Periprosthetic joint infection (PJI) is a major cause of morbidity and mortality in patients undergoing total joint arthroplasty (TJA). Concurrently, the economic burden of this disease is expected to reach $1.85 billion by 2030.1–3 Furthermore, as the annual volume of primary and revision TJA procedures continues to expand, the burden of subsequent infection is also expected to rise.3,4 Currently, main surgical treatment options for PJI include Debridement, antibiotics and implant retention, one stage exchange arthroplasty and two stage exchange arthroplasty.5–7 Although there has been renewed interest in single stage exchange arthroplasty, two stage exchange arthroplasty remains the preferred treatment option for patients with chronic PJI, at least in North America.8–10
The first stage of a two-stage exchange for the management of chronic PJI involves extraction of the infected prosthesis, removal of other foreign material such as bone cement, followed by chemical and mechanical debridement of nonviable necrotic tissues, and insertion of antibiotic impregnated cement spacer. Following this procedure, tailored antimicrobial therapy is administered for about 6 weeks. The goal of the first stage is to reduce bioburden and remove the infected prostheses. The interval separating the first and second stage is to allow for administration of local and systemic antimicrobials to further reduce bioburden. The second stage, reimplantation, is performed when infection is considered to be under control.
To date, no concrete protocol for optimal timing of reimplantation exists.11 The purpose of this article is to provide an overview on the prognostic utility of parameters that may guide the optimal timing of reimplantation.
Historical recommendations for timing of reimplantationTwo stage exchange arthroplasty first came to prominence in the 1990s following descriptions by Nelson12 and Insall.13 Nelson described a small number of PJI cases treated with incision and drainage, single stage exchange, and two stage exchange. Importantly, only patients undergoing two stage exchange arthroplasties had successful eradication of infection. Similarly, Insall et al. also described a small series of patients with PJI of the knee managed with a two-stage exchange and reported a success rate of 100%.13 However, there was no description of the method by which the duration of the interval was established, nor any methods recommended for deciding the optimal timing for reimplantation.13 Over the subsequent decades, several series were published with a focus on comparing outcomes of two stage exchange arthroplasty to single stage exchange arthroplasty. A study in 1995 by Garvin and Hanssen reviewed existing literature and found that patients undergoing two-stage exchange procedure had success rates of 82%, compared to 58% for those undergoing single-stage exchange arthroplasty.14 However, early reports were unable to establish an optimal timeframe for reimplantation. In a study by McDonald et al., the authors found that >1 year interval between resection and reimplantation was associated with better treatment outcomes.15 In contrast, Colyer et al. showed that a short interval between resection and reimplantation (1 month) was more suitable for elderly patients.16 The decision to introduce an antibiotic holiday was first described in Insall's article as a measure to guard against an infection that is paradoxically suppressed rather than eradicated. This, along with other measures such as serial serological markers, pre-operative aspirates and intraoperative samples were reported as being useful when determining timing of reimplantation. From these early reports, the most common two stage revision protocol emerged which involved a six week interval following the first stage, often followed by a two week antibiotic holiday, and then subsequent reimplantation when no clinical sign of infection recurrence was apparent.17
Serological markersThe efficacy of serological markers to guide optimal timing of reimplantation has been widely studied. The methodology for most studies investigating serological markers have been retrospective reviews of patients undergoing two-stage exchange with identification of those which failed compared to those in whom infection was successfully controlled.
CRP and ESR are established serological markers utilized in diagnosis of PJI.18 As such, they have been the focus of multiple studies investigating their ability to guide optimal timing of reimplantation. To our knowledge, a cut off level for ESR or CRP that would indicate persistent infection has not been established.19–28 Although early reports on two stage exchange outcomes recommended CRP and ESR return to normal levels prior to initiation of the second phase to limit the possibility of residual infection, there has been little to no data to support the implementation of this practice.17,20,29 In one study, CRP and ESR were found to have little to no utility in predicting failure following reimplantation with a reported AUC of 0.545 and 0.503 respectively.19 Further investigations have found similar results with reported AUC for CRP and ESR between 0.39–0.63 and 0.47–0.76, respectively.20,21,23,27,28 Furthermore, a systemic review found a pooled sensitivity of 53% and specificity of 72% for CRP. For ESR, a pooled sensitivity of 56% and specificity of 60% was reported.26
Failure to establish an ideal cut-off value for CRP and ESR has led some to believe that the use of CRP and ESR trends, rather than a cut-off value, may be more suitable in determining infection control. Unfortunately, several studies in the literature have failed to identify a correlation between the percentage change in CRP and ESR levels and infection control prior to reimplantation.19,24,27,30,31 However, a recent study observed that consistently elevated CRP levels (>10mg/L) was associated with a higher rates of subsequent failure.32 In addition, patients with persistently elevated CRP were often subjected to prolonged antimicrobial treatment and delayed reimplantation, who also experienced a higher rates of subsequent failure.33
An early study by Shahi et al. supported the use of plasma D-dimer in predicting failure of reimplantation. They found that in a group of 245 patients, a D-dimer threshold above 850ng/mL was 89% sensitive, 93% specific for diagnosing PJI and could also predict the presence of infection at the time of reimplantation. Interestingly, there were five patients in that cohort with elevated D-dimer and normal ESR and CRP, two of whom had positive cultures taken during reimplantation.34 In contrast, a study by Xu et al. found that at a threshold of 820 ng/mL provided a sensitivity of 83.3% but a lower specificity of 41%, with an AUC of 0.565.35 A higher plasma D-dimer threshold of 3070ng/mL demonstrated a sensitivity of 90% and a negative predictive value of 94% but a low specificity of 47% and a positive predictive value of only 33%.36 Further studies attempting to correlate trends in D-dimer with optimal timing for reimplantation have revealed no consensus. In some cases, D-dimer levels would remain the same or paradoxically increase between 1st and 2nd stages,37–39 while other cases within the same cohort would have down trending D-dimer levels.39 Based on the current evidence it appears that elevated D-dimer at the time of reimplantation is suggestive of persistent infection, however, normal d-dimer level does not exclude the possibility of a latent infection.
Due to the absence of an alternative marker, the prognostic utility of serum fibrinogen in the setting of reimplantation has also been investigated.35,40 One study observed that in contrast to D-dimer, CRP, and ESR, fibrinogen levels declined significantly in the interval between the first and second stage.40 In addition to this, Xu et al. found that a plasma fibrinogen cut-off of 3.61g/L demonstrated an AUC of 0.773, sensitivity of 87.5%, and specificity of 62.8% for predicting failure following reimplantation.35 Furthermore, Shao et al. reported that a similar threshold of 365mg/dL demonstrated an AUC of 0.831, sensitivity of 72.7% and specificity of 83.2%. However, the latter study was limited by a small sample size of 119 patients with only 11 patients failing treatment.41 Conversely, another study found that fibrinogen levels were similar between patients that failed treatment, and those that had successful treatment with little to no prognostic utility in determining failure following reimplantation with an AUC of 0.586.30 In conclusion, larger prospective studies are required in order to determine the role of fibrinogen in this setting.
Interleukin-6 (IL-6) is a cytokine that drives the production of acute-phase reactants, including CRP, by stimulating inflammatory cells. Several studies have attempted to determine whether serum IL-6 levels could aid in timing of reimplantation. In the majority of studies, serum IL-6 levels were found to be comparable in the failure and treatment success groups.30,42–44 Although multiple studies found serum IL-6 levels decreased significantly in the interval between resection and reimplantation, the Δ serum IL-6 was not capable of predicting treatment outcomes in this setting.30 Therefore, based on available data serum IL-6 cannot be relied on to determine timing of reimplantation.
Joint aspiration prior to reimplantation has been shown to have some prognostic value for predicting failure following reimplantation and was endorsed by the most recent International Consensus Meeting (ICM) on Musculoskeletal Infections.11 A variety of analysis techniques have been evaluated including cultures, white cell count, percentage of polymorphs, alpha defensin, and leukocyte esterase.
Studies on preoperative aspiration cultures have consistently reported a low sensitivity for identifying infection persistence with sensitivities ranging from 0 to 47%.45–50 Mont et al. claimed 100% infection control after a spacer exchange on all three patients with positive cultures prior to reimplantation.51 However other reports on the outcomes of spacer exchange have been far less favorable with high failure rates.52,53 Whilst a positive culture during reimplantation is highly predictive of a subsequent failure, the low sensitivity of culture means that in a high proportion of persistent infections culture may be negative.
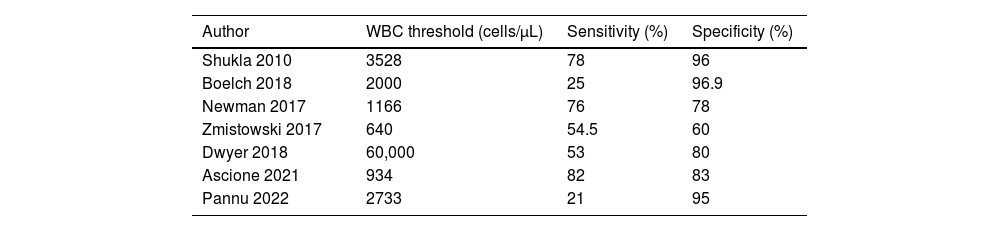
WBC count and percentage of polymorphonuclear (PMN) cells are metrics that have been investigated both for diagnosis of PJI as well as optimal timing of reimplantation. However, the literature remains disputed on the utility of these markers to diagnose persistent infection and guide timing of reimplantation. For WBC count, some studies claim moderately high sensitivities ranging from 53 to 82%,20,50,54–56 while other studies have reported significantly lower sensitivities ranging between 10 and 31.3%.23,47,48,57 Specificity for elevated WBC is reported to be high between 60 and 96.9%20,23,47,50,54–57 with only one study reporting a low specificity of 39.1%.48 These studies suggest that an elevated WBC count could be indicative of persistent infection. The thresholds calculated for a WBC count that would be suggestive of persistent infection varies across the studies ranging from 640 to 3528cells/μL (see Table 1).20,23,47,50,54–57 Sensitivities are generally improved when threshold is lowered to 1000cells/mm3 as compared to 2018 ICM thresholds of 3000cells/mm3.58
Reported WBC thresholds from joint aspirate to indicate persistent infection following 1st stage revision.
| Author | WBC threshold (cells/μL) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Shukla 2010 | 3528 | 78 | 96 |
| Boelch 2018 | 2000 | 25 | 96.9 |
| Newman 2017 | 1166 | 76 | 78 |
| Zmistowski 2017 | 640 | 54.5 | 60 |
| Dwyer 2018 | 60,000 | 53 | 80 |
| Ascione 2021 | 934 | 82 | 83 |
| Pannu 2022 | 2733 | 21 | 95 |
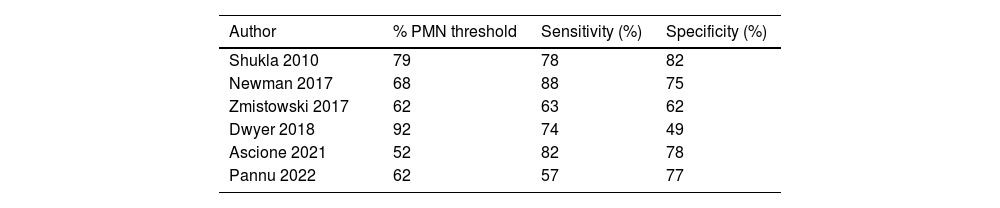
Findings in literature for percentage of PMNs are similar to that of WBC count, with some studies claim moderately high sensitivities ranging from 63 to 88%25,27,56,57 while others report lower sensitivities.59,60 Again, most studies reported high specificities ranging between 62 and 88%20,23,50,54,56,57 with only Dwyer et al. reporting a lower specificity of 49%.55 Calculated thresholds for percentage of PMNs also vary widely across studies, ranging from 52 to 92% (see Table 2).25,27,56,57,60 Based on these studies, as compared to the 2018 ICM threshold of 80%, a lower threshold of 65–70% may be more appropriate in the setting of an antibiotic spacer in situ.
Reported % polymorphonuclear cell thresholds from joint aspirate to indicate persistent infection following 1st stage revision.
| Author | % PMN threshold | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| Shukla 2010 | 79 | 78 | 82 |
| Newman 2017 | 68 | 88 | 75 |
| Zmistowski 2017 | 62 | 63 | 62 |
| Dwyer 2018 | 92 | 74 | 49 |
| Ascione 2021 | 52 | 82 | 78 |
| Pannu 2022 | 62 | 57 | 77 |
Other, synovial biomarkers that have also been investigated in the setting of reimplantation include alpha defensin and leukocyte esterase. Recent studies have reported a consistently low sensitivities (0–7%)59 but with high specificities (89–97.8%) for alpha defensin in detecting persistent infection.44,59,60 In one study by Kheir et al., synovial leukocyte esterase was shown to be very promising marker in detecting latent infection following resection arthroplasty. They reported on a series of 109 patients that underwent a two-stage exchange arthroplasty, of whom, 19 patients experienced subsequent failures. The leukocyte esterase test was negative in all patients who had successful outcome while it was positive in 5 of 19 patients who failed, subsequently accounting for a sensitivity, specificity, positive predictive value, negative predictive value, and AUC of 26.3%, 100%, 100%, 87.5%, and 0.632, respectively.61 A recent study by different investigators had similar findings with a sensitivity, specificity, positive predictive value, and negative predictive value 82%, 99%, 90%, and 97%, respectively and an AUC of 0.9044.62 Based on the available reports, synovial leukocyte esterase appears to have a promising utility in determining the presence of a latent infection at the time of reimplantation and should be exploited, whenever possible.
Antibiotic holidayA period of two weeks or so after the completion of antimicrobial treatment and prior to reimplantation has been termed as the “antibiotic holiday”.63 The rationale for having this period is to allow for latent infection to surface prior to reimplantation. In recent years the value and rationale for antibiotic holiday period has been questioned by several studies that have shown no difference in success of two-stage exchange between groups experiencing or not experiencing an antibiotic holiday.64–66 In fact, one study found that performing reimplantation without discontinuing antibiotics was associated with a more favorable outcome (OR=3.32), when compared to patients undergoing an “antibiotic holiday”. This led to the recommendation by the 2018 ICM to avoid subjecting patients to an antibiotic holiday.11
Duration of intervalGiven the elusive nature of a laboratory test that can act to inform optimal timing for re-implantation, there has been a focus on determining if the duration of the interval has a direct impact on treatment outcomes. As previously mentioned, early reports advocated for an interval of six weeks, however, varying durations have been reported from four weeks to greater than 12 months. Recently, there has been several reports that highlight increased failures associated with a longer time to reimplantation.67–73 The duration of the interval varies greatly between studies, with some reporting increased rates of infection with an interval greater than four weeks,67,71 greater than 11–14 weeks,68,69 and greater than 16–18 weeks.70,72 In addition, it is not clear whether extended intervals in the aforementioned studies were utilized in order to administer prolonged antibiotics (>8 weeks) or to allow for the implementation of an “antibiotic holiday”. Furthermore, patients with prolonged time to reimplantation may have demonstrated signs of infection persistence, introducing significant selection bias.
Benefits to shorter intervals clinically include faster mobilization, ability to treat within a single hospital stay, and less likelihood of contractures.71 As a result, short interval two stages procedures have garnered significant interest in this setting. In a study examining the outcomes of short-interval two-stage exchange, the authors found no increased failure rates when the time to reimplantation was reduced to just 3 weeks.74 Furthermore, Winkler et al. showed no difference in treatment failure rates between patients with a time to reimplantation of >4 weeks, compared to those with a time to reimplantation of <4 weeks.71 In contrast, Cochran et al. found that a time to reimplantation of <4 weeks was associated with an increased risk of treatment failure.75 Based on currently available data, there is little evidence to suggest any additional benefit from a prolonged time to reimplantation (>18 weeks) and an interval of 4–12 weeks may be optimal.
Frozen sectionsThe utility of frozen sections to determine persistence of infection in two-stage revision has low sensitivity but high specificity for identifying infection persistence at the time of reimplantation. Multiple definitions of a positive frozen currently exist, with the two most common ones developed by Mirra et al. (>5 polymorphonuclear (PMN) leukocytes per high power field (HPF) in ≥5 microscopic fields), which has been adopted by MSIS, and Athanasou et al. (>1 PMN per HPF in 10 microscopic fields).76
Most studies agree that the use of frozen section to determine persistence of infection prior to reimplantation has resulted in low sensitivities ranging from 25 to 28.5%.77–79 There is however, one study that reported high sensitivities of 90% with the Feldman criteria.70 The inconsistencies in reported sensitivities have been attributed to the virulence of organisms, as less virulent organisms such as coagulase-negative staphylococci generate a lower inflammatory response.80,81 Therefore, microbiology profiles of patient cohorts studied may influence sensitivities. There is less dispute for specificities of frozen section reported, with most falling in the range of 83.1–100% regardless of the cut-off criteria used.70,77–79 Furthermore, a positive frozen section has been correlated with increased likelihood (odds ratio [OR]=4.2) and rates (hazard ratio [HR]=4.2) of treatment failure, even in settings of prolonged antibiotic suppression and normal serum tests.70,82 There is one study that cautions against delaying reimplantation even in the presence of 5–20 PMNs in intraoperative frozen sections, reporting that they were still able to achieve 100% infection eradication despite positive frozen sections.83 The study was limited by a small sample size of 15 but noted that most positive frozen sections will likely exhibit>20 PMNs regardless of persistence of infection. Therefore, the use of frozen sections as a screening tool should be avoided, however most studies agree that it may be useful in confirming a diagnosis of persistent infection regardless of criteria utilized.
SummaryThere is a desperate need for well-established protocol that can guide optimal timing of reimplantation. In addition, studies are needed to better define the role, and the threshold, for common serum and synovial tests in determining the optimal timing of reimplantation. We believe such protocol needs to rely on clinical signs indicating soft tissue healing and absence of florid signs of infection, the use of common serum tests such as CRP, ESR and D-dimer, as well as synovial markers such as WBC, neutrophil percentage and leukocyte esterase. Frozen section may also be utilized in selective cases to confirm the presence of infection. Based on available evidence, subjecting patients to antibiotic holidays appears to be pointless and should be stopped. It is of paramount importance that the interval between the first and second stage is used to fully optimize patients and control comorbidities such as diabetes, anemia, and malnutrition.
Conflict of interestThe authors declare they have no conflict of interest.