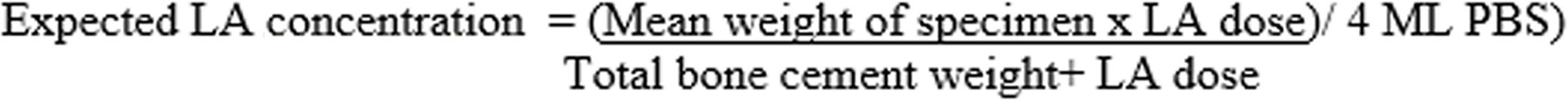Currently, we do not have a gold standard for pain management after total knee arthroplasty. We may use one of more drug delivery systems, none of which are ideal.
An ideal depot delivery system would provide therapeutic, nontoxic, doses of drug at the surgical side, especially during 72h postoperatively.
The bone cement used in arthroplasties has been used as a drug delivery system, especially antibiotics, since 1970. Based on this principle, we developed this study with the aim to characterise the elution profile of two local anaesthetics (lidocaine hydrochloride and bupivacaine hydrochloride) from PMMA (polymethilmethacrylate) bone cement.
Material and methodsPalacos® R+G bone cement and lidocaine hydrochloride or bupivacaine hydrochloride specimens were obtained depending on the study group. These specimens were immersed in PBS (phosphate buffered saline) and removed from the solution at different set times.
Subsequently, the concentration of local anaesthetic in the liquid was analysed by liquid chromatography.
ResultsThe percentage of lidocaine eluted from PMMA bone cement in this study was 9.74% of the total lidocaine content per specimen at 72h and 18.73% at 336h (14 days). In case of bupivacaine, the elution percentage was 2.71% of the total bupivacaine content per specimen at 72h and 2.70% at 336h (14 days).
ConclusionsLocal anaesthetics elute in vitro from PMMA bone cement, reaching doses at 72h close to the doses used in anaesthetic blocks.
Actualmente no disponemos de un gold standard para el manejo del dolor postoperatorio tras una artroplastia total de rodilla, dado que se pueden administrar analgésicos a través de diferentes vías y ninguna de estas está exenta de riesgos. El sistema ideal de administración de analgésicos debería proporcionar dosis terapéuticas, no tóxicas, en el sitio quirúrgico, especialmente durante las primeras 72 h.
El cemento óseo utilizado en las artroplastias se ha usado como un medio de liberación de fármacos, especialmente antibióticos, desde 1970. Basado en este principio, se desarrolló este estudio con el objetivo de conocer el perfil de elución de dos anestésicos locales (hidrocloruro de lidocaína e hidrocloruro de bupivacaína) desde el cemento óseo de polimetilmetacrilato (PMMA).
Material y métodosSe obtuvieron especímenes de cemento óseo Palacos® R + G e hidrocloruro de lidocaína o hidrocloruro de bupivacaína según el grupo de estudio. Estos especímenes se sumergieron en phosphate buffered saline (PBS) y se retiraron de la solución en diferentes cortes temporales establecidos. Posteriormente, se analizó la concentración de anestésico local en el líquido mediante cromatografía líquida.
ResultadosEl porcentaje de lidocaína eludida del cemento óseo PMMA de este estudio ha sido de 9,74% del contenido total de lidocaína por espécimen a las 72 h y de 18,73% a las 336 h (14 días). En el caso de la bupivacaína, el porcentaje de elución ha sido de 2,71% del contenido total de bupivacaína por espécimen a las 72 h y de 2,70% a las 336 h (14 días).
ConclusionesLos anestésicos locales eluyen in vitro desde el cemento óseo, alcanzando a las 72 h dosis cercanas a las dosis utilizadas en bloqueos anestésicos.
Total knee arthroplasty (TKA) is one of the most commonly used procedures in patients with chronic refractory knee pain in whom conservative treatment has failed.1
Postoperative pain after TKA implantation is usually moderate to severe and can be difficult to control, especially during the first 3 postoperative days.2 Achieving optimal pain control improves function, facilitates rehabilitation and attenuates the progression from acute to chronic pain.3 Currently, there is no gold standard for postoperative pain management after TKA,4–7 since analgesics can be administered through different routes and none of these is ideal, as adverse reactions are frequent and complications can arise.8 The ideal analgesic delivery system should be able to provide therapeutic, non-toxic doses at the surgical site, especially during the first 72h. In the management of pain following RKA implantation, polymethylmethacrylate (PMMA) bone cement could be used as a local drug delivery system to achieve an analgesic effect.
Since 1970, studies have been conducted analysing the ability of PMMA bone cement as a local drug release system, especially for antibiotics.9 The elution pattern of these drugs shows a high initial drug release followed by a decrease in drug release over the following days.10
The elution of drugs from the cement will be conditioned by some factors, such as the water uptake of the cement, the porosity of the cement matrix, the composition of the cement, the cement surface, the particle size of the drug and the drug content.11–13
Following these initial studies and taking into account that local anaesthetics (LAs) are drugs with an excellent safety and effectiveness profile, some authors have tested the elution capacity of LAs from PMMA bone cement,8,14 obtaining variable elution figures depending on the drug and the cement used.
The aim of this study is to analyse the elution profile of lidocaine hydrochloride and bupivacaine hydrochloride from PMMA bone cement with gentamicin.
Material and methodsThis study was conducted with the approval of the clinical research ethics committee (HCB 2020/0097) and following the regulations of the international standards ISO 5833:2022 Implants for surgery-Acrylic resin cements and the Standard Specification for Acrylic Bone Cement ASTM F451-16.
Two study groups were defined: cement group with lidocaine hydrochloride (GL) and cement group with bupivacaine hydrochloride (GB).
The LA dose was defined taking into account the elution percentages reported in previous studies8,14 and aiming for a total eluted dose of 020g lidocaine and .14g bupivacaine. These doses correspond to usual doses used in anaesthetic blocks and to the usual estimate of 3mg/kg and 2mg/kg for a patient with an average weight of 70kg for lidocaine15 and bupivacaine,16 respectively. The calculated doses were 2.48g in the lidocaine (GL) group and 3.58g in the bupivacaine (GB) group. Lidocaine hydrochloride and bupivacaine hydrochloride (Fagron®, Terrassa) were used.
Palacos® R+G (Heraeus Medical GMBH, Germany), a fast-setting, high-viscosity PMMA composite bone cement containing gentamicin, was used.
Moulds (Fig. 1) for the creation of the cylindrical specimens of 6mm diameter×12mm height were fabricated by machining in Teflon. As the standard does not specify the dimensions of the specimens for elution analyses, the references for mechanical studies on this type of cements, specified in ISO 5833:2002, were used.
For the preparation of the GL and GB specimens, the powdered LAs (lidocaine hydrochloride or bupivacaine hydrochloride) were mixed with the powdered component of the cement using the geometric dilution method. The liquid component of the cement was added to the mixture according to the manufacturer's recommendations. The mixture was allowed to stand for 30s and was introduced into the moulds, removing the excess. It was left to dry for 30min and then the specimens were removed.
A sample size of 3 specimens per study group and time cut-off was set arbitrarily, as there are no regulations governing the study of elution at present.
The elution process follows the law of diffusion (Fig. 2), is carried out from the surface and is directly related to the water absorption capacity of the cement. In order to obtain specimens with similar contact surfaces, the 3 most homogeneous specimens (A, B, C) of each group in terms of size and weight were selected.
Specimens were placed in cryovials, immersed in 4ml of saline (PBS) at room temperature and placed on an oscillating shaker. At the set cut-off points (1h, 3h, 6h, 24h, 48h, 72h, 168h and 336h) the specimens were removed from the solution and the cryovials were stored at −80°C. In the obtained samples, the concentration of AL in the liquid was analysed by liquid chromatography (HPLC).
To give internal validity to the determination method, the analysis of the AL concentration was repeated in triplicate, in each study group and time cut-off.
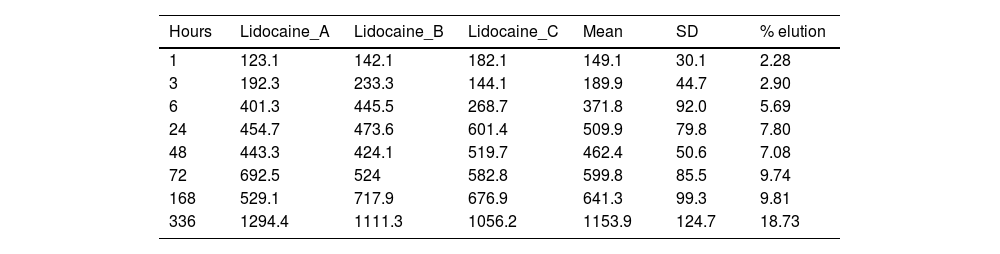
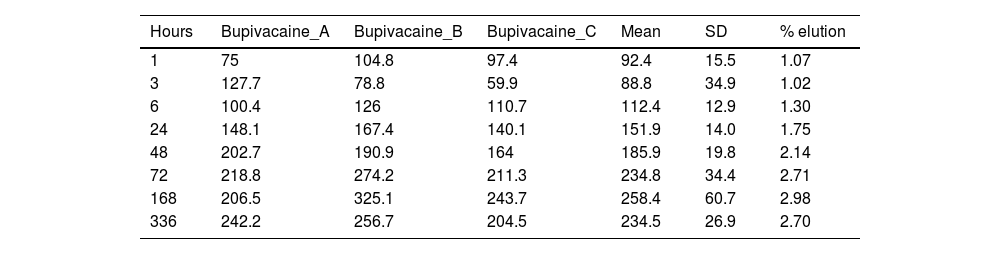
ResultsTables 1 and 2 show the concentration of lidocaine and bupivacaine in μg/ml in each of the samples analysed, and the mean and standard deviation (SD) of concentration at each time point. At the same time, the percentage of lidocaine and bupivacaine released with respect to the total LA present in the cement mixture is shown.
Concentration (μg/ml) and mean elution (%) of lidocaine.
| Hours | Lidocaine_A | Lidocaine_B | Lidocaine_C | Mean | SD | % elution |
|---|---|---|---|---|---|---|
| 1 | 123.1 | 142.1 | 182.1 | 149.1 | 30.1 | 2.28 |
| 3 | 192.3 | 233.3 | 144.1 | 189.9 | 44.7 | 2.90 |
| 6 | 401.3 | 445.5 | 268.7 | 371.8 | 92.0 | 5.69 |
| 24 | 454.7 | 473.6 | 601.4 | 509.9 | 79.8 | 7.80 |
| 48 | 443.3 | 424.1 | 519.7 | 462.4 | 50.6 | 7.08 |
| 72 | 692.5 | 524 | 582.8 | 599.8 | 85.5 | 9.74 |
| 168 | 529.1 | 717.9 | 676.9 | 641.3 | 99.3 | 9.81 |
| 336 | 1294.4 | 1111.3 | 1056.2 | 1153.9 | 124.7 | 18.73 |
Concentration (μg/ml) and mean elution (%) of bupivacaine.
| Hours | Bupivacaine_A | Bupivacaine_B | Bupivacaine_C | Mean | SD | % elution |
|---|---|---|---|---|---|---|
| 1 | 75 | 104.8 | 97.4 | 92.4 | 15.5 | 1.07 |
| 3 | 127.7 | 78.8 | 59.9 | 88.8 | 34.9 | 1.02 |
| 6 | 100.4 | 126 | 110.7 | 112.4 | 12.9 | 1.30 |
| 24 | 148.1 | 167.4 | 140.1 | 151.9 | 14.0 | 1.75 |
| 48 | 202.7 | 190.9 | 164 | 185.9 | 19.8 | 2.14 |
| 72 | 218.8 | 274.2 | 211.3 | 234.8 | 34.4 | 2.71 |
| 168 | 206.5 | 325.1 | 243.7 | 258.4 | 60.7 | 2.98 |
| 336 | 242.2 | 256.7 | 204.5 | 234.5 | 26.9 | 2.70 |
For the calculation of the percentage of lidocaine released with respect to the total lidocaine present in the specimen, the following estimation was made: in the GL group, 2.48g of lidocaine was added to 40.8g of powdered cement, representing a total powder weight of 43.28g. In group GB, 3.58g of bupivacaine was added to 40.8g of powder cement representing a total powder weight of 44.38g. If the average weight of the specimens was .43g and they were immersed in 4ml of PBS, the expected maximum concentration, if 100% lidocaine eluted, would be 6160μg/ml in the GL group and 8672μg/ml in the GB group. These calculations have been made using the formula shown in Fig. 3.
The percentage of lidocaine eluted from PMMA bone cement in this study was 9.74% of the total lidocaine content per specimen at 72h and 18.73% at 336h (14 days).
Fig. 4 shows the graph of the average eluted concentration of lidocaine in the different time slices.
The percentage of bupivacaine eluted from PMMA bone cement in this study was 2.71% of the total bupivacaine content per specimen at 72h and 2.70% at 336h (14 days). Fig. 5 shows the graph of the average eluted concentration of bupivacaine in the different time slices.
DiscussionTo carry out this study we chose the bone cement Palacos® R+G, a high viscosity cement containing gentamicin sulphate like the cements we usually use in our environment. Palacos® (Heraeus) is one of the most widely used brands on the market for arthroplasty fixation.17
We selected two LAs to evaluate their elution in bone cement with antibiotics. On the one hand, lidocaine, as an anaesthetic with a short half-life and, on the other, bupivacaine, as an anaesthetic with a longer half-life. Both have good thermal resistance, an essential condition, since in vitro PMMA can reach temperature peaks during curing of up to 80–90°C.11,18
Two previous studies have been performed analysing the elution of different LAs added to PMMA bone cements. In the study by Bond et al.8 they added lidocaine, prilocaine, bupivacaine and tetracaine to 5 PMMA bone cements without antibiotic. A percentage elution of the different LAs between .05 and 1.10% at 72h was reported. In the study by Balaguer et al.14 they added lidocaine and bupivacaine to a medium viscosity PMMA bone cement containing gentamicin. They reported a lidocaine elution percentage of 25.49% at 72h and 38.48% at 14 days. At the same time, they reported a bupivacaine elution rate of 3.18% at 72h and 4.53% at 14 days.
In our study, lidocaine elution percentages were 9.74% at 72h and 18.73% at 14 days (336h). In the case of bupivacaine, the elution percentage was 2.71% at 72h and 2.70% at 14 days (336h).
In the two study groups GL and GB and in the two time slices, elution was lower than that reported by Balaguer et al. These differences in results could be justified by the fact that they did not use the same bone cement as we did and used a different LA dose. Despite having used higher doses of LA in our study, the percentage of elution is lower. This result could be justified by the fact that the elution of drugs from the bone cement takes place from the surface10 and may be limited despite the increase in drug dose.
Our results and those of Balaguer et al. show a higher elution than that reported by Bond et al. In Bond's study, bone cement without antibiotic, physiological saline instead of PBS and other geometrical characteristics of the samples were used.
Regarding the interpretation of our results and from a theoretical point of view, we should take into account two main factors: the maximum total amount of drug eluted and the dose of drug needed to block the A and C fibres that are responsible for pain conduction.
If we were to use the entire cement (40.8g), the maximum eluted dose of lidocaine would be reached after 14 days (336h) with an elution percentage of 17.66%, corresponding to .438g of AL. As for bupivacaine, the maximum dose would be reached at 7 days (168h) with an elution percentage of 2.98%, corresponding to .106g bupivacaine.
In the case of lidocaine, the maximum dose would exceed the toxic dose of 3.5mg/kg in a 70kg adult. In contrast, bupivacaine would not exceed the toxic dose of 2mg/kg in a 70kg adult.19 In this sense, we have to take into account that this interpretation disregards the phenomena of absorption, metabolisation and elimination of LAs that would be present in in vivo studies.
If we interpret the elution results obtained at 72h, we observe that the amount of lidocaine eluted corresponds to .22g and that of bupivacaine to .09g. These figures are close to the usual doses used in anaesthetic blocks of .20g lidocaine and .14g bupivacaine.
With regard to the dose of LA required to achieve a therapeutic effect, we can use as a reference the classic electrophysiological studies20–24 that determine the amount of LA in vitro that blocks nerve conduction of the A and C fibres responsible for the transmission of painful stimuli. These in vitro studies establish doses of .084–08mM25,26 for lidocaine and .048–0.200mM24,27 for bupivacaine.
The elution results obtained in our study at 72h in the two study groups GL and GB exceed the minimum doses established for the two drugs.
LAs can be administered in the context of knee arthroplasty by different techniques such as anaesthetic blockade, LAI (local anaesthetic infiltration), joint puncture or experimentally using bone cement as a drug carrier. The latter technique would avoid the adverse effects associated with the techniques listed above and could allow drug elution to be maintained for a longer period of time.
Subsequent to the elution analysis of these drugs, we should analyse how their addition to bone cement acts on workability and mechanical properties.
Previous studies have reported that the manual addition of 2g of antibiotic powder to the powdered component of bone cement reduces the flexural strength by 20% and the impact strength by 23%.10 The regulations of the international standards ISO 5833:2022 Implants for surgery-Acrylic resin cements and the Standard Specification for Acrylic Bone Cement ASTM F451-16 set minimum values for flexural strength of 50MPa, compressive strength of 70MPa and Young's modulus of 1800MPa.
Regarding LAs, Giordano et al.28 reported that the addition of LAs to bone cement increases its impact strength. Lotfi et al.29 reported that bone cements that set in a liquid environment containing ropivacaine decrease their compressive strength with respect to those set in air.
The main limitation of this study is the fact that it is not possible to generalise the results to in vivo conditions due to the in vitro study and the small sample size analysed.
Our future lines of research include carrying out workability and mechanical tests to confirm that the addition of these drugs does not alter these properties of PMMA bone cement. In parallel, the possibility of using other anaesthetics such as levobupivacaine or ropivacaine, with a medium half-life and a high safety profile.
Level of evidenceLevel of evidence v.
FundingThis research was partially funded by the Spanish Society of Orthopaedic Surgery and Traumatology (SECOT) with the “Aid for Research Projects in Orthopaedics and Trauma Surgery” of the SECOT Foundation and by the Josep Trueta Grant of the Catalan Society of Orthopaedic Surgery and Traumatology (SCCOT).
Conflict of interestsThe authors declare that there is no conflict of interest with regard to obtaining the bone cements used from the Palacos® R+G brand or the local anaesthetics (lidocaine hydrochloride and bupivacaine hydrochloride) from the Fagron® brand.