Blood cobalt (Co) and chromium (Cr) ion levels have been used as surveillance tools for adverse reaction to metal debris in metal-on-metal (MoM) hip arthroplasty. The aim of our study was to present serial 7–13 year blood Co and Cr levels in a cohort of MoM total hip arthroplasties in asymptomatic patients.
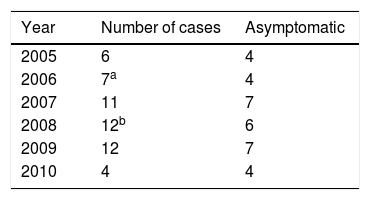
Material and methodsA total of 52 MoM surface total hip arthroplasties were included in this study with data collected prospectively. Annual follow-up with blood Co and Cr measurements was performed. Revision surgery was necessary for 16 patients and therefore they dropped out of the study. The metal ion levels were analysed separately in 31 asymptomatic patients.
ResultsHigh Cr or Co levels were not found continuously in any of the asymptomatic patients. The median Cr in blood was maintained between 1.3 and 5.4μg/L and that of Co between 0.5 and 1.2μg/L. After 7 years, there was no significant change in Co and Cr values.
ConclusionIn the medium term and in asymptomatic patients, the metallic blood levels remained unchanged; therefore, it does not seem appropriate to repeat these tests on a permanent basis in annual controls.
La determinación periódica de los niveles de cromo (Cr) y cobalto (Co) en sangre ha sido utilizada para evaluar las reacciones adversas secundarias al desgaste metal-metal en las artroplastias de superficie de cadera. El objetivo de nuestro estudio es evaluar los niveles de Cr y Co en sangre en una serie de pacientes asintomáticos con artroplastias de superficie con un seguimiento de 7 a 13 años.
Material y métodosHemos analizado la evolución de los niveles metálicos en sangre en una serie de 52 artroplastias de superficie metal-metal. Se realizó un control anual de Co y Cr en sangre. En 16 pacientes fue preciso realizar una cirugía de revisión, por lo que salieron del estudio. Los niveles de iones metálicos en sangre fueron analizados en 31 pacientes asintomáticos.
ResultadosEn ninguno de los pacientes asintomáticos aparecieron cifras elevadas de Cr o Co de manera continuada. La mediana de Cr en sangre se mantuvo entre 1,3 y 5,4μg/L y la de Co entre 0,5 y 1,2μg/L. Después de 7 años tras la cirugía no se han apreciado cambios en los niveles referidos.
ConclusiónA medio plazo y en pacientes asintomáticos, los niveles metálicos en sangre se mantienen sin cambios, por lo que no parece adecuado repetir anualmente estas determinaciones de manera permanente.
The good results obtained at the end of the last century with metal-on-metal (MoM) hip arthroplasties were overshadowed by the appearance of high levels of metal ions in the blood and urine. Together with the formation of periarticular cysts and a high level of failures, this forced certain companies to withdraw some models of implants from the market in 2010. The presence of high levels of metals in the blood is a serious complication and its effects over the long-term are still unknown.1 Due to this situation scientific societies (the Hip Society, BOA, AOA, EFORT, SECOT and SECCA), international agencies (the FDA) and health authorities (the Ministry of Health in Spain)2 published monitoring protocols for patients who had been operated using these procedures, indicating when revision surgery should be recommended and how patients with these arthroplasties should be monitored. These protocols are based on the clinical situation of patients, radiographic findings, raised levels of chrome (Cr) and cobalt (Co) in the blood. They are also based on the presence, detected by imaging techniques, of periarticular cysts known as ALVAL (aseptic lymphocytic vasculitis associated lesions), the histology of which is dominated by B-lymphocytes and plasma cells, suggesting an immune response similar to a delayed hypersensitivity reaction. These complications deriving from high level of metal in the blood are grouped in the literature under the name ARMD (adverse reactions to metal debris).
During the first years after the said alarm the necessary revision surgical operations were performed to remove MoM prostheses and implant conventional arthroplasties. The other patients have continued to regularly attend Hospital out-patient departments for evaluation using series of analytical checks. Although the literature states that MoM surface models have a shorter long-term lifespan than conventional arthroplasty models,3 a good number of patients with MoM arthroplasty implants are asymptomatic and show excellent evolution over the medium term.
Although discrepancies exist regarding the usefulness of determining metal levels in the blood to indicate revision surgery,4 this technique is still accepted as a means of evaluating whether these arthroplasties are functioning well or not in asymptomatic patients. It also supplies a criterion accepted by the majority to detect the presence of adverse reactions. The figure of 7μg/L or ppb of Co or Cr in the blood is considered to mark the limit of normality.1,5,6
The correct guideline for monitoring these patients is unknown, as is how often analysis should take place for metals in the blood and how long this should continue. The main question of our work is whether it is necessary to monitor levels analytically during the whole lifetime of asymptomatic patients who have MoM surface arthroplasties. We analysed blood levels of Cr and Co and how they evolved over the years in patients who have shown no clinical or radiographic alterations.
Material and methodsA case study – monitoring diagnostic techniques. Our series is composed of 51 patients (52 arthroplasties, with one bilateral case) operated from 2005 to 2010 using the same type of arthroplasty (ASR, DePuy Orthopaedics, Inc., Warsaw, IN, U.S.A.), in the same hospital and by the same surgeons. The indication was highly selective, given that these cases amounted to only 4% of all the total hip arthroplasties (THA) implanted the 6 years in question in the Department.
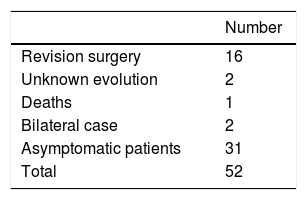
The average age in the group of patients was 57.5±9.05 years old (35–76) and the average body mass index was 29.42±5.37 (19.48–43.58). Two patients were women and 49 were men. Sixteen revision surgeries were performed in as many patients. The causes were: 8 due to high levels of metals in the blood and the presence of ALVAL, 2 due to the presence of ALVAL with normal levels, 2 due to fracture of the neck of the femur, one due to osteolysis in the neck of the femur, one due to instability with repeated luxations, and 2 due to pain with an unexplained cause. 5 cases were lost: 2 whose evolution is unknown, and 3 patients died, 2 of them after the switch to a conventional THA (Table 1). The bilateral case was excluded from this study because of the possibility of introducing distortions in blood metal levels.
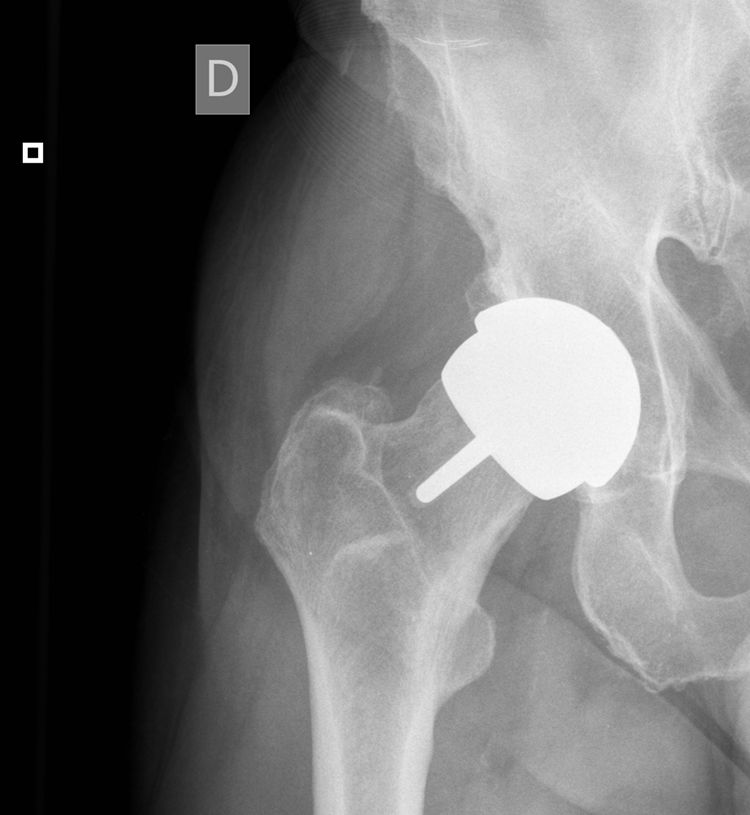
Thus during all of the years in question we monitored 31 MoM arthroplasties implanted from 2005 to 2010 (Table 2). At the time we undertook our work the arthroplasties had an age of from 7 to 13 years (Fig. 1). The patients attended the department clinic annually for analytical studies of the blood and 24 hour urine, a clinical examination (filling out the WOMAC, SF-12 and Oxford Hip questionnaires) and a standard radiographic study of the operated hip. Blood was extracted and transported to the laboratory under the conditions set for this process. Samples were analysed using mass spectrometry with an inductively coupled source (ICP-MS). Until the year 2013 molybdenum in blood and urine was also measured, together with detection of metals in the hair thanks to a SECOT research grant (results already published). Magnetic resonance (MR) imaging was indicated to rule out ALVAL or osteolysis if the patient showed functional reduction and pain, or when levels above 20μg/L were detected in annual analysis. If levels of Co and Cr were from 7 to 19μg/L and the patient was asymptomatic then they visited again after 6 months for another metal ion analysis. This work was approved by the Regional Ethics Committee (Reference 037/2011) and the patients signed an informed consent document prepared specifically for this study.
The prosthesis sizes used in the series were above all those with a 58mm diameter acetabular cup (19 cases) and 56mm (12 cases). Taking into account that this model has a range of sizes from 50mm to 64mm in diameter, it can be said that medium sized were implanted above all.
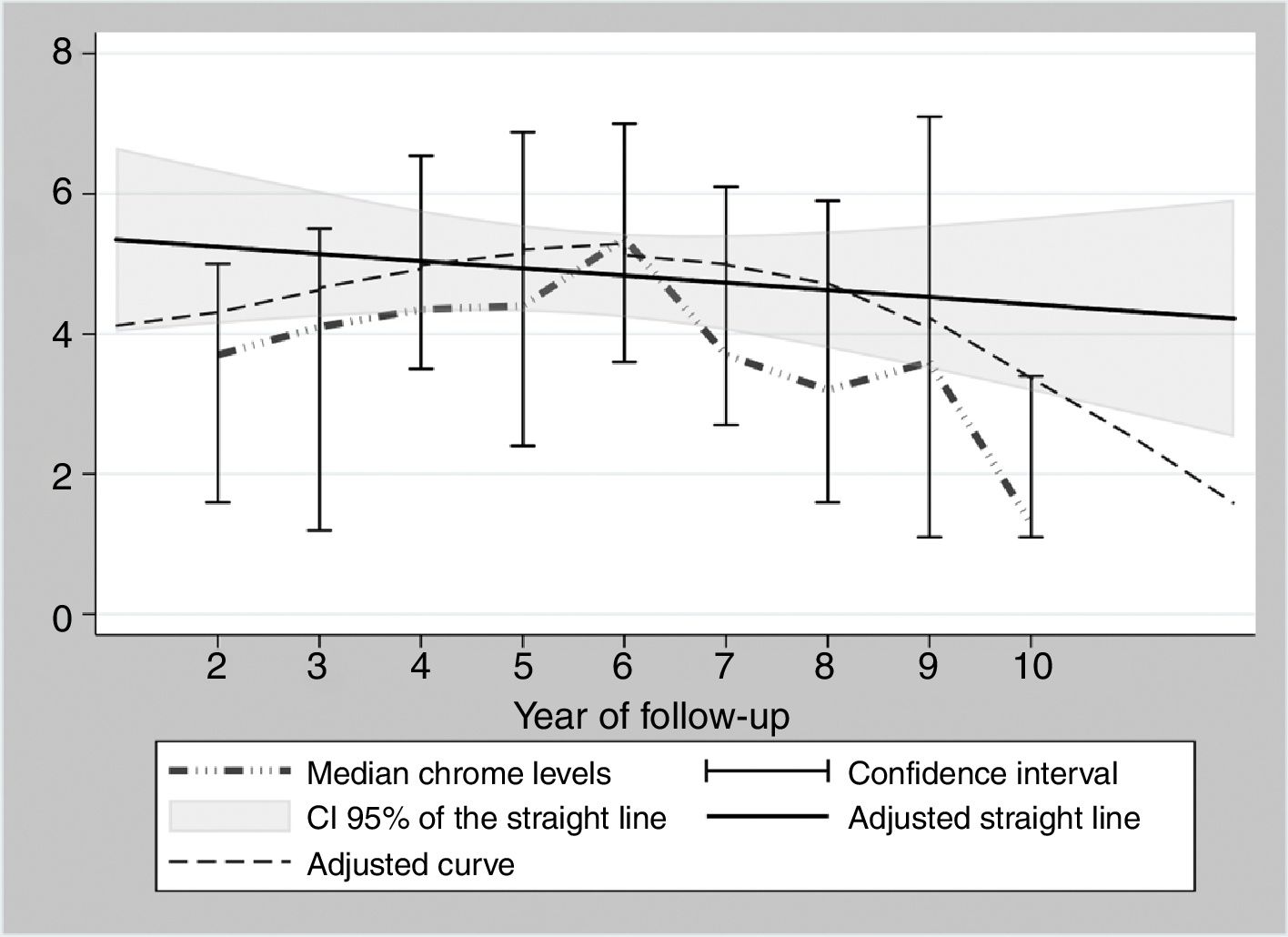
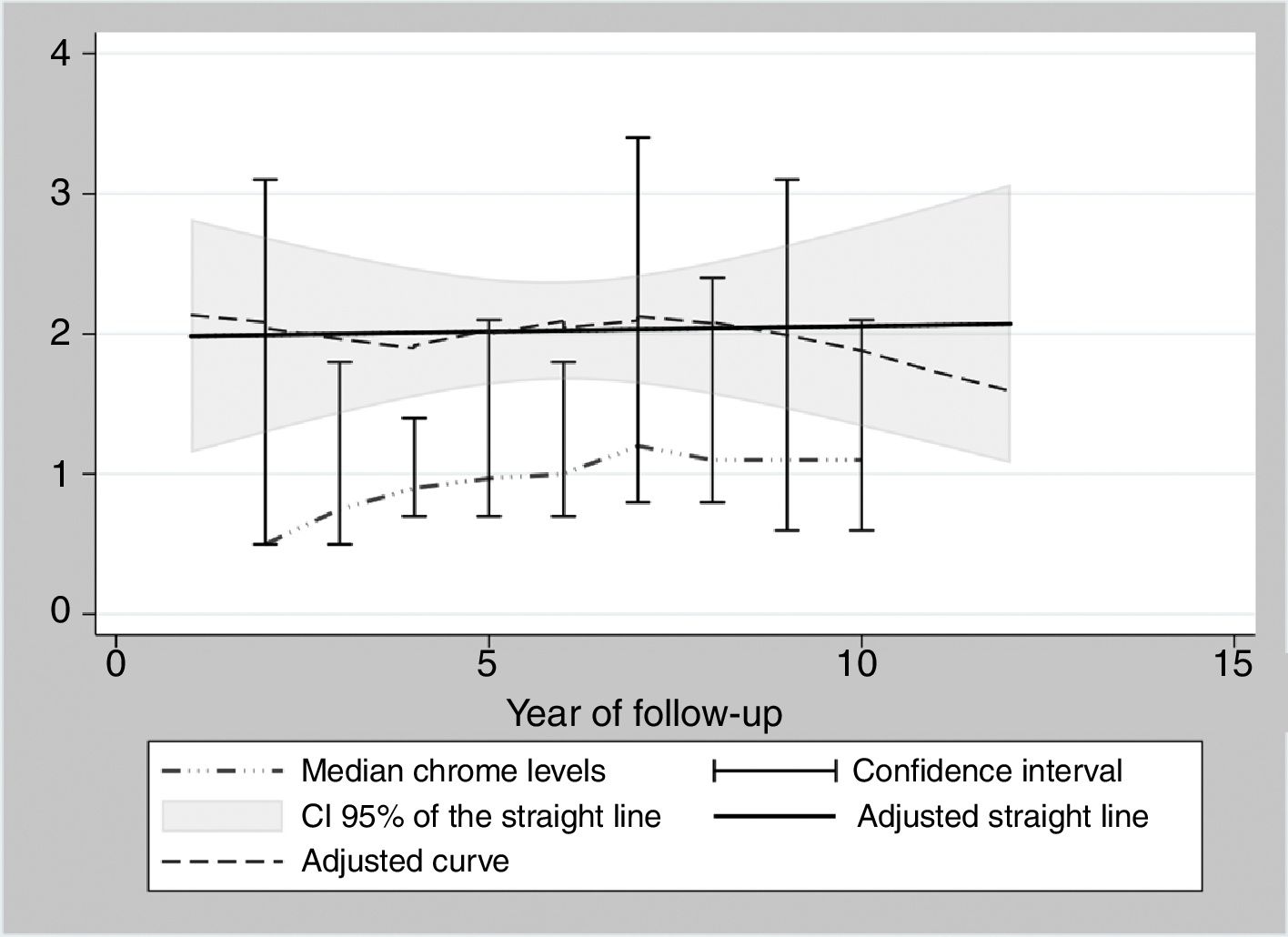
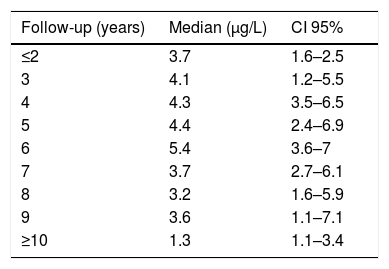
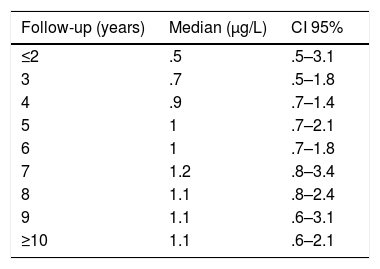
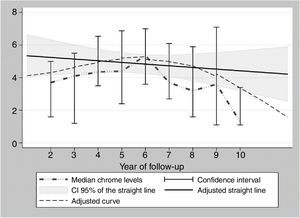
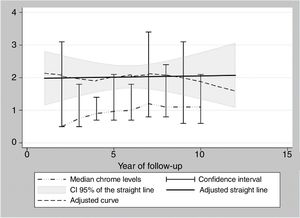
During the years in question Cr Levels in the blood remained at from .5 to 14μg/L, with a median level from 1.3 to 5.4μg/L (Table 3). Co levels were from .5 to 13.8μg/L, with a median at from .5 to 1.2μg/L (Table 4). After 5 years a reduction in both metals was detected, although they increased during the sixth year before falling again. No changes were detected after 7 years (Figs. 2 and 3).
Under the circumstances described above (raised levels of Cr or Co in the blood or change in clinical and functional situation) MR imaging was performed with normal results in all cases, without detecting ALVAL-type periarticular cysts. The levels of both metals were not found to increase continuously in successive annual checks in any patient.
One patient with high levels of Cr and Co in annual checks refused to undergo hip MR imaging to search for periarticular cysts; he remains asymptomatic and regularly attends for clinical, radiographic and analytical tests. In this case figures for Cr have evolved from 11.4 to 14μg/L, while those for Co have evolved from 13.8 to 6.6μg/L.
Data analysis and representation. As there are a small number of highly variable data, the levels of Cr and Co per year are shown as their median together with their confidence interval at 95%. These figures were calculated using the binomial method that does not make suppositions on the underlying distribution of the variable. Box graphs were created according to the levels of Cr and Co for each year of monitoring. These graphs adjust data to a straight line, using the squared minimums method and a curve using the “lowess” command in STATA v.15 (STATA Corp., TX, U.S.A.).
DiscussionAfter time the true scope of the failures of MoM surface arthroplasties is becoming clear. A period in which they were used for a wide range of indications has been followed by another period of almost complete restriction due to health alarms. As longer-term monitoring bears fruit we are able to learn the true place these models should occupy in the surgical arsenal for hips. They are less aggressive and offer better bone conservation, easier revision surgery and many patients describe their good evolution. In the future these factors will lead to the design of new types of surface once problems deriving from the materials used have been resolved.
The percentage of failures of MoM arthroplasties in our series is similar to those published by other authors.7,8 As is the case in other series, when revision surgery was carried out during the early years after implant, the most common cause was progressive pain together with high levels of metals in the blood and the finding of ALVAL in MR imaging. Other complications were less common and coincide with those published in broader series. We do not know the reason why patients progress in different ways. While in some patients failure occurs in the short term, in others the clinical result is excellent and patients request this same type of prosthesis for their other hip when an arthroplasty becomes necessary.
Although the presence of metals in other organic structures such as hair9 has been analysed, as has the concentration of other metals like molibdenum,10 the studies usually used to rule out metal intoxication after MoM arthroplasties centre on analysing blood Cr and Co. Although the literature on this respect is not fully in agreement, it is accepted that patients with low levels of metal ions in the blood are at low risk of ARMD following MoM hip arthroplasty.11,12 Nevertheless, the decision to perform revision surgery must depend on individual factors and not exclusively on a certain specific analysis.13 Several associated findings are considered to be necessary for this: clinical, imaging and analytical. For the latter, series of measurements and the tendency they display are considered to be more important than momentary values in predicting failure for arthroplasties of this type.
There is no agreement in the literature on acceptable limits for blood metal levels; although some works do not accept that >7μg/L indicates a potential soft tissue reaction,14 this is the threshold recommended in monitoring protocols. Figures higher than 7μg/L of Cr and Co in the blood after the implant of a MoM arthroplasty are considered to be raised and need special monitoring.6 Levels up to 10μg/L are considered to indicate risk and should be evaluated together with the results of other examinations. Co values ≥10μg/L indicate an increase in joint wear, with a specificity of 100% and a sensitivity of 93%. A Co concentration of from 10 to 20μg/L has to be considered highly abnormal, while above concentrations higher than 20μg/L Co in repeated measurements are unacceptable due to the risk of systemic intoxication, and surgical treatment in the short term must be considered.15 Intoxication by this metal is known as cobaltism, and it may also occur in the case of other types of prosthesis surfaces.16 In our study we consider different alternatives depending on the levels found, considering 7 to 19μg/L to be the range which demands careful monitoring.
The cause of high blood levels of metals in cases of MoM arthroplasties has not been determined. It has been blamed on wear of the surfaces, an exaggerated acetabular angle, large cup size, gender, individual hypersensitivity, defective treatment of the metal surfaces or their corrosion.17–19 Nor is it known why metal levels vary over time. Some studies20 detect a fall in Cr but not in Co over the years. Other studies21 detect raised levels of both metals in the first two years that then fall, although a rise in the level of Co was detected after 4 years together with a rise in Cr 6 years after the implant, followed by a fall. In our study we did not find such high levels in the first years, although there was an increase after 6–7 years which went on to normalise (Figs. 2 and 3). We also found higher levels of Cr than Co throughout the follow-up, as well as a wider range between the upper and lower limits and less uniform data for Cr. As we pointed out above, the causes of these variations are unknown; it seems that they are not important if the curve remains flat or falls over time. Although our study is limited by its short duration, as are others,21 it seems that after 7 years levels of these ions either stabilise or continue falling.
Although recommendations for monitoring these patients have changed over the years, they have not been agreed. Some scientific societies recommend increasing the time between analytical studies in asymptomatic patients 7 years after the implant. Given how these patients are observed to progress, annual analysis is not thought to be cost-effective if they have no pathological clinical data.22,23 Other organisations recommend continuing regular checks only in certain cases, due to patient sex, cup size or the type of implant.24,25
Our work has limitations. Its greatest limitation is due to the small number of patients studied, as this also prevents appropriate statistical analysis and therefore may reduce the validity of the conclusions. Our patients have a limited follow-up. Nevertheless, the period studied is of similar length to those of other published studies.21,26 Men predominate in our series, so this may lead to major distortion if the results are extrapolated to the general population. On the other hand, this work does not analyse how hip arthroplasties evolve over the years radiographically, clinically or functionally; our study is solely concerned with series of analytical determinations of blood Cr and Co.
A slight rise in blood Cr and Co levels in a single annual evaluation should not lead to revision surgery in patients with MoM arthroplasties. On the other hand, it is more important to observe how levels change in a series of analyses over time than it is to base our decision on a single isolated high level of metals. It is also indispensible to evaluate other findings and most especially the clinical state of the patient.
For asymptomatic patients blood metal levels remain stable over the medium term, so that it seems unnecessary to continue repeating annual determinations of the same.
Level of evidenceLevel of evidence IV.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Hernández-Vaquero D, García-Pascual M, Iglesias-Fernández S, Escandon-Rodríguez A. Artroplastias de superficie metal-metal en cadera. ¿Es necesario realizar seguimiento anual mediante determinación de metales en sangre? Rev Esp Cir Ortop Traumatol. 2018;62:436–441.