To analyse the efficacy and safety after the application of platelet-rich-plasma (PRP) as an adjuvant in arthroscopic rotator cuff repairs.
Material and methodsA bibliographic search of the literature of prospective studies with level of evidence one or two was carried out from January 2004 to December 2021, including studies that compare the functional and re-tear results after arthroscopic cuff repair rotator with or without PRP.
ResultsA total of 281 articles were identified, of which 14 met the inclusion criteria. The overall re-rupture rate was 24%. In the PRP group, a decrease in the re-rupture rate and better functional results were demonstrated, although these differences were not significant.
ConclusionsAdjuvant treatment with PRP has shown promising results, although there is not yet enough evidence to provide a clear advantage for routine use in clinical practice.
Analizar la eficacia y la seguridad tras la aplicación de plasma rico en plaquetas (PRP) como coadyuvante en las reparaciones artroscópicas del manguito rotador.
Material y métodosSe realizó una búsqueda bibliográfica de la literatura de estudios prospectivos con nivel de evidencia uno o dos desde enero de 2004 hasta diciembre de 2021, incluyendo los estudios que comparan los resultados funcionales y de rerrotura tras la reparación artroscópica del manguito rotador con o sin PRP.
ResultadosSe identificaron un total de 281 artículos, de los cuales 14 cumplieron los criterios de inclusión. La tasa general de rerrotura fue del 24%. En el grupo del PRP se observó una disminución en la tasa de rerrotura y unos mejores resultados funcionales, aunque estas diferencias no fueron significativas.
ConclusionesEl tratamiento coadyuvante con PRP ha mostrado resultados prometedores, aunque todavía no hay suficiente evidencia para proporcionar una ventaja clara para el uso rutinario en la práctica clínica habitual.
Rotator cuff tear is a major cause of limited mobility and decreased strength.1 This injury is one of the most common causes of shoulder pain in the general population, with a prevalence of 2.5–62%, which increases with age.2,3
When conservative treatment fails in patients with a degenerative rotator cuff tear, the treatment of choice (gold standard) is arthroscopic repair of the tear.
Although the different mechanical problems have been addressed in recent years, and different techniques have been developed to improve the strength of the fixation of the repair, such as the double-row technique or the use of a more resistant suture material, the re-tear rate remains high, between 34% and 97%.4–6 It should be borne in mind that this rate is higher with degenerative cuffs (typical in older patients), with larger tears, or when there is fatty infiltration at the level of the rotator cuff musculature.7,8
It is well accepted that the biological problem is one explanation for the high rate of re-tear after repair of degenerative rotator cuff tears.9 An explanation for this phenomenon is that with increasing age there is an imbalance at the tissue matrix level, leading to increased degradation through apoptosis of the tendon cells.10 Thus, in these tendons there is poor vascularisation of the lesional edges, which decreases the capacity to create an enthesis and causes the formation of scar tissue that involves loss of the original structure of the tendon insertion, since the scar tissue is incapable of completely regenerating the original biomechanical properties.11,12
For these reasons, studies are being conducted and new biological strategies are being proposed to accelerate the tendon repair mechanisms and reduce postoperative recurrence rates, with the consequent improvement in the patient's function and clinical condition. One of the most widely used biological preparations is platelet-rich plasma (PRP) or plasma rich in growth factors (PRGF).13–16
PRP is an autologous blood product, acquired from part of the plasma fraction obtained after centrifugation of whole blood, to achieve a platelet concentration above physiological levels (2–5 times the normal value).17 Platelets harbour a large number of proteins, including growth factors, immune system messengers, enzymes, and other bioactive compounds involved in various aspects of tissue repair. Thus, by attracting undifferentiated cells to the site of injury and through neoformation of a matrix that stimulates cell division, the aim is to reduce scar tissue formation and effectively restore the biological structure and biomechanical strength of the tendon.18,19 Supraphysiological levels of growth factors may therefore stimulate the resolution of pathological processes in tissues that lack optimal blood supply.20
Numerous studies have been published on the clinical efficacy of PRP in patients undergoing arthroscopic rotator cuff repair,15,21,22 although there is disparity in the number of participants, the methods used, the size of the tear, the injured tendon, the type of PRP used, and the type of surgical procedure, and also the site and timing of PRP administration.14,15,23–26
We conducted a systematic review and meta-analysis to evaluate the efficacy of PRP as an adjunct in arthroscopic rotator cuff repair, to study the clinical and functional outcomes, the re-tear rate, and to investigate the role of different related factors.
Material and methodsSearch strategyWe conducted a systematic review of the literature following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and using the three main online database sources, PubMed, Google Scholar, and Science Direct.
The databases were searched for the use of platelet-rich plasma as an adjunct in arthroscopic rotator cuff repair up until December 2021. Data were uniformly extracted and compiled in Microsoft Excel 2019, version 16.54.
The search equation used in PubMed was (((rotator cuff repair) AND ((PRGF) OR (PRP) OR (platelet rich plasma) OR (growth factor))) NOT (animals); for Google Scholar: (rotator cuff repair + PRP OR PRGF OR platelet OR rich OR plasma or growth or factor - animal), and for Science Direct: (rotator cuff repair) AND (PRP OR growth factors OR platelet-rich plasma OR PRGF OR platelet-rich growth factors).
The literature references of the selected articles were also analysed to rescue other studies for potential inclusion in the review. These studies were located through PubMed and Google Scholar.
Inclusion and exclusion criteriaAll prospective clinical studies with level of evidence one or two that incorporated the use of platelet-rich plasma as adjuvant treatment in arthroscopic rotator cuff repair, compared to a control group also undergoing arthroscopic rotator cuff repair but not receiving adjuvant treatment with PRP, and with imaging control after a period of time to assess tendon re-tear rate and at least one patient-reported outcome measure (ASES, Constant–Murley, VAS) were used as inclusion criteria.
We excluded any form of research that was not a prospective clinical study (meta-analysis, review articles, case–control study, descriptions of techniques, letters to the Editor, expert opinion, in vitro studies, animal research). Articles in a language other than English or Spanish were not evaluated. We did not limit by year of publication. Related articles that included the same patients were not considered.
All studies describing any pathology other than complete rotator cuff tendon tear, or treated conservatively, were excluded. Studies using biological augmentations other than PRP or those involving PRP matrix, platelet membrane or PRP in gel form were not considered. Articles that did not include the administration of PRP in the same surgical procedure were also excluded.
Data extractionData collected included sex, mean age, and number of patients in each group.
The size of the tendon tear (small, medium, large, or massive), the tendons injured (supraspinatus, infraspinatus, and/or subscapularis), leucocyte concentration in PRP (leucocyte-poor or leucocyte-rich PRP), site of infiltration (tendon–bone interface, intra-articular, or subacromial) were obtained, type of surgical technique used (single or double-row suture), re-tear (assessed by arthro-MRI, MRI, or ultrasound at the end of follow-up), postoperative immobilisation time and follow-up time (6 months as short term, 12 months as medium term, and 24 months as long term). The various patient-reported functional outcome measures (Constant score, UCLA, and VAS) were also collected.
Risk of bias assessmentThe assessment of the methodological quality of the included studies was based on the Cochrane risk of bias criteria. The seven domains used to assess bias in each trial included generation of randomisation sequence, allocation concealment, blinding of participants and staff, blinding of outcome assessors, incomplete outcome data, selective reporting of results, and other biases. Items were classified as low risk, high risk, or uncertain risk. Included studies were evaluated independently by two investigators, and differences of opinion between the two were resolved by discussion and consultation with a third author.
Statistical analysis of the dataData from the included studies were analysed using Review Manager 5.3 software. Dichotomous variables (re-tear rate) were expressed by risk ratio (RR) and 95% confidence interval (CI), while the weighted mean difference (WMD) was calculated for continuous data (Constant–Murley, UCLA, and VAS pain scores). Q and I2 tests were used to estimate between-study heterogeneity. The I2 test was used to assess heterogeneity according to the thresholds reported in the Cochrane Handbook of Systematic Reviews of Interventions27: 0%–40%, not significant; 30%–60%, moderate heterogeneity; 50%–90%, substantial heterogeneity, and 75%–100%, considerable heterogeneity. When I2<50% or p>.1, a fixed-effects model was applied for meta-analysis, and when I2>50% or p>.1, a random-effects model was used.
Subgroup analysis of re-tear rate was performed according to the following factors: follow-up time (short term, medium term, or long term), rupture size (small to medium or large to massive), number of tendons affected (one or two [supraspinatus and/or infraspinatus] vs. three [supraspinatus, infraspinatus, and subscapularis]), leucocyte concentration of PRP (leucocyte poor vs. leucocyte rich), surgical procedure (single row vs. double row) and site of PRP infiltration (intratendinous or intra-articular). For all outcomes, forest plots were used to present individual study results and pooled estimates of effect size.
ResultsSearch resultsA literature search was conducted, and 443 published articles were located; after excluding duplicate articles, 281 articles were collected. After reviewing the titles and abstracts, a total of 44 studies met the established inclusion criteria. After a complete and detailed review of the remaining studies, in order to decide whether or not the information they contained was related to our objective, 19 papers were excluded because they were studies that used biological augmentations other than autologous PRP. Another five papers were excluded because they were related studies involving the same patients, and a further four studies were excluded because of eligibility problems (the full text could not be retrieved). We excluded one article because it was a retrospective study and another because it was an unfinished pilot study. In the end, a total of 14 studies were selected for this systematic review and meta-analysis. The process of article selection is shown in Fig. 1.
Study characteristicsWe considered the re-tear rate and patient-reported clinical and functional recurrence rate as outcome measures. Surgical repair was performed by arthroscopy in all included studies, and subacromial decompression and tenotomy or tenodesis of the long portion of the biceps tendon were performed when deemed necessary by the surgeon. When PRP was administered, saline was removed from the joint so that the application was relatively dry to minimise the washout effect.
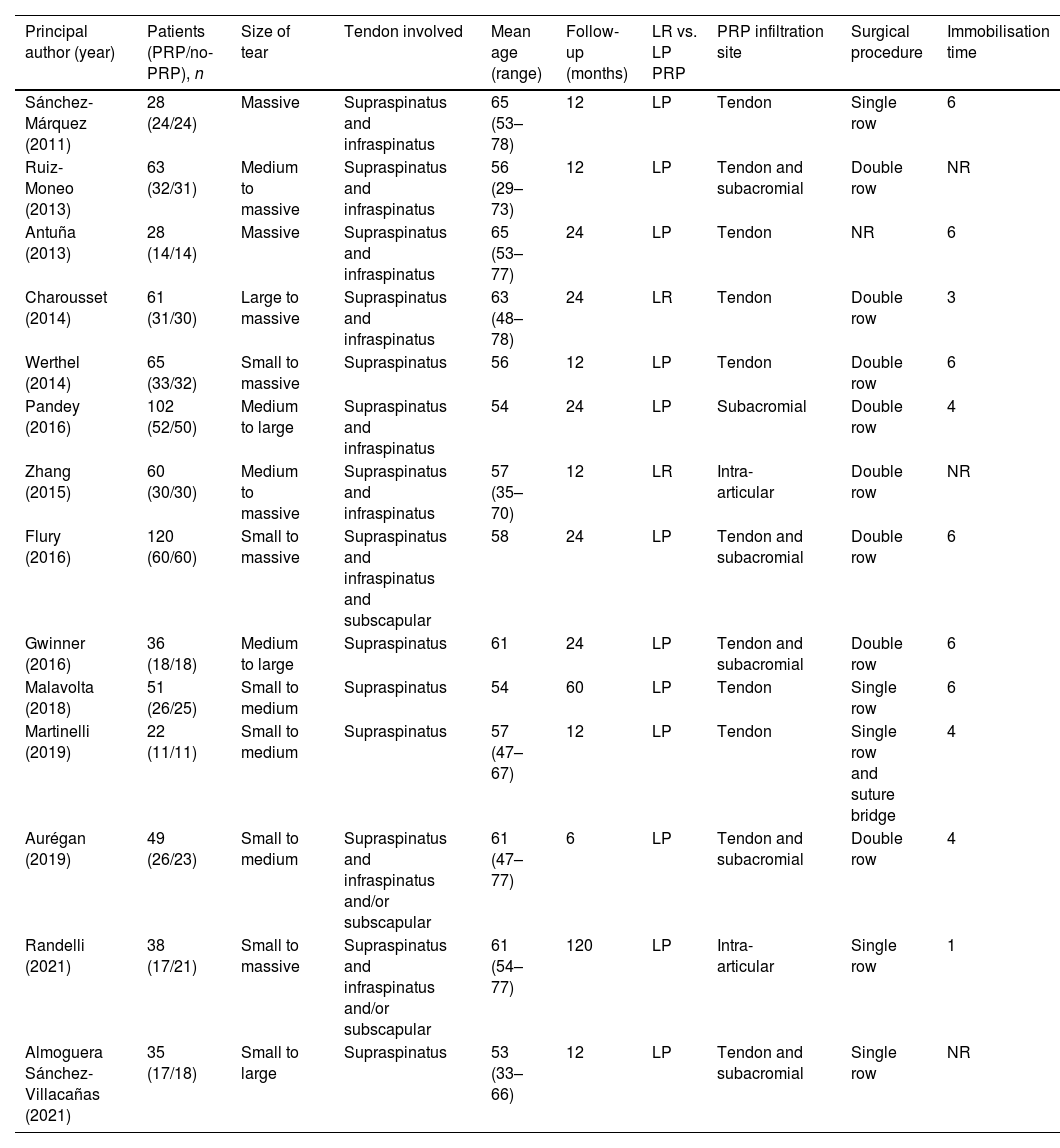
All studies included were published between 2011 and 2021 and included 778 patients (391 patients in the PRP group and 387 in the control group). Two studies used ultrasound findings to diagnose tendon re-tear,28,29 while the rest relied on MRI or arthro-MRI.14,21,22,27,30–37 Five studies performed tendon repair using the single-row surgical repair technique14,27–30 and seven studies used the double-row technique.21,31,33–36
The main characteristics of the 14 studies included are summarised in Table 1.
Characteristics of the studies included.
| Principal author (year) | Patients (PRP/no-PRP), n | Size of tear | Tendon involved | Mean age (range) | Follow-up (months) | LR vs. LP PRP | PRP infiltration site | Surgical procedure | Immobilisation time |
|---|---|---|---|---|---|---|---|---|---|
| Sánchez-Márquez (2011) | 28 (24/24) | Massive | Supraspinatus and infraspinatus | 65 (53–78) | 12 | LP | Tendon | Single row | 6 |
| Ruiz-Moneo (2013) | 63 (32/31) | Medium to massive | Supraspinatus and infraspinatus | 56 (29–73) | 12 | LP | Tendon and subacromial | Double row | NR |
| Antuña (2013) | 28 (14/14) | Massive | Supraspinatus and infraspinatus | 65 (53–77) | 24 | LP | Tendon | NR | 6 |
| Charousset (2014) | 61 (31/30) | Large to massive | Supraspinatus and infraspinatus | 63 (48–78) | 24 | LR | Tendon | Double row | 3 |
| Werthel (2014) | 65 (33/32) | Small to massive | Supraspinatus | 56 | 12 | LP | Tendon | Double row | 6 |
| Pandey (2016) | 102 (52/50) | Medium to large | Supraspinatus and infraspinatus | 54 | 24 | LP | Subacromial | Double row | 4 |
| Zhang (2015) | 60 (30/30) | Medium to massive | Supraspinatus and infraspinatus | 57 (35–70) | 12 | LR | Intra-articular | Double row | NR |
| Flury (2016) | 120 (60/60) | Small to massive | Supraspinatus and infraspinatus and subscapular | 58 | 24 | LP | Tendon and subacromial | Double row | 6 |
| Gwinner (2016) | 36 (18/18) | Medium to large | Supraspinatus | 61 | 24 | LP | Tendon and subacromial | Double row | 6 |
| Malavolta (2018) | 51 (26/25) | Small to medium | Supraspinatus | 54 | 60 | LP | Tendon | Single row | 6 |
| Martinelli (2019) | 22 (11/11) | Small to medium | Supraspinatus | 57 (47–67) | 12 | LP | Tendon | Single row and suture bridge | 4 |
| Aurégan (2019) | 49 (26/23) | Small to medium | Supraspinatus and infraspinatus and/or subscapular | 61 (47–77) | 6 | LP | Tendon and subacromial | Double row | 4 |
| Randelli (2021) | 38 (17/21) | Small to massive | Supraspinatus and infraspinatus and/or subscapular | 61 (54–77) | 120 | LP | Intra-articular | Single row | 1 |
| Almoguera Sánchez-Villacañas (2021) | 35 (17/18) | Small to large | Supraspinatus | 53 (33–66) | 12 | LP | Tendon and subacromial | Single row | NR |
LP: leucocyte poor; LR: leucocyte rich; NR: not reported; PRP: platelet-rich-plasma.
The methodological quality of the studies included was assessed based on the Cochrane risk of bias criteria. The risk of bias in each study is illustrated in Fig. 2.
Seven studies14,27–32 were identified as low risk in the randomisation process. Blinding of treatment allocation, participants, and personnel was low in eight studies.14,22,27–32 Two studies were identified as high risk34,35 and three studies as uncertain risk21,27,37 for the blinding of the assessors. All the studies were low risk for incomplete outcome data and selective outcome reporting.
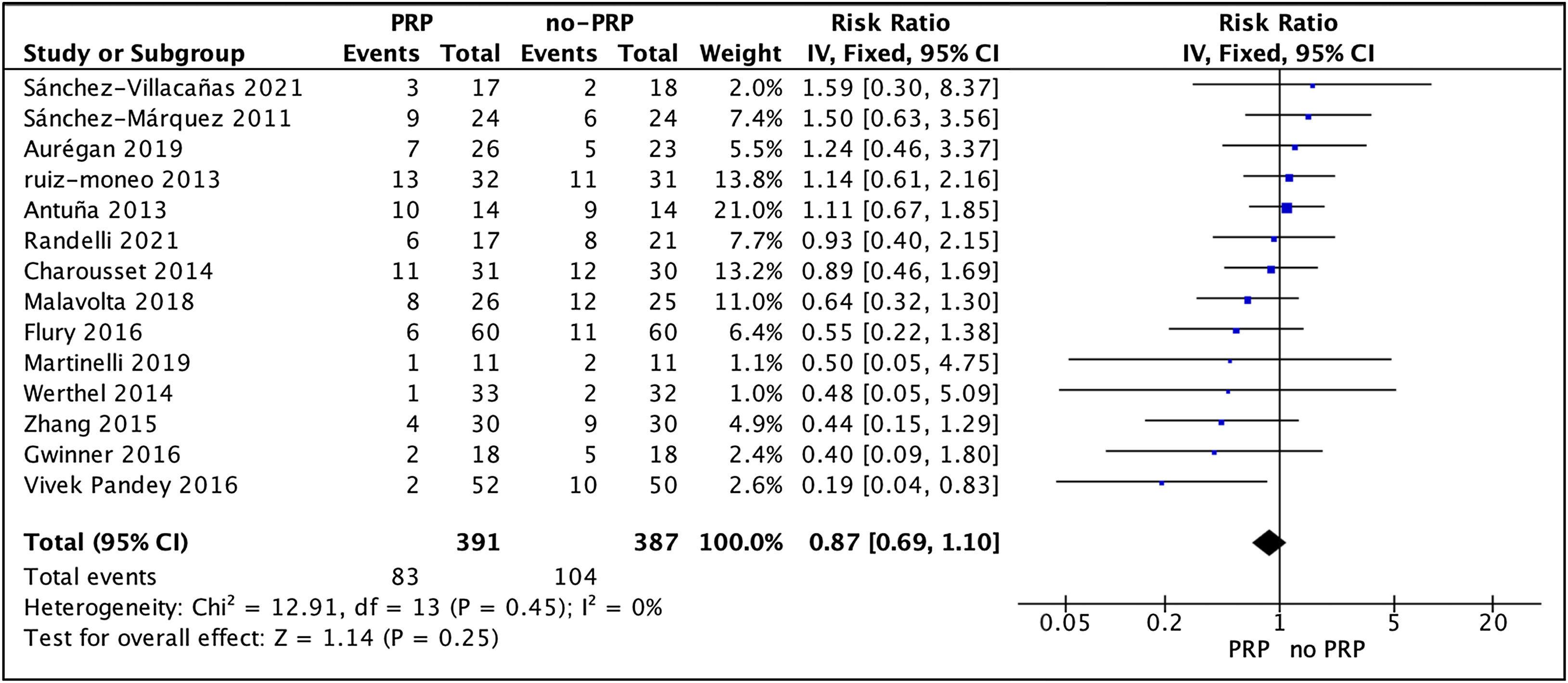
Re-tear resultsAll 14 studies reported re-tear rates. Homogeneity across the studies was good (I2=0%, p=.45). The overall rate of new tears was 24% (21.2% for the PRP group vs. 26.9% for the non-PRP group).
The overall effect of the pooled results indicated that patients in the PRP group had a lower rate of new tears compared with the no-PRP group (RR, .87 [95% CI: .69–1.10]; p=.45; I2=0%), although this difference was not significant (Fig. 3).
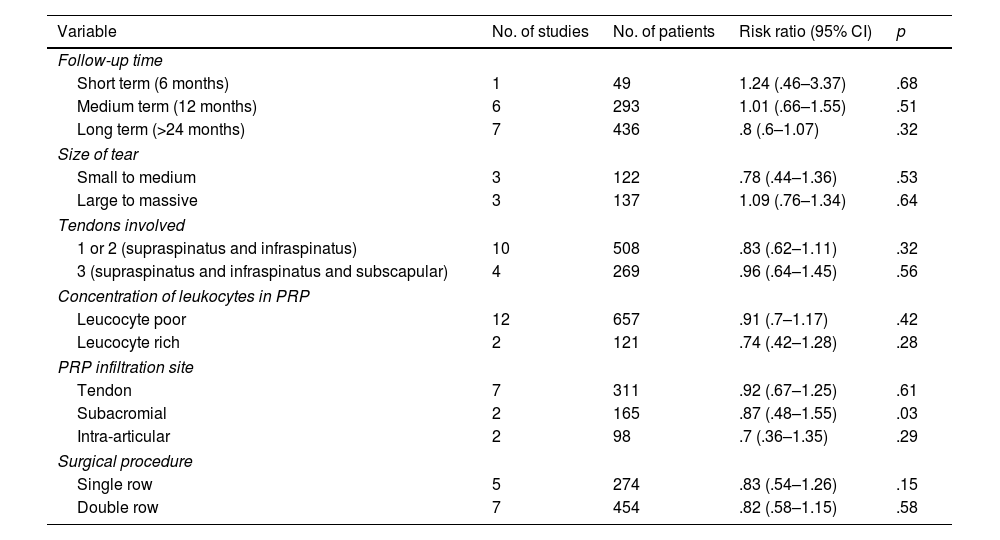
Subgroup analysis showed a significantly lower rate of new tears in the PRP group when infiltrated at the subacromial level compared to the no-PRP group. The remaining analysis revealed no statistically significant difference between groups, but there was a trend in the effectiveness of PRP when follow-up was long term, only one or two tendons were injured, when the tear was small or medium, when the PRP was leucocyte rich, or when the tendon suture was performed using single-row. The results of the subgroup analysis for the re-tear rate are shown in Table 2.
Subgroup analysis of re-tear rate.
| Variable | No. of studies | No. of patients | Risk ratio (95% CI) | p |
|---|---|---|---|---|
| Follow-up time | ||||
| Short term (6 months) | 1 | 49 | 1.24 (.46–3.37) | .68 |
| Medium term (12 months) | 6 | 293 | 1.01 (.66–1.55) | .51 |
| Long term (>24 months) | 7 | 436 | .8 (.6–1.07) | .32 |
| Size of tear | ||||
| Small to medium | 3 | 122 | .78 (.44–1.36) | .53 |
| Large to massive | 3 | 137 | 1.09 (.76–1.34) | .64 |
| Tendons involved | ||||
| 1 or 2 (supraspinatus and infraspinatus) | 10 | 508 | .83 (.62–1.11) | .32 |
| 3 (supraspinatus and infraspinatus and subscapular) | 4 | 269 | .96 (.64–1.45) | .56 |
| Concentration of leukocytes in PRP | ||||
| Leucocyte poor | 12 | 657 | .91 (.7–1.17) | .42 |
| Leucocyte rich | 2 | 121 | .74 (.42–1.28) | .28 |
| PRP infiltration site | ||||
| Tendon | 7 | 311 | .92 (.67–1.25) | .61 |
| Subacromial | 2 | 165 | .87 (.48–1.55) | .03 |
| Intra-articular | 2 | 98 | .7 (.36–1.35) | .29 |
| Surgical procedure | ||||
| Single row | 5 | 274 | .83 (.54–1.26) | .15 |
| Double row | 7 | 454 | .82 (.58–1.15) | .58 |
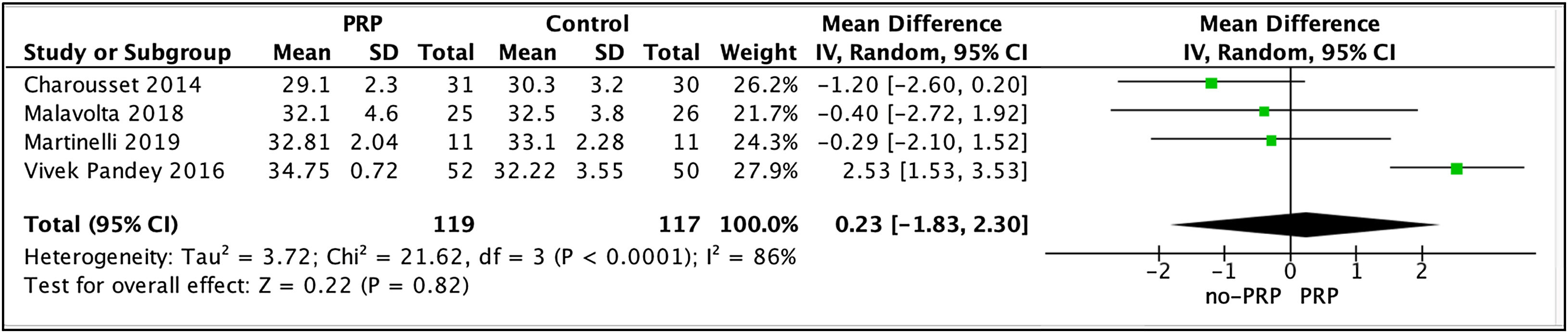
A total of four studies14,29,33,37 with 236 patients reported outcomes using the UCLA score. Participants were evaluated at the end of each of the studies, which could be at 6, 12, 24, or 120 months of follow-up. The results indicated that patients in the PRP group had a higher score compared to the no-PRP group (WMD, .23 [95% CI: −1.83 to 2.30]; p=.82; I2=86%), although this difference was not significant (Fig. 4).
Eight studies14,21,29,33–37 with 446 patients reported outcomes using the Constant–Murley score. Participants were evaluated at the end of each study. The results indicated that patients in the PRP group scored higher compared with patients in the no-PRP group (WMD, .72 [95% CI: −1.78 to 3.22]; p=.57; I2=63%), although this difference was not significant (Fig. 5).
VAS pain scores were reported in seven studies14,21,29,33–35,37 with 410 patients. Participants were evaluated at the end of follow-up. Both groups were found to have similar results, although with a trend to lower scores in the PRP group compared to the control group (WMD, −.08 [95% CI: −1.31 to .14]; p=.46; I2=56%) (Fig. 6).
Subgroup analysis showed no significant rate in terms of UCLA, Constant, or VAS scores, but higher scores were observed in the PRP group compared to the control group in all subgroups analysed.
DiscussionAccording to the literature, the re-tear rate after rotator cuff repair ranges from 2.5% to 62%.2,3
We conducted this systematic review and meta-analysis to evaluate the effect of PRP as an adjuvant in rotator cuff repairs and to investigate whether it is related to decreased re-tear rates and improved patient function.13–16
The results of our study revealed better scores in the PRP group compared to the control group with no increase in adverse effects, although not significantly, in the Constant–Murley test, the UCLA test, and the VAS test for pain. The PRP group had a lower rate of new tears compared to the non-PRP group, although this difference was not significant.
Previous studies have shown that double-row repair has better outcomes compared to single-row tendon repairs due to improved biomechanical properties, and provides a better environment for tendon healing.38 This may explain why the effect of PRP is not evident when administered in patients undergoing double-row repair. In contrast, there is a trend in favour of PRP, as seen in our study, when a single-row repair is performed, as the latter provides a lower biomechanical strength.
The concentration of leukocytes in PRP is an important factor to consider. Zhao et al.39 suggest that leucocyte-poor PRP is better than leucocyte-rich PRP both functionally and for preventing re-tears. In our analysis there are no differences in this aspect, however there is a trend in favour of LR-PRP, although only two studies included LR-PRP, which could be due to a lack of data.
Previous studies have shown that PRP is more beneficial in larger tears; however, in our study there are no differences.
A review of the different studies in the literature showed great heterogeneity in the data. In the systematic review and meta-analysis conducted by Xu and Xue40 comparisons are made between PRP and biological augmentations other than PRP, such as PRP matrix, platelet membrane, or the gel form of PRP. This study also does not consider the time of application of PRP, or the number of infiltrations administered, since it compares indistinctly whether the administration of PRP was intraoperative or extraoperative, or the number of infiltrations administered.
In our study, we used strict eligibility criteria to avoid bias derived from the factors mentioned above and to achieve a more homogeneous study. Thus, we only included studies that included the administration of PRP, and this administration was intraoperative.
In our study, as in others published, articles were included without considering the PRP infiltration site. Comparisons were made between infiltrations at the level of the tendon–bone interface, at the intra-articular level, or at the subacromial level, to then perform the subgroup analysis and thus be able to evaluate the real usefulness of PRP, and whether it is related to the infiltration site.
Even so, we believe that this is still a heterogeneous study among the different studies analysed and among the different variables, such as platelet concentration and the amount of PRP injected. Thus, the limitations of this study are probably the absence of a standard PRP preparation and the use of varying concentrations and amounts of PRP in the different studies, which may have been the reason for the different results.
ConclusionsThe administration of PRP as an adjunct treatment in arthroscopic repairs of complete rotator cuff tears has shown promising results. Although there is not yet sufficient evidence to support its routine use in clinical practice, there are some proven benefits, such as short-term pain reduction and improved function. A reduction in the rate of re-tear was also observed, which could be due to improved tendon healing, and in this aspect, it can be considered superior to the other available treatments.
Level of evidenceLevel of evidence I.
FundingThis research received no specific grant from any funding agency in the public, commercial or not-for-profit sector.
Conflict of interestsThe authors have no conflict of interests to declare.