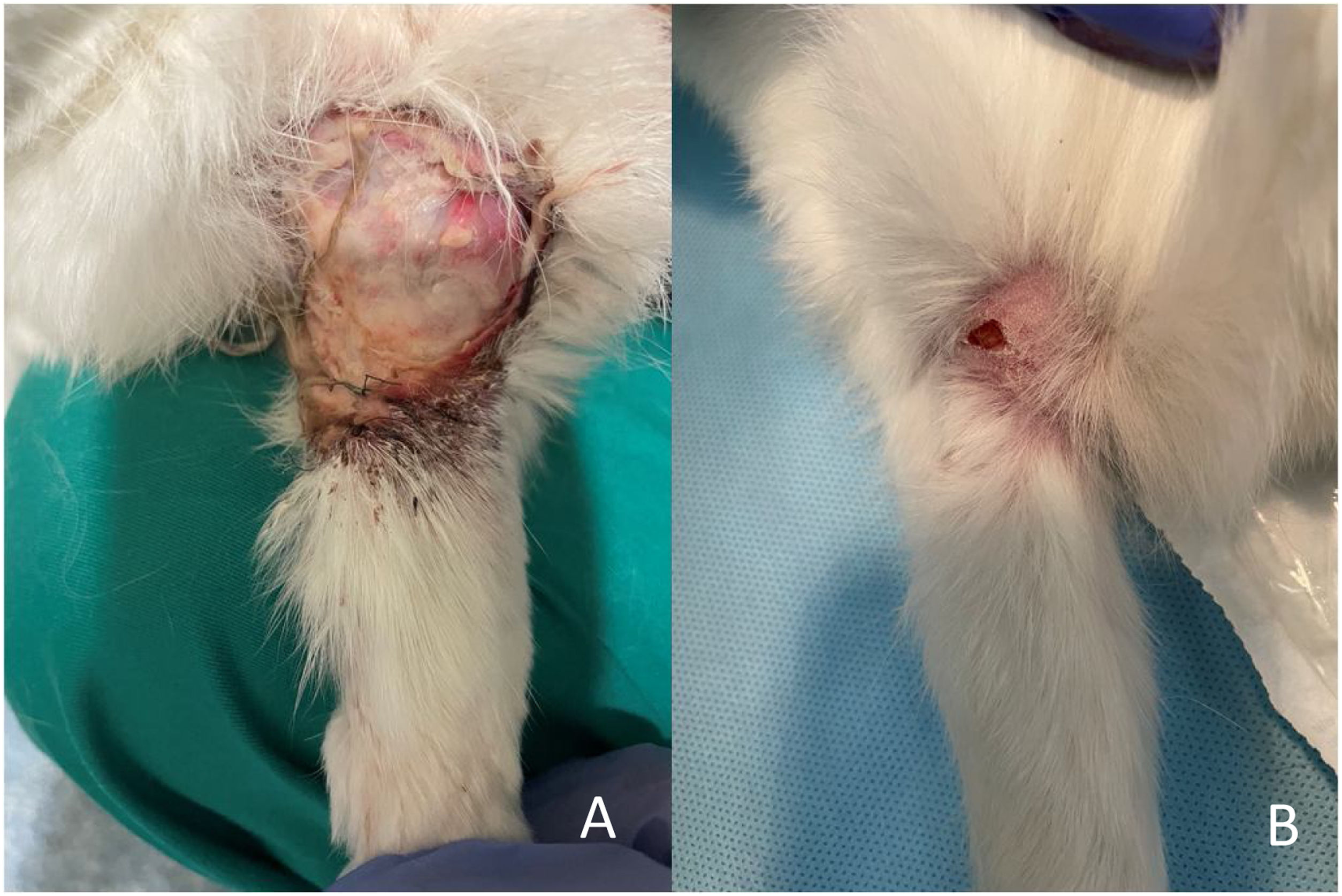
ResultsClinical follow-upNo deaths occurred during surgery. An early euthanasia was performed seven weeks post-operatively on a rabbit that suffered a spinal cord injury during a change of location for cleaning of the housing enclosure, belonging to the iliac crest autograft group (G2). Early euthanasia of this animal was decided for humane reasons. There was also a suture dehiscence with no signs of infection in the G2 group which was resolved by serial dressings and an infection in the G1 group which was washed, debrided and treated with antibiotics and resolved after two weeks (Fig. 5A and B).
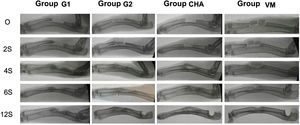
Results of plain radiologyBone formation according to the modified Sandhu and Lane scale increased over time in all groups (Fig. 6). At all follow-up times the iliac crest autograft group (G2) had the highest scores, this difference being statistically significant at four (p=0.03) and six weeks (p=0.04) follow-up. Both the mesoporous glass group and the NCHA group showed higher bone formation after four weeks than the no-material control group (G1), although always lower than in the G2 group. The radiological results are shown in Table 2.
Results of plain radiology.
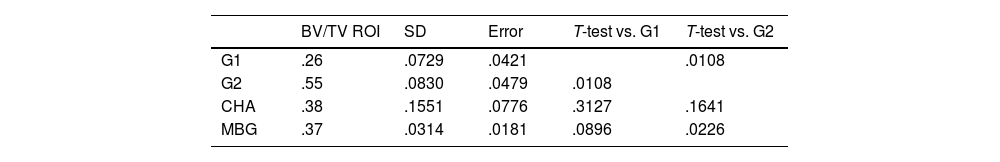
Bone volume in the study area (BV/TV, region of interest [ROI]) was significantly higher (p=.012) in the G2 group compared to the rest of the groups. Both the MBG and NCHA groups presented greater bone formation than the G1 group, although this difference was not statistically significant (Table 3).
Bone volume measurements within the defect by groups.
| BV/TV ROI | SD | Error | T-test vs. G1 | T-test vs. G2 | |
|---|---|---|---|---|---|
| G1 | .26 | .0729 | .0421 | .0108 | |
| G2 | .55 | .0830 | .0479 | .0108 | |
| CHA | .38 | .1551 | .0776 | .3127 | .1641 |
| MBG | .37 | .0314 | .0181 | .0896 | .0226 |
BV/TV ROI: bone volume in the study area; CHA: carbonate hydroxyapatite group; G1: no material control group; G2: iliac crest autograft control group; MBG: mesoporous bioactive glass group; SD: standard deviation.
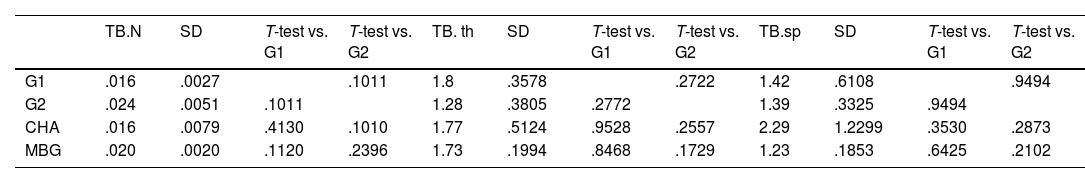
Regarding the trabecular study, no statistically significant differences were found in any of the parameters studied, which were the number of bone trabeculae (TB.N), thickness (TB.Th mm) and trabecular separation (Tb.sp mm). The results of the statistical study are shown in Table 4.
Trabecular measurements from micro-CT study.
| TB.N | SD | T-test vs. G1 | T-test vs. G2 | TB. th | SD | T-test vs. G1 | T-test vs. G2 | TB.sp | SD | T-test vs. G1 | T-test vs. G2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | .016 | .0027 | .1011 | 1.8 | .3578 | .2722 | 1.42 | .6108 | .9494 | |||
| G2 | .024 | .0051 | .1011 | 1.28 | .3805 | .2772 | 1.39 | .3325 | .9494 | |||
| CHA | .016 | .0079 | .4130 | .1010 | 1.77 | .5124 | .9528 | .2557 | 2.29 | 1.2299 | .3530 | .2873 |
| MBG | .020 | .0020 | .1120 | .2396 | 1.73 | .1994 | .8468 | .1729 | 1.23 | .1853 | .6425 | .2102 |
BV/TV ROI: bone volume in the study area; CHA: carbonate hydroxyapatite group; G1: control group without material; G2: iliac crest autograft control group; MBG: mesoporous bioactive glass group; TB.N: number of bone trabeculae; SD: standard deviation.
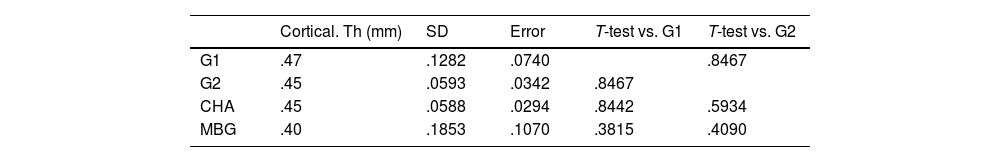
Regarding the cortical study, no significant differences in cortical thickness were found in any of the study groups. The results are shown in Table 5.
Cortical thickness measurements by micro-CT study.
| Cortical. Th (mm) | SD | Error | T-test vs. G1 | T-test vs. G2 | |
|---|---|---|---|---|---|
| G1 | .47 | .1282 | .0740 | .8467 | |
| G2 | .45 | .0593 | .0342 | .8467 | |
| CHA | .45 | .0588 | .0294 | .8442 | .5934 |
| MBG | .40 | .1853 | .1070 | .3815 | .4090 |
CHA: carbonate hydroxyapatite group; cortical. Th (mm): cortical thickness in mm; G1: control group without material; G2: iliac crest autograft control group; MBG: mesoporous bioactive glass group; SD: standard deviation.
To evaluate the bone regeneration potential of the designed biomaterials, some based on MBG and others on CHA, a critical defect was generated on rabbit radial bone. Furthermore, it was compared with the gold standard (iliac crest autograft) and with the biomaterial-free defect.
The New Zealand rabbit was chosen as the study model, as previous studies have shown it to be ideal for bone substitute studies,11 given the similarities between rabbit and human limbs, its medium size, its relative ease of handling and housing, as well as its affordable cost. It also exhibits an increased rate of bone regeneration, which has led it to be widely used as a model in numerous bone regeneration studies.12,13
A critical defect was defined as “the smallest intraosseous defect in a particular bone and animal species that cannot regenerate itself during the animal's entire lifetime.”14 Although the minimum size that a defect must be to be considered critical is not yet well established, it has been defined as a length of 2.5–3 times the diameter of the long bone where the defect is located.15 The radius bone was chosen as a model for a bone defect in long bone, as its shape is tubular, which allows the creation of a segmental defect. Furthermore, despite the defect, the ulna continues to support the weight of the forearm, allowing immediate postoperative loading and avoiding the need for stabilisation with additional osteosynthesis material. The diameter of the radius in New Zealand rabbits is between 4 and 5mm, so, according to other authors,16,17 a 15mm radial diaphysis defect was created. Numerous investigators have shown that this size defect is filled with a fibrous scar with no bony connection.16,17
Regarding the composition and design of the biomaterials used in this study, two of the most promising families in the field of biomaterials engineering were chosen. One based on bioglasses and the other based on apatites.18 The groups developing bioglass-based materials consider these to be the ideal scaffolds for the development of bone substitutes. The same is promulgated by groups specialising in research in the field of apatite-based substrates. In this work, two types of three-dimensional porous scaffolds developed by the Intelligent Biomaterials Research Group (GIBI) were compared. Tests were performed under the same in vivo conditions for both types of biomaterials, as these materials were implanted in the same animal model, same defect, and same surgeon in the same facilities.
Furthermore, Oonishi et al.19 showed that there are two classes of bioactivity. Materials with class A bioactivity exhibit both osteoconductive and osteoproductive effects, as a result of a rapid surface reaction including the local release of critical concentrations of ions, mainly Si, Ca, P and Na, causing intra- and extracellular reactions with the biological environment. MBGs belong to this class. On the other hand, biomaterials with class B only produce an osteoconductive effect and allow direct bone apposition on the support due to their low rate of interface reactions. CHA belong to this group. Furthermore, the design of the substrates was based in both cases on a three-dimensional architecture of interconnected pores of different sizes in order to simulate the bone structure,20 which has a porosity between 1 and 3500μm, where different pore sizes imply different functions. Pores larger than 100μm, (macropores) allow for progenitor cells, tissue growth, nutrition and vascularisation. Pores<2nm in size (micropores) or those between 2 and 50nm (mesopores) promote cell adhesion, metabolite uptake and controlled resorption rates of biomaterial necessary for tissue repair.21 In addition, this porosity was created in the design phase in an anisotropic manner,22 as it has demonstrated greater bone formation than when designed in an isotropic manner for both materials.
Regarding the behaviour of the MBGs, they show a rapid bioactive response, due to their partial solubility in contact with biological fluids, releasing significant quantities of ions into the surrounding medium, especially Si ions, which enhance angiogenesis, and Ca2+ ions which contribute to cell proliferation and also show osteogenic activity.23 The constitution of the mesoporous glasses used for this work was 78.5% SiO2–15% CaO–5% P2O5–2.5% SrO (mol-%). The MBG mesomacroporous supports were enriched with SrO and showed mesoporosity (6nm), macroporosity (1–100μm) and giant channels (1mm). Under in vitro conditions they have shown excellent properties for bone replacement, including ordered mesoporous structure, high textural properties, rapid bioactive response and the ability to release concentrations of strontium ions, which can stimulate the expression of early markers of osteoblastic differentiation.24
For all these reasons, and given the extensive experience of the study group in the design and manufacture of this type of support, they were considered ideal candidates for carrying out this in vivo study.
The results obtained in the MBG group, both in the case of the simple radiology study and in the micro-CT study, showed that there was greater bone formation than in the case of the control without material, although not as much as in the bone marrow autograft group. This suggests that these scaffolds help in the formation of new bone, but are not sufficient on their own to replace bone marrow autograft. Gil-Albarova et al.25 reported in a study on a defect in a rabbit femur that bioactive glasses are capable of generating bone, although given their high bioactivity and porosity, could also be ideal for antibiotic release.
The interest in apatite-based materials for the development of bone substitutes is marked by their high acceptance as a biocompatible material given their similarity to the mineral component of bone,3 their osteoconductive capacity26 and their good integration into the bone defect. The CHA component that makes up this scaffold, integrated within an agarose matrix, can be easily degraded, favouring its progressive replacement by neoformed tissue. The porosity of these supports is between 50 and 800μm. This type of biomaterial, in turn, has been shown to present a surface suitable for the adhesion of pre-osteoblastic cells that could grow on the support without toxicity from any of the components that make up this material.3 For these reasons, this material was considered ideal for this in vivo study.
As for the results obtained from the radiological and micro-CT studies in the CHA group, they were essentially similar to those obtained in the case of the MBG. Again, more bone formation was shown than in the no-material control group, but not as much as in the bone marrow autograft group. These data are in agreement with those published by Oryan et al.27 in which a study with the same model comparing hydroxyapatite-based scaffolds with control and PRP-enriched hydroxyapatite showed that the hydroxyapatite scaffolds showed greater bone formation than the controls without material, although less than in the case of the PRP-enriched hydroxyapatite scaffolds. In another in vivo study on rabbit femur using this type of scaffold, it was concluded that they allow osteoconduction with an appropriate range of resorption and with wide intraoperative adaptability, obtaining bone formation in all defects.28
Once again, the scaffolds studied seem to improve bone formation with respect to the control group, but not enough to be considered bone substitutes with characteristics similar to autograft. This response may be due to the lack of osteoinductivity of this type of scaffold, which has been previously reported,29 even with the same experimental model.30
Although in terms of composition and macroscopic characteristics they are very different, no differences have been found in this preliminary study with regard to bone formation between the MBG-based biomaterials and those based on CHA. However, these macroscopic characteristics could make each of them more suitable for a specific type of defect. In the case of MBG-based scaffolds, they have greater initial strength, although on the other hand, they have less intraoperative adaptability to the defect, and the size of the defect must be planned in advance for the scaffold to fit it. For these reasons, this type of scaffold would be more suitable for defects involving cortical bone as well as medullary bone.However more in vivo studies are needed in this respect to confirm this assertion. In the case CHA-based scaffolds, they have a coralline consistency, which makes them less resistant initially, although they can be adapted to the defect intraoperatively, with the possibility of cutting the scaffold in the surgical act to adapt it to the defect. These characteristics, a priori, would position this type of scaffold as a more suitable option for defects that mainly involve cancellous bone.
In conclusion, we observed that both CHA-based and MBG-based scaffolds favour bone formation, but it is still far from being affirmed that they alone are capable of reproducing the characteristics of bone autograft. Our research group is working on the enrichment of these scaffolds with osteoinductive substances and progenitor cells to optimise the biological behaviour of these biomaterials and get closer to our ultimate goal of being able to use them as a substitute for bone autograft in our routine medical practice.
Level of evidenceLevel of evidence i.
FundingThis project was funded by the Foundation of the Spanish Society of Orthopaedic Surgery and Trauma by means of the grant for research initiation projects 2020 and by the Carlos III Health Institute with the FIS grant number PI20/01384, IP co-financed with European Regional Development Fund (ERDF) funds from the European Union.
Conflict of interestsThe authors have no conflict of interests to declare.
Ethics committee approvalIn compliance with RD 1090/2015, the study was evaluated and approved by the Ethics Committee in research with experimental animals of the I+12 Research Institute, the Ethics Committee/ animal experimentation subcommittee of the Autonomous University of Madrid, registration number: ES 28079-0001164 and by the Directorate General of Agriculture, Livestock and Food of the Community of Madrid, registration number: ES280790001164.





![Graph of the synthesis of MBG-based scaffolds. Scaffolds are designed using AutoCAD (BioploterTM 3D priter rapid prototyping equipment [EnvisionTEC, Gladbeck, Germany]) for subsequient printing by rapid prototyping. Graph of the synthesis of MBG-based scaffolds. Scaffolds are designed using AutoCAD (BioploterTM 3D priter rapid prototyping equipment [EnvisionTEC, Gladbeck, Germany]) for subsequient printing by rapid prototyping.](https://static.elsevier.es/multimedia/18884415/0000006700000004/v3_202404150749/S1888441523001030/v3_202404150749/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)