Treatment paradigms for patients with spine metastases have evolved significantly over the past two decades. The most transformative change to these paradigms has been the integration of spinal stereotactic radiosurgery (sSRS). sSRS allows for the delivery of tumoricidal radiation doses with sparing of nearby organs at risk, particularly the spinal cord. Evidence supports the safety and efficacy of radiosurgery as it currently offers durable local tumor control with low complication rates even for tumors previously considered radioresistant to conventional external beam radiation therapy. The role for surgical intervention remains consistent, but a trend has been observed toward less aggressive, often minimally invasive techniques. Using modern technologies and improved instrumentation, surgical outcomes continue to improve with reduced morbidity. Additionally, targeted agents such as biologics and checkpoint inhibitors have revolutionized cancer care by improving both local control and patient survival. These advances have brought forth a need for new prognostication tools and a more critical review of long-term outcomes. The complex nature of current treatment schemes necessitates a multidisciplinary approach including surgeons, medical oncologists, radiation oncologists, interventionalists and pain specialists. This review recapitulates the current state-of-the-art, evidence-based data on the treatment of spinal metastases and integrates these data into a decision framework, NOMS, which is based on four sentinel pillars of decision making in metastatic spine tumors: Neurological status, Oncologic tumor behavior, Mechanical stability, and Systemic disease burden and medical co-morbidities.
Los paradigmas de tratamiento para pacientes con metástasis de columna vertebral han evolucionado significativamente en las últimas dos décadas. El cambio más transformador de estos paradigmas ha sido la integración de la radiocirugía estereotáctica espinal (sSRS). La sSRS permite la administración de dosis de radiación lítica con preservación de los órganos cercanos en riesgo, particularmente la médula espinal. La evidencia apoya la seguridad y la eficacia de la radiocirugía, ya que actualmente ofrece un control tumoral local duradero con bajas tasas de complicaciones, incluso para tumores que anteriormente se consideraban radiorresistentes a la radioterapia convencional de haz externo. El papel de la intervención quirúrgica sigue siendo consistente, pero se ha observado una tendencia hacia técnicas menos agresivas, a menudo mínimamente invasivas. Utilizando tecnologías modernas e instrumentación mejorada, los resultados quirúrgicos continúan mejorando con una morbilidad reducida. Además, los agentes dirigidos, como los productos biológicos y los inhibidores de puntos de control, han revolucionado la atención del cáncer al mejorar tanto el control local como la supervivencia del paciente. Estos avances han dado lugar a la necesidad de nuevas herramientas de pronóstico y a una revisión más crítica de los resultados a largo plazo. La naturaleza compleja de los esquemas de tratamiento actuales requiere un enfoque multidisciplinario que incluya cirujanos, oncólogos médicos, oncólogos radioterápicos, intervencionistas y especialistas en dolor. Esta revisión recapitula los datos actuales basados en la evidencia sobre el tratamiento de las metástasis espinales e integra estos datos en un marco de decisión, NOMS, que se basa en cuatro pilares centinela de la toma de decisiones en tumores metastásicos de la columna vertebral: estado neurológico, comportamiento oncológico del tumor, estabilidad mecánica, y carga sistémica de la enfermedad y comorbilidades médicas.
Spinal metastases are a common oncologic challenge as 20–40% of cancer patients are affected during the course of their illness and up to 20% of those will become symptomatic from spinal cord compression.1–5 The magnitude of this problem is expected to grow commensurate with the exponential rise in the use of targeted therapies which have demonstrated markedly improved survivals for virtually all malignant tumors. Additionally, the increased availability of advanced diagnostic imaging such as magnetic resonance imaging and 18-FDG PET scans will also serve to increase detection of spine metastatic disease. Despite extended survivals conveyed by biologics and checkpoint inhibitors, the treatment goals for patients with spine metastases remain palliative and focused on the preservation or restoration of neurological function and spinal stability, improved pain control and health related quality of life (HRQOL), and durable tumor control. Scoring systems such as the Tomita score6 and Tokuhashi revised score7 historically have been used to estimate survival and dictate treatment but increasingly have become obsolete due to their inability to incorporate and account for advances in all domains of cancer treatment.
Over the past fifteen years, the development of spine stereotactic radiosurgery (sSRS) has fundamentally changed the spine tumor treatment paradigm.8–13 The ability of sSRS to deliver an ablative radiation dose that is histology independent in its control rates fundamentally changed the indications for and types of surgery required.10,14,15 Whereas treatment in the early 2000s was predicated on aggressive surgical approaches due to the local control limitations of external beam radiation, sSRS has caused the pendulum to swing back toward radiation as the principal treatment modality. Surgery is currently used in selective cases as an adjuvant to sSRS for the recovery of neurologic function, improvement in radiation target volume coverage within spinal cord dose constraints, and spinal reconstruction in cases of instability.8,16,17 Intralesional gross total or en bloc excisions are no longer required and have largely been supplanted by separation surgery to create a safe sSRS target through epidural decompression.9,18
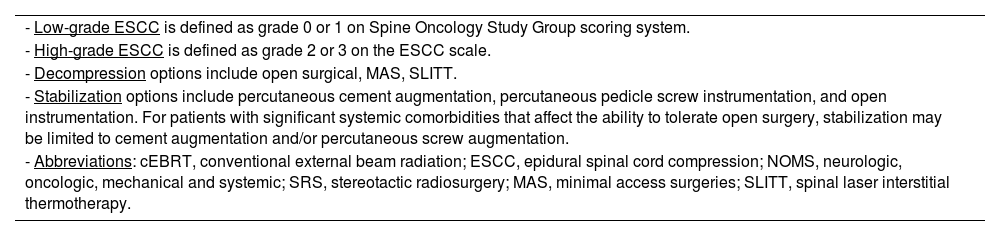
As radiation and surgical techniques evolved, so too did patient treatment algorithms. The NOMS decision framework developed at Memorial Sloan Kettering Cancer Center (MSKCC) in 2004 is very commonly used and is updated every 2 years to integrate new evidence-based medicine and advances in technology.19 The four sentinel decision points in NOMS are Neurologic, Oncologic, Mechanical (stability) and Systemic disease. The Neurologic assessment considers both clinical and radiographic parameters, including the presence of myelopathy, functional radiculopathy and the degree of epidural spinal cord compression (ESCC). The Oncologic consideration reflects the most effective strategy for achieving local tumor control, which is principally based on the expected radiation and systemic treatment responses. Mechanical stability assesses the impact of symptomatic pathologic fractures to determine the need for an interventional procedure or external orthosis. The final consideration is the extent of systemic disease and medical co-morbidities which are major determinants in a patient's suitability for surgery or even radiation therapy based on risk stratification of the proposed procedure and expected survival. In this paper, the evolution of critical advances leading to current treatment paradigms will be assessed in the context of the NOMS decision framework (Table 1).
Current NOMS decision framework.
| - Low-grade ESCC is defined as grade 0 or 1 on Spine Oncology Study Group scoring system. |
| - High-grade ESCC is defined as grade 2 or 3 on the ESCC scale. |
| - Decompression options include open surgical, MAS, SLITT. |
| - Stabilization options include percutaneous cement augmentation, percutaneous pedicle screw instrumentation, and open instrumentation. For patients with significant systemic comorbidities that affect the ability to tolerate open surgery, stabilization may be limited to cement augmentation and/or percutaneous screw augmentation. |
| - Abbreviations: cEBRT, conventional external beam radiation; ESCC, epidural spinal cord compression; NOMS, neurologic, oncologic, mechanical and systemic; SRS, stereotactic radiosurgery; MAS, minimal access surgeries; SLITT, spinal laser interstitial thermotherapy. |
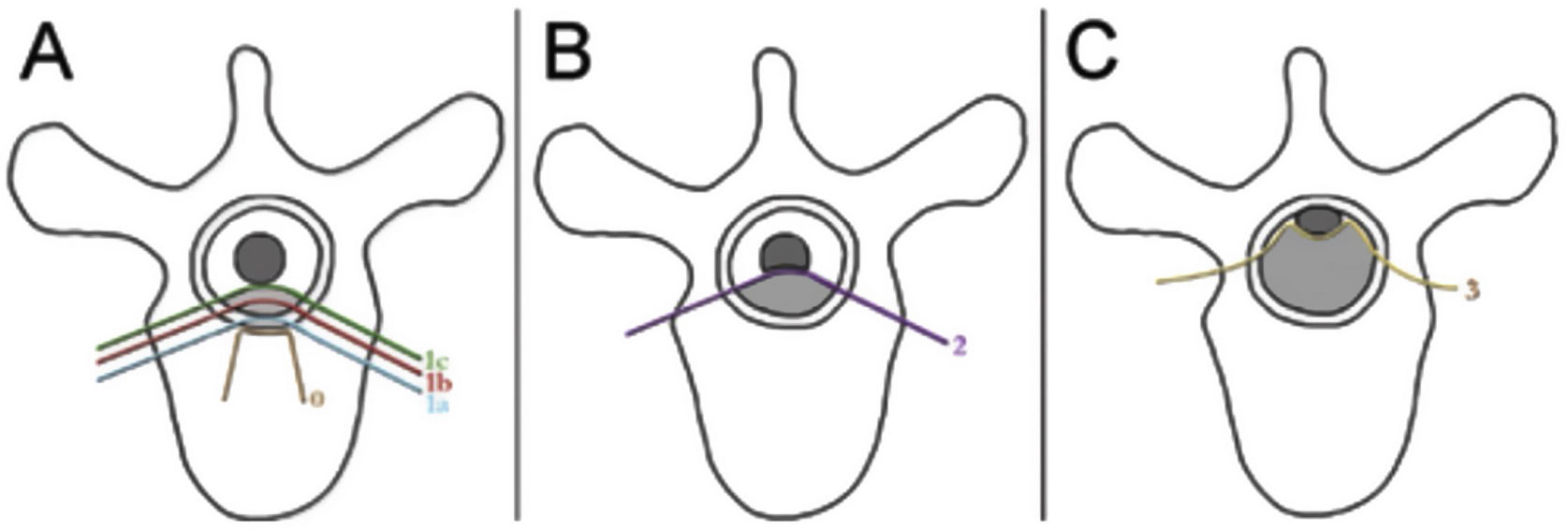
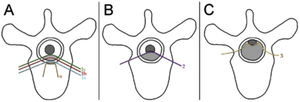
The neurologic and oncologic assessments are made in combination with the focus on preservation of neurologic function and local tumor control. The neurologic considerations consider both clinical parameters reflecting the presence of myelopathy, cauda equina syndrome, and functional radiculopathy and radiographic parameters assessing the degree of epidural spinal cord compression. The Spine Oncology Study Group (SOSG) validated an Epidural Spinal Cord Compression (ESCC) score, eponymously referred to as the Bilsky score in the literature, which uses T2-weighted axial MR imaging to define the degree of epidural spinal cord compression for spine tumors.20 The ESCC score is a 6-point scale ranging from 0 to 3, with scores of 0 to 1c constituting low-grade compression and scores of 2 and 3 constituting high-grade compression (Fig. 1). The scoring system was developed to standardize reporting in the literature, but also to critically examine SBRT outcomes based on a consistent assessment of ESCC. Whereas neurologic status is an important part of the decision-making algorithm in planning treatments, many patients undergo spinal imaging before they develop neurologic deficits. Therefore, the ESCC score provides the description of radiographic ESCC that covers the full range of symptomatic and asymptomatic tumors.
ESCC scale. The Epidural Spinal Cord Compression (ESCC Scale).10 Grades 0–1c represent tumor involving the bone only or varying degrees of thecal sac compression without spinal cord compression. Grades 2 and 3 are considered to be high-grade spinal cord compression and are differentiated by whether spinal fluid signal is obliterated on T2-weighted images.
The oncologic consideration is predicated on predicted cytotoxic and durable tumoral response to radiation therapy in the form of either conventional external beam radiation therapy (cEBRT) or sSRS.21,22 In general systemic chemotherapy and the newer targeted agents (e.g., biologic and checkpoint inhibitors) are ineffective for treating osseous spine tumors, so tumor control remains largely dependent on the response to radiation. Historically, cEBRT, often defined as 30Gy in 10 fractions, was the mainstay of treatment for spine tumors.8,23,24 With cEBRT, 1–2 beams are delivered to a treatment field, but organs at risk (OAR's) remain within the radiation field; thus, the dose of radiation is constrained by the toxicity to OAR's.21 The dose fractionation scheme was selected to minimize toxicity to organs at risk (OARs) such as the spinal cord, kidneys, bowel and esophagus, but was largely ineffective at controlling the vast majority of spine metastases. Based on the treatment response to cEBRT, tumors are classified as either radioresistant or radiosensitive. Moderately to highly radiosensitive tumors to cEBRT include most hematologic malignancies (i.e., lymphoma, multiple myeloma, and plasmacytoma) as well as selected solid tumors (i.e., breast, prostate, ovarian, neuroendocrine carcinomas and seminoma).8,25 However, most solid tumors are radioresistant to cEBRT including renal cell carcinoma (RCC), colon cancer, non-small cell lung cancer (NSCLC), thyroid cancer, hepatocellular carcinoma, melanoma and sarcoma with response rates of approximately 30% 1-year local control.23–26
Defining responsiveness to cEBRT is critical in terms of predicting clinical outcomes. In a number of series, favorable responders (i.e., those with more radiosensitive tumors) are more likely to maintain ambulation or remain ambulatory longer than patients with unfavorable histologies, (i.e., those with radioresistant tumors) after radiation treatment.25,27,28 Maranzano et al. prospectively demonstrated that 67% of breast cancer patients with symptomatic metastatic epidural spinal cord compression regained ambulation compared to only 20% in hepatocellular carcinoma.24 Further, they showed that myeloma, breast and prostate histologies had response durations of 16, 12 and 10 months, respectively. Others found a low success rate of only 33% in radioresistant tumors compared to 72% in patients with favorable histologies; moreover, patients with favorable histologies also demonstrated improvements in their motor strength, functional ability, and pain scores.27 Hence, patients with radiosensitive tumors can be treated effectively with cEBRT obviating the need for surgical intervention, regardless of the degree of ESCC.8,24 However in practice, patients with radiosensitive solid tumor malignancies who are myelopathic are usually considered for upfront surgery as the potential to achieve immediate decompression and maximize neurological recovery with cEBRT is limited.
Radiosurgery is a “game-changer”The technical evolution and integration of spine stereotactic radiosurgery (sSRS) has been a true paradigm changer for the treatment of spinal metastases. The safe and effective implementation of sSRS is the result of technological advances in non-invasive patient immobilization, intensity modulated image-guided radiation (IGRT) delivery systems, and sophisticated planning software.29,30 High-dose hypofractionated radiation delivery (i.e., sSRS) overcomes the radioresistance observed with cEBRT in part by creating more lethal double-stranded DNA breaks, but also by inducing significant damage in the tumor vasculature via the acid sphingomyelinase pathway.15,31,32 Additionally, sSRS is typically delivered in one to three factions, shortening the treatment time and improving patient compliance.
Radiosurgery as definitive therapyRecent data demonstrates that sSRS yields a clinical benefit regardless of tumor histology and volume, providing durable symptomatic responses and high local-control rates.10,33,34 The high-dose hypofractionated sSRS overcomes the radioresistance seen in most solid-tumor malignancies to cEBRT rendering all tumors essentially radiosensitive. In patients without spinal cord compression (ESCC 0-1C), sSRS can be used as definitive therapy and has largely replaced en bloc resection favored by the Tokuhashi and Tomita scoring systems even for solitary metastases.9,35 (Fig. 2). Based on the superb outcomes using sSRS, Bilsky et al. in a Cochran review from the SOSG made a strong recommendation that patients with RCC in the absence of spinal cord compression (i.e. ESCC 0 to 1c) undergo stereotactic radiosurgery rather than en bloc resection.17 This transition is based on a plethora of outcome data demonstrating excellent outcomes with sSRS for traditionally radioresistant histologies such as renal cell carcinoma,36–38 sarcoma39 and melanoma.40 Local control rates of 88% in the non-cervical spine have been shown prospectively, independent of histology.41 A multi-institutional retrospective analysis of 387 cases treated with SBRT, reported local control of 84% at 2 years. The cohort was comprised of various solid tumor histologies and the median treatment dose was 8Gy in 3 fractions.42 Other series have demonstrated similar conclusions.43,44 Yamada et al. described a case series of 811 lesions presenting wtih ESCC 0 to 1c treated in 657 patients with a single-fraction SSRS in which dose was analyzed as a continuous variable ranging from 18 to 26Gy.10 The median dose that covered 95% of the planning target volume (PTV D95) was 16.44Gy in the low-dose group compared to 22.40Gy in the high-dose group. Local failure rates for the low- and high-dose groups were 5% versus 0.41% at 12 months, 15% versus 1.6% at 24 months, and 20% versus 2.1% at 48 months, respectively. In this study, 82% of the tumors were traditionally radioresistant, but tumor responses were found to be independent of both tumor histology and prior radiation for the high-dose cohorts. Thus, SSRS yields a clinical benefit regardless of histology, providing a durable symptomatic response and high local-control rates, but these responses appear to be dose dependent.10,33,34
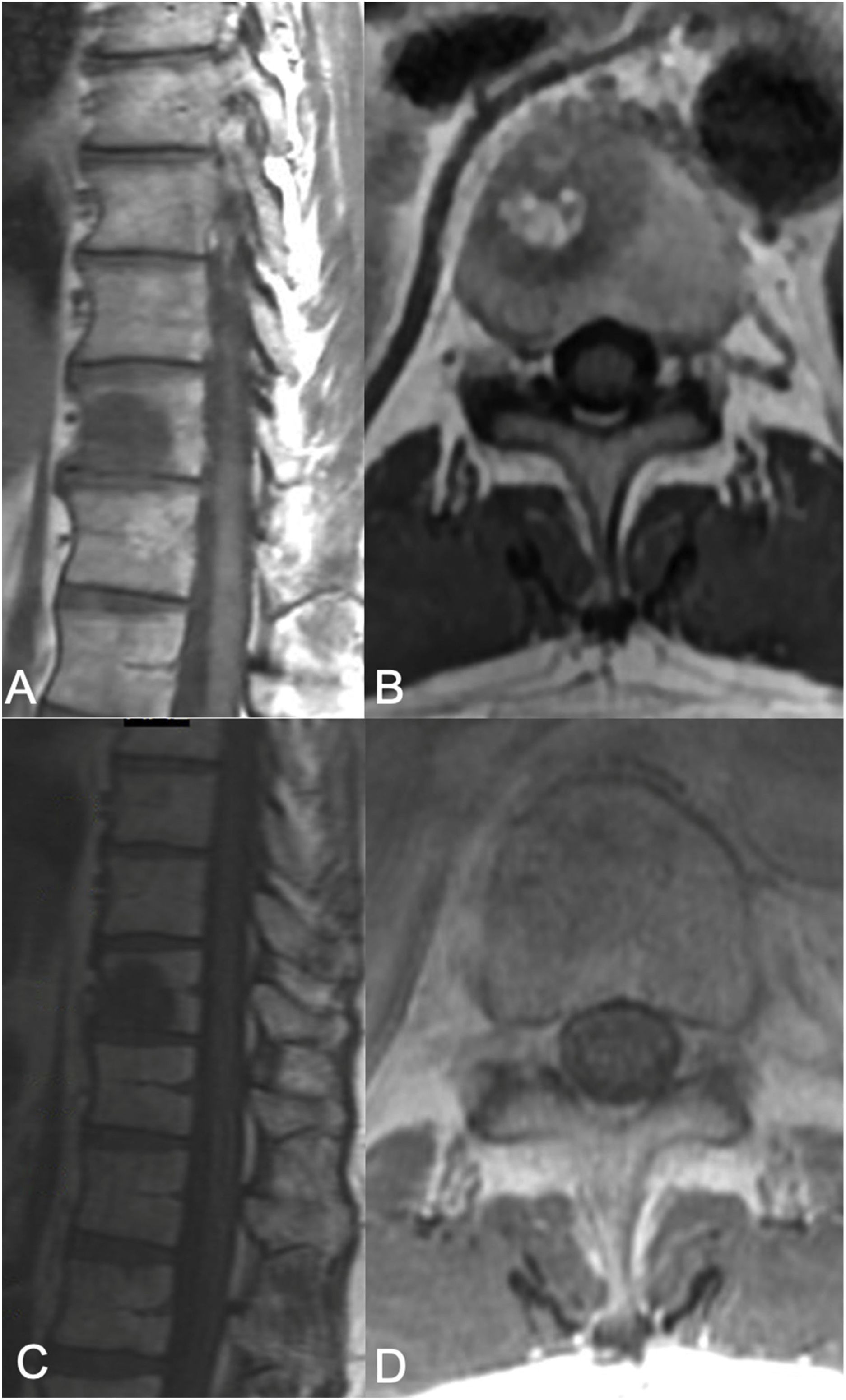
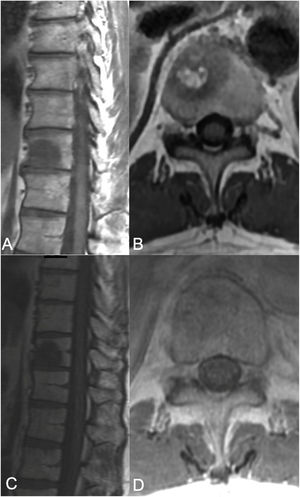
sSRS treatment. 68-Year-old female, without a previous history of cancer, presented with back pain and was found to have a T8 lesion and a lung lesion. Due to her symptomatic presentation, she underwent kyphoplasty along with a biopsy from T8 yielding metastatic adenocarcinoma consistent with adeno-carcinoma of lung. No epidural tumor extension was found (ESCC grade 0) but a left paraspinal extension was noted. Along with systemic treatment, the patient underwent SSRS treatment of 24Gy in a single treatment fraction. (A) Axial MR with contrast enhancement at T8 showing the vertebral body lesion with extension to the left posterior elements with a paraspinal component. No epidural cord compression seen. (B) 13 month follow up MR showing good local tumor control. (C) Radiosurgery treatment plan color wash. The minimum dose in the color wash (dark blue) is set to 1920cGy or 80%.
Dose constraints have been established for all major OAR's.45,46 The balance between underdosing the tumor margins resulting in tumor progression versus overdosing and damaging organs at risk (OARs) is extremely delicate. Fortunately, high-grade toxicity after sSRS occurs infrequently and most of the observed complications are mild, including esophagitis, mucositis, dysphagia, diarrhea, paresthesias, transient laryngitis, and radiculitis.41,47–52 Vertebral compression fractures (VCF) following SSRS have been described in up to 40% of treatments compared with a less than 5% risk following cEBRT.53 In a follow-up analysis from the same group, the fracture rate of 40% captured all radiographic fractures, but the symptomatic fracture rate requiring an intervention was only 7% at 5-year follow-up.54 A multi-institutional analysis found that VCF following SSRS is more likely to occur following treatment with high doses.55 Saghal et al. suggested that caution be observed when treating with ≥ 20Gy per fraction, in particular, for high-risk patients.56 Risk factors identified are older age, a lytic lesion, vertebral malalignment or the presence of a pre-existing VCF; as a result, some advocate pre-treatment kyphoplasty in select patients.53,57,58 An ongoing controversy regarding dose-dependent fracture risk has yet to be resolved, but the demonstrated control rates at higher doses combined with the ability to stabilize most fractures with the low-risk of morbidity associated with percutaneous cement augmentation may justify more aggressive dosing.
The most significant concern with regard to sSRS toxicity is radiation-induced myelitis (i.e., spinal cord injury). Gibbs et al. reported outcomes from a multi-institutional review of sSRS in which 0.6% (6/1075) developed myelitis.59 Similarly, MSKCC reviewed a series of 476 patients undergoing sSRS where the spinal cord dose was limited to a Dmax of 14Gy and reported a 0.42% risk of reversible spinal cord injury.10 However, this spinal cord constraint prevents effective treatment of tumors with high-grade ESCC even in the absence of myelopathy or cauda equina syndrome. Michael Lovelock et al. conducted a dose failure analysis in which they found that all patients with post-sSRS tumor progression had received less than 15Gy to even a small percentage of the planning target volume.21 Accepting a cord Dmax of 14Gy with a 10% per mm dose falloff, the consequence would be either underdosing at the margin of the spinal cord with a high-risk of epidural tumor progression and progressive ESCC or conversely overdosing the spinal cord with resultant myelitis. In 2010 Ryu et al. reviewed a series of 62 patients presenting with high-grade ESCC tumors treated with a median dose of 16Gy single fraction and with almost a 50% loss to follow-up still reported a 20% risk of neurologic progression.60 However, the use of high-dose hyprofactionated radiation may improve the therapeutic window and allow for the safe, effective treatment of higher-grade ESCC. Rothrock et al. recently reported outcomes in 31 tumors presenting with ESCC grade 2 were treated with hypofractionated radiation doses (i.e. 24–50Gy in 3–5 fractions).61 The 1- and 2-year incidence of loco-regional failure in their study was 10.4% and 22%, respectively and 1- and 2-year incidence of same level salvage surgery was 6.8% and 14.5%, respectively. Treatment related radiographic fractures occurred in 12% of patients. This study suggests that the treatment of high-grade metastatic ESCC 2 is possible, but progress in this sphere has been incremental to date. ESCC grade 3 compression and those presenting with myelopathy from solid tumor malignancies continue to require surgical intervention.
Neurologic/oncologic surgical indications: high-grade ESCC from RT-resistant tumorsGiven the poor responses observed following cEBRT and the inability to deliver a cytotoxic sSRS-dose within spinal cord constraints, the SOSG utilized a Cochran review to make a strong recommendation for surgical decompression and stabilization followed by radiation therapy for patients with high-grade ESCC with radioresistant tumors.9 This recommendation was based largely on the landmark study by Patchell et al., which reported outcomes from a prospective randomized trial comparing cEBRT to surgery followed by cEBRT for patients presenting with high-grade spinal cord compression due to radioresistant solid-tumor malignancies.62 Exquisitely radiosensitive hematologic malignancies and germ cell tumors were excluded. In every outcome variable, the surgical-arm demonstrated improved outcomes compared to the cEBRT cohort including maintenance or recovery of ambulation and bowel and bladder function, lower narcotic requirements, and improved survival. Whereas neurologic outcomes were better with surgery, it also became clear that cEBRT did not provide durable tumor control. Klekamp and Samii reviewed a series of 101 patients undergoing aggressive partial or complete resection followed by adjuvant cEBRT.63 The local recurrence rate was 70% at 1-year and 96% at 4-years. The most significant predictors of recurrence were non-ambulatory status, completeness of resection and tumor histology. These high-recurrence rates are reflective of the ineffectiveness of cEBRT with respect to overcoming radioresistant tumor histologies, even after cytoreductive surgery.
The presence of myelopathy or cauda equina syndrome due to ESCC shifts the decision-making paradigm. Whereas in the absence of neurological deficits, some patients with high grade ESCC may be candidates for radiation treatment alone, the presence of deficits increases the likelihood that urgent to emergent surgical decompression is necessary for the preservation or recovery of function as these patients can experience rapid neurological decompensation. Additionally, whereas relatively radiosensitive tumors, such as breast and prostate carcinoma, can be treated with cEBRT to achieve durable tumor control in the setting of high-grade ESCC, these solid tumors typically will not respond expeditiously enough to achieve neurologic recovery in the setting of myelopathy or cauda equina syndrome. An apoptotic tumor response is seen in the exquisitely radiosensitive hematologic malignancies (e.g., multiple myeloma and lymphoma), leading to a fairly immediate spinal cord decompression resulting in a high probability of neurologic recovery; however, this phenomenon is not seen with radiosensitive solid tumor malignancies which often takes months to see a radiographic response even with well controlled disease.
The second scenario that needs to be addressed specifically is a patient who presents with myelopathy but no history of cancer, and thus no known tumor histology. An unknown tumor could be anything from a radiosensitive hematologic malignancy to a radioresistant solid tumor, including primary bone tumors such as chordoma. Establishing a definitive diagnosis in a patient presenting with a neurological emergency is often very difficult. Recently, we biopsied a neurologically intact patient with Grade 3 ESCC tumor that appeared radiographically and histologically on a biopsy touch prep to be consistent with lymphoma. Forty-eight hours after the biopsy, flow-cytometry determined the tumor definitively was not lymphoma, and 10-days later, the final pathology was confirmed to be a radioresistant neuroblastic tumor. The patient had no radiographic resolution of the spinal cord compression at 1-month follow-up and thus no effective decompression although the patient remained at neurologic baseline.
Another issue arises when a newly diagnosed tumor has a histology consistent with a primary bone tumor, such as a chordoma, which traditionally is considered for an en bloc resection to achieve wide margins. Chordomas resulting in high-grade ESCC and myelopathy by definition are not candidates for a curative en bloc resection; however, chordoma can be effectively treated with a safe intralesional resection followed by sSRS. Jin et al. demonstrated 90% 5-year local control rates for chordomas treated with 24Gy single fraction using sSRS as a postoperative adjuvant following intralesional resection, neoadjuvant followed by en bloc resection, or as definitive radiation without resection.64 In our practice, patients with myelopathy from an unknown primary are considered radioresistant until proven otherwise and are surgically decompressed urgently or emergently to ensure the best chance of neurologic recovery. This treatment paradigm works because most radioresistant tumors, including chordoma, can be effectively treated with postoperative sSRS to achieve local durable tumor control.
Hybrid therapy: separation surgery and postoperative SSRSWhereas the Patchell study provides a road map for decompressive surgery for high-grade ESCC with radioresistant tumors, sSRS as a postoperative adjuvant has changed the goals of surgery and therefore the surgical approach taken. In recent years, we have seen a transition from treatment with aggressive cytoreductive surgeries, such as en bloc spondylectomy or gross total resection, to reliance on sSRS to provide the oncologic goal of tumor control.9 Hybrid therapy refers to the combination of separation surgery followed by sSRS.14 The term “separation surgery” describes a posterolateral approach that allows for stabilization and circumferential decompression of the thecal sac and nerve roots. Spinal cord decompression is ensured by resecting the posterior longitudinal ligament with subsequent reconstitution of the thecal sac. To safely deliver an appropriate radiation dose, patients with high-grade ESCC caused by radioresistant tumors undergo separation surgery.14,18,65 Due to the highly conformal nature of sSRS, large paraspinal masses and vertebral body tumors do not need to be resected in order deliver ablative doses to the tumor volume and achieve durable local tumor control. The importance of achieving adequate surgical decompression to reconstitute the thecal sac has been emphasized by Al-Omair et al., who showed that postoperative patients who had continued compression of the spinal cord (i.e., residual ESCC grade 2 or 3) had a significantly higher risk of local recurrence after post-operative SSRS compared to patients with sufficient separation between the tumor and the spinal cord.66
In a retrospective review of 186 patients, Laufer et al., found postoperative adjuvant sSRS following separation surgery is a safe and effective in achieving durable local tumor control.18 In this series, patients who received high dose hypofractionated sSRS (i.e., 24–30Gy in 3 fractions) demonstrated 1-year local progression rates of less than 5%. In those receiving single-fraction sSRS (i.e., 24Gy), the local progression rate was less than 10%. There was no impact of radioresistant tumor histology, prior radiation or the degree of preoperative epidural extension on recurrence rates and no patient suffered a neurological complication; however, it should be noted that these results were superior to the results of low-dose hypofractionated sSRS (i.e., 30Gy in 5 fractions). Similarly, Molding et al. reported a 1-year local failure risk of only 6.3% using high-dose (18–24Gy) single fraction sSRS after separation surgery65 and Rock et al., reported a 92% local control rate in patients treated with radiosurgery following open surgical procedures.67 (Fig. 3) More recently, Hussain et al. reported hybrid therapy for RCC using the same dose strategies with a median postoperative dose of 27Gy in 3 fractions (i.e., high-dose hypofractionated sSRS).14 The 1-yr and 2-yr cumulative incidence of recurrence were 4.6% and 8.2%, respectively. Overall, 90% of patients remained ambulatory with an ECOG of 0 to 2 at 1-yr follow-up. Similarly, Chakravarthy et al. reported hybrid therapy for non-small lung carcinoma in 103 patients with a median postoperative dose of 27Gy in 3 fractions.68 In their series, the 2-yr cumulative incidence of recurrence was 5.4%. Of note, an EGFR mutation in the metastatic tumor conveyed a 50% survival advantage in those not previously treated with an EGFR-inhibitor.
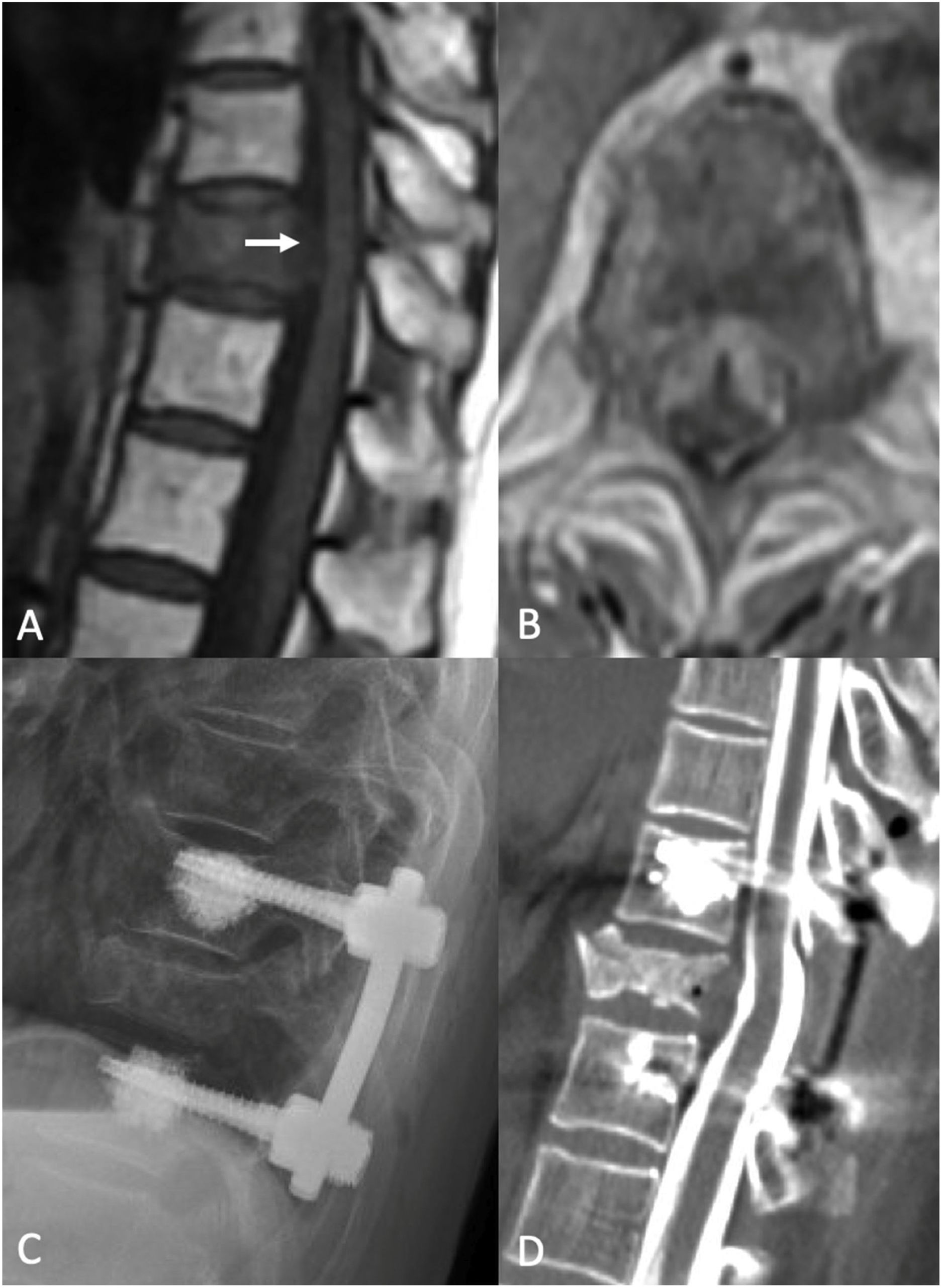
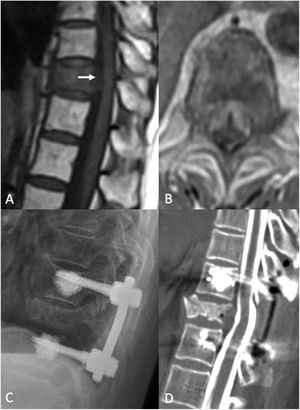
Separation surgery. 83 year old female with a history of non-small cell lung cancer (NSCLC). She underwent a routine PET-CT which demonstrated pathologic fracture of T10 and a PET avid lesion with erosion of the posterior cortex of the vertebral body suggestive of spinal canal involvement. MRI demonstrated high grade spinal cord compression (ESCC 3) at T10. Neurologically intact at presentation with mild chronic back pain. Due to the high-grade cord compression with a radioresistant tumor she underwent separation surgery followed by sSRS. (A) Pre operative sagittal T1 non contrast MRI. Note the compression at T10 (white arrow). (B) Pre operative Axial MRI with contrast enhancement demonstrating the ESCC3 compression. (C) Post op X-ray demonstrating a construct extending from one level above to one level below the index level with cement augmentation of the fenestrated pedicle screws. (D) Post-operative CT Myelogram showing re-constitution of the thecal sac at the index level.
Separation surgery often requires ventral decompression of the thecal sac. In the cervical and thoracic spine, accessing the ventral epidural space from a posterolateral approach requires pedicle resection while in the lumbar spine, a medial facetectomy may aid in achieving this access.14,16 Historically, the loss of spinal stability that accompanies pedicle resections and medial facetectomies was managed with long-segment posterior fixation in order to distribute the load born by the instrumentation and decrease the risk of hardware failure—particularly screw pullout—due to poor patient bone quality. These long-segment surgeries typically were at a minimum two levels above and two levels below the index level and were associated with significant blood loss and increased operative times, both of which contribute to patient morbidity. Using this strategy, in an analysis of 318 patients who underwent separation surgery for solid malignancies, 2.8% experienced hardware failure.16
The technological advance of fenestrated screws allowed for polymethyl methacrylate (PMMA) instillation through the screw fenestrations and into the vertebral body, thereby increasing screw pullout strength.69–72 This increased screw pullout strength allowed for the consideration of shorter posterior constructs for spine stabilization.73–75 This has allowed us to shorten constructs to single level above and below the index level. PMMA-augmented screws overcome osteoporosis and adjacent level fracture progression. In a series of 44 patients undergoing separation surgery reconstructed with adjacent single-level PMMA augmented screws, the fixation failure rate requiring a reoperation was 2.2%.75 Preliminarily, these shorter, PMMA-augmented constructs do not appear to be associated with an increased risk of hardware failure, but further studies are needed.
From a radiographic standpoint, surgical implants are also being designed to improve postoperative imaging quality in order to be able to better evaluate for local tumor recurrence. Polyether ether ketone (PEEK) and carbon-fiber-reinforced PEEK are currently available materials used for pedicle screw-rod systems and vertebral body replacement cages. Their radiolucent properties generate postoperative imaging with significantly reduced artifact commonly seen with titanium-based constructs. The modulus of elasticity for PEEK is similar to that of bone, which lessens the risk of subsidence while still providing strength similar to that of titanium constructs.76
In the event that anterior column support or reconstruction is necessary, PMMA bone cement can be used to create custom shaped supports and constructs.77 Preliminary reports demonstrate the safety and efficacy of radiation using both PEEK78 and PMMA79 materials, making these appropriate for use in the oncologic population. PEEK reconstruction is particularly appealing due to its benefits relating to planning proton beam radiation treatments, which is gaining increased interest even in the domain of metastatic tumors.
Timing of postoperative radiationRadiation therapy is known to impair wound tissue repair through multiple mechanisms, and surgical wound complications following radiation treatment remains a major concern.80,81 Keam et al. evaluated wound complication rates occurring in patients receiving cEBRT compared to sSRS before undergoing spine surgery and found no significant differences.82 Importantly, they concluded that preoperative sSRS is associated with clinically acceptable rates of wound morbidity.
Surgeons tend to wait several weeks before operating after cEBRT. A systematic review emphasized the lack of uniform data reporting, but suggested a 1 week interval between surgery and sSRS based on animal models and limited human studies.83 Keam et al. reported a wound dehiscence/infetion rate in those undergoing radiation followed by surgery of 16% with cEBRT vs. 6% with sSRS.82 This finding is consistent with the notion that sSRS largely spares the operative corridor, thereby reducing wound complication rates.
NO spinal instability SIn NOMS, mechanical instability is a separate consideration from the neurologic and oncologic assessments as radiation does not play a role in the treatment of pathologic fractures. Using a modified Delphi approach, the SOSG defined, codified and validated the Spine Instability Neoplastic Score (SINS) to assess tumor-related fractures.84 The weighted system combines the presence of mechanical pain with 5-radiographic criteria including tumor location, bone quality, vertebral body fracture, posterior element involvement, and degree of deformity. SINS has been critically important in standardizing the assessment and treatment of pathologic fractures. High SINS scores (13–18) reliably predict the need for surgical stabilization while low SINS scores (0–6) are considered stable. The intermediate SINS (7–12) tumors need further assessment, with the need for surgical intervention based on the discretion and experience of the spine surgeon.84 Mechanical instability can be addressed with an external orthosis, but cancer patients have physical restrictions that limit brace tolerance: therefore, patients are often treated with percutaneous cement augmentation, either kypho- or vertebroplasty, percutaneous pedicle screws or open surgery.
Minimally invasive percutaneous PMMA augmentation procedures were developed early in the 2000s to stabilize thoracic and lumbar burst and compression fractures. The most commonly used techniques, vertebroplasty and kyphoplasty, are controversial regarding pain relief in the treatment of osteoporotic fractures. Two prospective trials demonstrated no difference between percutaneous bone cement augmentation and best medical management.85,86 Conversely, in 2011, Berenson et al. reported outcomes from the CAFE study, a prospective randomized trial comparing kyphoplasty to non-operative therapy for pathologic vertebral compression fractures.87 Patients undergoing kyphoplasty demonstrated a significant reduction in pain, improvements in quality of life, and functional recovery at one month that was maintained at one year follow-up.
A failure analysis of standalone percutaneous bone cement augmentation demonstrated that patients with vertebral compression fractures who additionally had posterior element disease did not experience significant pain improvement. This problem led to the strategy of kyphoplasty at the index fracture level and the placement of percutaneous cement-augmented pedicle screws to provide an additional posterior tension band. In a review of 44 patients, Moussazadeh et al. reported outcomes demonstrating that all patients presenting with severe pain resolved to minimal or no pain postoperatively.73 This construct was durable with the exception of one asymptomatic screw pull-out and one adjacent-level vertebral body fracture.
NOM systemic diseaseThe final consideration in the management of spine metastases is the assessment of systemic disease and medical co-morbidities. These factors directly impact the ability of patients to tolerate a proposed treatment and also determine if a proposed intervention is reasonable within the context of their disease. Therefore, even if a patient has a firm indication for surgery based on the NOM considerations, they may be excluded based on their inability to tolerate surgery or even radiation from a medical or cancer standpoint. However, it must be recognized that many treatment decisions are made urgently or emergently, creating situations in which these decisions must be made with limited information and incomplete work-up. In metastatic disease, expected survival is often used as a major determinant for the type of treatment offered. Survival has been extended for virtually every metastatic tumor histology due to the development and integration of biologics and checkpoint inhibitors. Rothrock et al. demonstrated the impact of newer systemic agents in a 20-year review of metastatic spine surgical data at MSKCC.88 Their work demonstrated a 20% improvement in survival over that time period. Newer predictive survival models also have been developed and validated in the era of biologic and checkpoint inhibitors such as the SORG nomogram89 and the New England Metastatic Spine Score.90,91 Massad et al. demonstrated that these models were better at predicting 1-year survival compared to traditional scoring systems such as the Tomita, revised Tokuhashi, and revised Bauer scores.92 Additionally, Massad et al. developed machine learning algorithms to assess frailty, mortality and complications related to metastatic spine tumor surgery.93 This analysis demonstrated that patients with sarcopenia and lower visceral and subcutaneous adiposity had significantly worse postoperative outcomes and more limited survival.
Although it seems somewhat counterintuitive that local radiation can impact systemic disease control, the integration of sSRS into the treatment of oligometastatic tumors (i.e. 1–5 metastases) has now been demonstrated to improve survival in multiple studies.12,13,94,95 Palma et al. reported outcomes from the SABR-COMET trial, a randomized phase 2 trial assessing overall survival in patients with a controlled primary tumor who were treated with standard of care (SOC) vs. SOC plus stereotactic ablative radiotherapy (SABR) for 1–5 metastases.95 The 5-year overall survival rate was 17.7% in those receiving SOC vs. 42.3% in those receiving additional SABR (p=.006). Zelefsky et al. reported outcomes from a phase 3 randomized trial examining the utility of high-dose single fraction radiation (24Gy) compared to high-dose hypofractionated radiation (9Gy×3 fraction) in the treatment of oligometastatic bone disease.13 Single fraction radiation demonstrated lower rates of local recurrence compared to the hypofractionated regimen with rates of 2.7% and 5.8% at 2 and 3 year vs. 9.1% and 22%, respectively (p=.0048). A significant difference was also seen in the 2 and 3-year cumulative incidence of distant metastatic progression which was 5.3% in the single fraction cohort vs. 10.7% and 22.5% in the hypofractionated cohort, respectively (p=.010).
ConclusionsDespite major radiation and medical advancements in cancer care, surgery still plays a major role in the treatment paradigm for patients with spinal metastases. Surgery is particularly important for those with high grade ESCC necessitating separation of the epidural tumor from the spinal cord, but also for spinal stabilization as facilitated by SINS. MAS techniques and improved implants and technologies offer less surgical related morbidity and rapid continuation of systemic therapies. The integration of sSRS has revolutionized treatment by overcoming radioresistance and providing for durable local tumor control. Targeted therapies are re-defining cancer care yet their precise role for spinal tumors is yet to be fully determined.
Optimal management of spinal metastases requires a multidisciplinary team effort. The NOMS framework provides a decision framework in which medical innovation can be readily incorporated for optimal patient care.
Level of evidenceLevel of evidence ii.
Conflict of interestsThe authors declare they have no conflict of interest.