The fat of the synovial joints can be used to maintain the joint structure. Our objective is to analyse the evolution of joint degeneration in knees with and without adipose pack.
Material and methodologyIn six sheep, the anterior cruciate ligament was sectioned in both knees, to cause osteoarthritis. In one group the fat pack was preserved and in another group it was completely removed. We performed a histological and molecular biology study analyzing the expression, in the synovial membrane, subchondral bone, cartilage, fat, meniscus, and synovial fluid, of RUNX2, PTHrP, cathepsin-K, and MCP1.
ResultsWe did not find morphological differences. We found increased expression of RUNX2 in synovial membrane, PTHrP and Cathepsin K in synovial fluid in the group without fat, and increased expression of RUNX2 in the meniscus and MCP1 in synovial fluid in the group with fat.
ConclusionInfrapatellar fat participates in the inflammatory process that accompanies osteoarthritis, since Hoffa fat pad resection alters pro-inflammatory markers, while the model with intact fat increases the pro-inflammatory marker MCP1 in synovial fluid.
La grasa de las articulaciones sinoviales puede servir para el mantenimiento de la estructura articular. Nuestro objetivo es analizar la evolución de la degeneración articular en rodillas con y sin paquete adiposo.
Material y metodologíaEn 6 ovejas se efectuó la sección del ligamento cruzado anterior en ambas rodillas, para provocar una artrosis. En un grupo se preservó el paquete adiposo y en otro grupo se extirpó completamente. Realizamos un estudio histológico y de biología molecular analizando la expresión —en la membrana sinovial, el hueso subcondral, el cartílago, la grasa, el menisco y el líquido sinovial— de RUNX2, PTHrP, catepsina-K y MCP1.
ResultadosNo encontramos diferencias morfológicas. Se detectó aumento de la expresión de RUNX2 en la membrana sinovial, PTHrP y catepsina-K en el líquido sinovial en el grupo sin grasa y aumento de la expresión RUNX2 en el menisco y MCP1 en el líquido sinovial en el grupo con grasa.
ConclusiónLa grasa infrapatelar participa en el proceso inflamatorio que acompaña en la artrosis, pues la resección de la grasa de Hoffa altera los marcadores proinflamatorios, mientras que el modelo con la grasa intacta incrementa el marcador proinflamatorio MCP1 en el líquido sinovial.
Osteoarthritis (OA) is a degeneration of the synovial joint, which affects more than 25% of the population over the age of 65 years1; it is characterised by progressive deterioration of the cartilage, abnormal bone growth, due to subchondral bone remodelling, sclerosis with osteophyte formation, and synovial inflammation, accompanied by joint pain, all of which can lead to functional disability. Its clinical presentation, however, is variable and does not always correlate with imaging. It has a multifactorial aetiology that includes factors such as previous injuries and surgeries of the joints, trauma, obesity, and ageing, not to mention genetic inheritance. It is a chronic condition featuring pain, inflammation and loss of cartilage, and is the result of an imbalance between degeneration phenomena and tissue repair when mechanical stresses exceed the level tolerated by the joint tissues.2
Synovial inflammation leads to cartilage destruction, pain, and functional disability3 and alters chondrocytes by inhibiting cartilage extracellular matrix remodelling.4 Inflammatory factors such as IL-1β and TNF-α, as well as other cytokines, activate NF-κB in synovial cells and chondrocytes.4 Inflammatory cytokines like interleukin-1 beta (IL-1β) and tumour necrosis factor alpha (TNF-α) stimulate the production of matrix metalloproteinases (MMPs), enzymes that can then degrade all the components of the extracellular matrix. The collagenases MMP-1 and MMP-13 augment collagen degradation. MMP-1 is primarily produced by the synovial cells of the synovial membrane, while MMP-13 is a product of chondrocytes.5
Hoffa's infrapatellar fat pad (IFP) is a specialised type of adipose tissue that serves as a mechanical shock absorber and guides the patellar tendon and even the patella itself during flexion–extension movements; however, under normal conditions, the IFP is a source of mesenchymal cells and growth factors that maintain and nurture the integrity of the joint tissues.6–8 Later, when the degenerative process begins, the IFP is modified and contributes to joint deterioration. To prove this hypothesis, osteoarthritis was provoked in the knee of animals. In one group, the fat in the IFP was completely excised, while in the other group it was left intact.
We have studied molecules such as RUNX2, a key transcription factor associated with osteoblast differentiation that impacts inflammatory processes in the joint as well as the degeneration of the articular cartilage.9 The PTH-related protein PTHrP is a receptor that is upregulated in osteoarthritic inflammatory processes.10 Cathepsin K is a clinical biomarker of osteoarthritic activity and can be used as a relevant predictive factor for the development of this condition,11 and MCP1, monocyte chemotactic protein, is an adipokine implicated in the regulation of monocyte migration and infiltration and participates as a pro-inflammatory cytokine.12
Material and methodsAnimal modelAll procedures were undertaken in accordance with current legislation (RD 53/2013 dated 1 February) and European legislation (Directive 2010/63/EU) and after obtaining the approval of the centre's Ethics Committee.
Two-year-old Assaf sheep (n=6) with an average weight of 60±10kg from the farm at our centre were used. All the animals underwent surgery on both knees and were included in two experimental groups. The anterior cruciate ligament (ACL) of the right knee was severed section in group A and all the fat was resected from the IFP. In group B, only the ACL was resected in the left knee. ACL section is a widespread methodology used to induce osteoarthritis within a short period of time.
All the animals were moved to the hospitalisation unit facilities, one week prior to the procedure, to ensure the quarantine period and their acclimation to their new housing. During this time, regular clinical and laboratory examinations were carried out to monitor for diseases that could preclude them from being included in the study. After their arrival, they were weighed and subsequently housed in groups of 2–3 animals, thereby lowering stress. Their diet was based on hay and feed concentrate with free access to water.
The day before surgery, the surgical field was shaved and the animals were placed in individual cages and were not allowed to eat any solid food for 24h. Access to drinking water was ad libitum.
Anaesthetic procedureAs pre-anaesthesia medication, a combination of midazolam (Midazolam Normon®), at a dose of 0.25mg/kg, and methadone (Metasedin®), at a dose of 0.2mg/kg, were administered intramuscularly. After a waiting period of 10–15min, in a quiet environment without stimuli, and once the proper state of sedation had been confirmed, they were moved to the operating theatre, where they received preoxygenation by means of a mask with 0.5 FiO2 and venoclysis of the cephalic vein was performed with a 16GA endovenous catheter.
Once the permeability of the intravenous lines was verified, anaesthetic induction was carried out using propofol (Propofol Lipuro®), at a dose of 2–3mg/kg, by slow intravenous administration, monitoring relaxation and the disappearance of reflexes. Conventional endotracheal intubation was then performed, placing the animal in supine decubitus.
Anaesthesia was maintained throughout the procedure using sevofluorane (Sevoflo®), at a dose of 1–1.5 CAM. Fentanyl (Fentanest®) was used as intraoperative analgesic therapy, administering a loading dose of 5mg/kg, followed by continuous infusion at a dose of 6mg/kg/h, and with rescue doses administered if required.
A 20mg/kg/24h dose of amoxicillin–clavulanic acid (Synulox®) was administered intramuscularly as prophylactic antibiotherapy and 4mg/kg of carprofen (Rimadyl®) intravenously for analgesia and anti-inflammatory therapy.
Surgical procedureA conventional bilateral lateral parapatellar mini-arthrotomy was performed, with incision of the joint capsule, internal dislocation of the patella, flexion of the joint and section of the ACL, and resection of the fat in Hoffa's fat pad, depending on the study group. Once the experimental protocol had been completed, the joint was closed plane by plane with resorbable suture.
After confirming that the animal's haemodynamic and respiratory status was correct, it was transferred to an acclimatised room and monitored until complete recovery, at which point it was returned to the hospitalisation unit.
Postoperative carePostoperative medication consisted of subcutaneous administration of carprofeno (Rimadyl®), 4mg/kg/24h for five days as anti-inflammatory-analgesic therapy and an intramuscular antibiotic, amoxicillin–clavulanic acid (Synulox®), at a dose of 20mg/kg/24h, for nine days. In addition, intramuscualr buprenorphine (Buprex®) 0.01mg/kg, every 8h was administered for the first 72h to provide postoperative analgesia.
Throughout the postoperative period, checks were made to ensure that there were no signs of illness, pain, or discomfort, and the proper intake of food and water was monitored at all times.
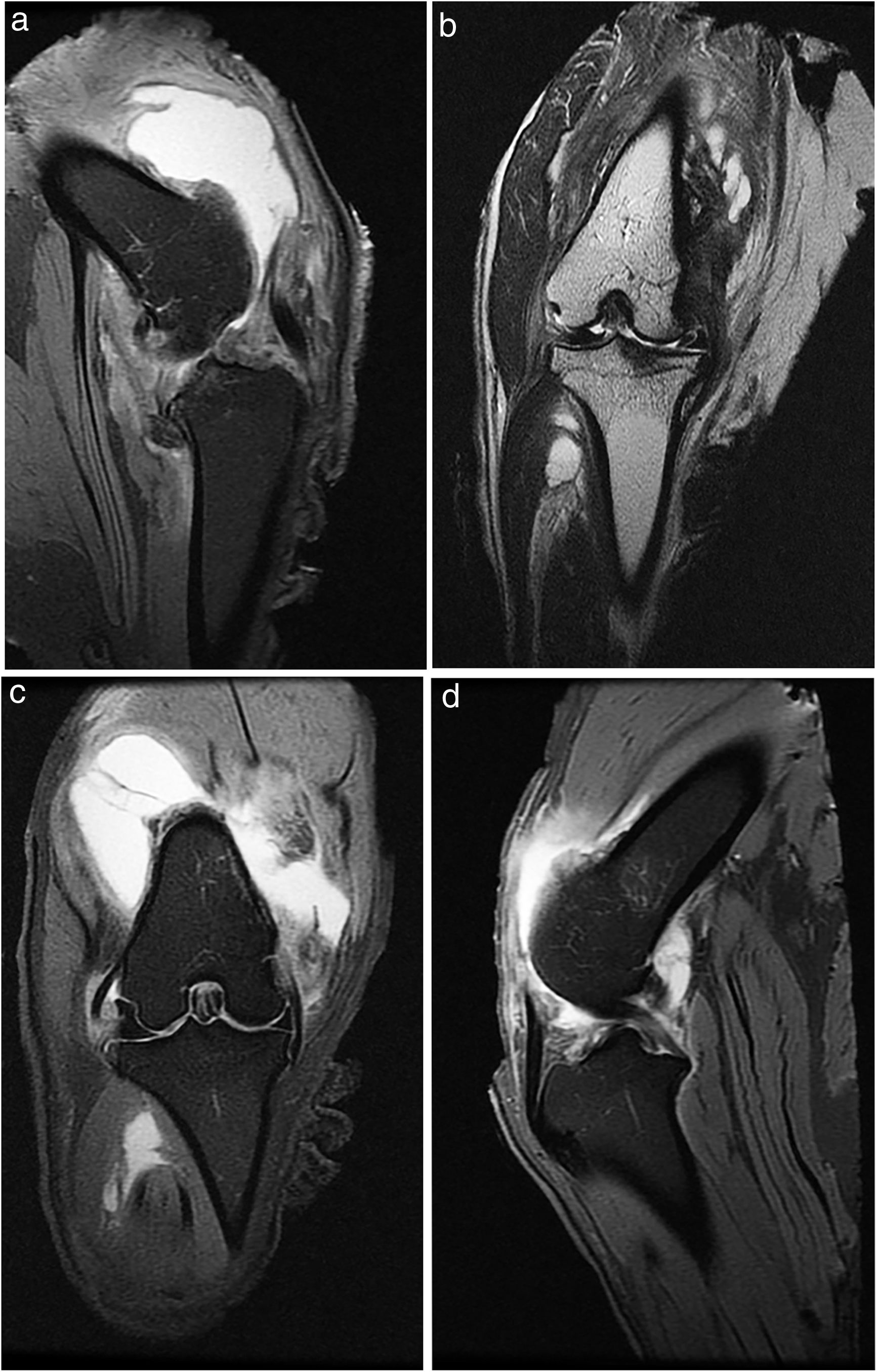
All the animals underwent X-ray and MRI prior to euthanasia (Fig. 1).
Euthanasia procedureTo sacrifice the animals, the sheep were premedicated with a mixture of intramuscular midazolam (0.35mg/kg) and ketamine (5mg/kg). Once the appropriate state of sedation was verified, venoclysis of the jugular vein was performed, injecting propofol (4mg/kg) intravenously, until the animal was completely tranquillised. The euthanasic solution of sodium pentobarbital (Dolethal), at a dose of 133mg/kg PV (1ml/1.5kg), was then administered intravenously.
After confirming the complete absence of cardiorespiratory activity by auscultation and ECG, the animal was removed to collect samples.
Studies performedAfter sacrificing the animal, the knee was completely removed which was severed at the femoral and tibial diaphysis with a saw; samples were obtained for histology and molecular biology studies of the synovial membrane, subchondral bone, articular cartilage, synovial fluid, and periarticular fat (genetic analysis by PCR of inflammatory factors, matrix metalloproteases, and inhibitors) and proteomics, Western blotting: electrophoresis, transfer, and immunodetection. RUNX2, PTHrP, Cathepsin K, Actin, and MCP1 were all assayed.
Histology studiesThe specimens were fixed in 4% phosphate-buffered formalin for 24h. Following fixation, the specimens were decalcified in a solution of polyvinylpyrrolidone (PVP) and EDTA for at least 2 months at 4°C.
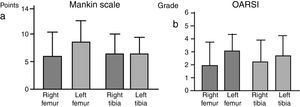
The specimens were dehydrated using alcohols of increasing gradation, placed in xylene for 4h, and embedded in paraffin at a temperature of 60°C. Finally, 4μm thick sections were sliced and stained with Masson's trichrome and safranin-O. Histological analysis was performed according to the Mankin Scale13 to assess the condition of the cartilage and subchondral bone. The final score was the result of adding the values corresponding to the changes in: (a) cartilage structure, (b) cellularity, (c) matrix staining, and (d) the integrity of the “tidemark” or basophilic limiting line. Normal cartilage scored 0 points, and the most severe cartilage deterioration would yield a score of 14 points. The OARSI14 scale contemplates the overall situation of the joint as a semi-quantitative assessment with each preparation fitting into a grade, taking into account the status of the various joint tissues, normal being grade 0 and the most severely degraded being grade 6. The histopathological evaluation was carried out by two independent observers.
Gene expression. RNA extractionTotal RNA from meniscus, synovial membrane, and periarticular fat samples was extracted using Trizol Reagent (Ambion). The meniscus samples were mechanically separated using a scalpel and then processed with the TisueLayser with two spheres for 20min at an intensity of 50Hz.
In the case of synovial membrane and fat samples, 1ml of Trizol was added per 100mg of tissue, after mechanical dissection with a scalpel. To each resulting sample, one-fifth of chloroform (Carlo Ebra Reagents) was added. To precipitate RNA, half isopropanol (Fisher Chemical) was added to each ml of Trizol. Ethanol was used to wash the precipitate. Prior to retrotranscription, the samples obtained were quantified by analysing their purity with the NanoDrop 2000 (Spectrophotometer, Thermo Scientific), always using DEPC (Treated water, Ambion).
To analyse the expression of our proteins, we used the cDNA kit (Applied Biosystems). Each sample was diluted 1:100. We used the mixture with 1μl of RNA per sample. Finally, we placed the tubes in the Mastercycler for retrotranscription.
PCRThe cDNA samples obtained were prepared for PCR. All samples were analysed in triplicate. Beta Actin Rv and Fw (Sigma–Aldrich), RunX2 Rv and Fw (Sigma–Aldrich), TB Green Premix (Takara) and DEPC were added to the mixture. The ratios were determined by the software itself, depending on the concentration of each sample. The PCR equipment was programmed (700HT Fast Real-Time PCR System, Applied Biosystems).
Western blotSynovial fluid samples were processed and analysed by Western blot. In a first step, the samples were freeze-dried to optimise them. The samples were placed in the freeze dryer and incubated “over-night” (vacuum freezing). After 12h, Ripa Buffer (Sigma–Aldrich) was added to the samples, which were dry after freeze-drying and dissolved in Ripa buffer.
For protein quantification, serial dilutions were prepared in a 96-well plate to obtain a standard curve with BSA, Comassie (Thermom Scientific), and distilled water (miliQ), maintaining the same volume in all wells. Colorimetric measurement was performed on the Varioskan (Thermo Fisher); absorbance was determined at 595mm. MQ water (distilled), Resolvin gel Buffer pH 8.8 (Bio-Rad), Tris HCL Buffer pH 6.8 (Bio-Rad), SDS (Sigma–Aldrich), TEMED (Bio-Rad), APS (Sigma–Aldrich), Acrylamide (Bio-Rad) were used as the separating gel. A wet transfer was conducted at 100V for 1h (Bio-Rad). At the end of the transfer, the membranes were obtained and immersed in 5% milk with 1× TBS for blocking. They were left “over night” with the primary antibody (MCP1 (Abcam), RUNX2 (Santa Cruz), Cathepsin K (Abcam), PTHRP (Santa Cruz), Actin (Abcam)) at 4°C under gentle agitation. The corresponding secondary antibodies were added (for MCP1: Rabbit (Abcam); for RUNX2: Rabbit (Abcam); for cathepsin K: Rabbit (Abcam); for PTHRP: Rabbit (Abcam); for actin: Rabbit (Abcam). ECL (Prime Western Blotting Detection Reagents from Amersham) was used to develop the assay, in the Varioskan flash (Thermo Scientific) for chemiluminescence reading.
Statistical analysisTo compare the different groups, the Student's t-test was used for independent samples if the variable under study followed a normal distribution, and the Kruskal–Wallis test followed by the Mann–Whitney U test if it did not. All statistical analyses were performed with SPSS 9.00 for Windows.
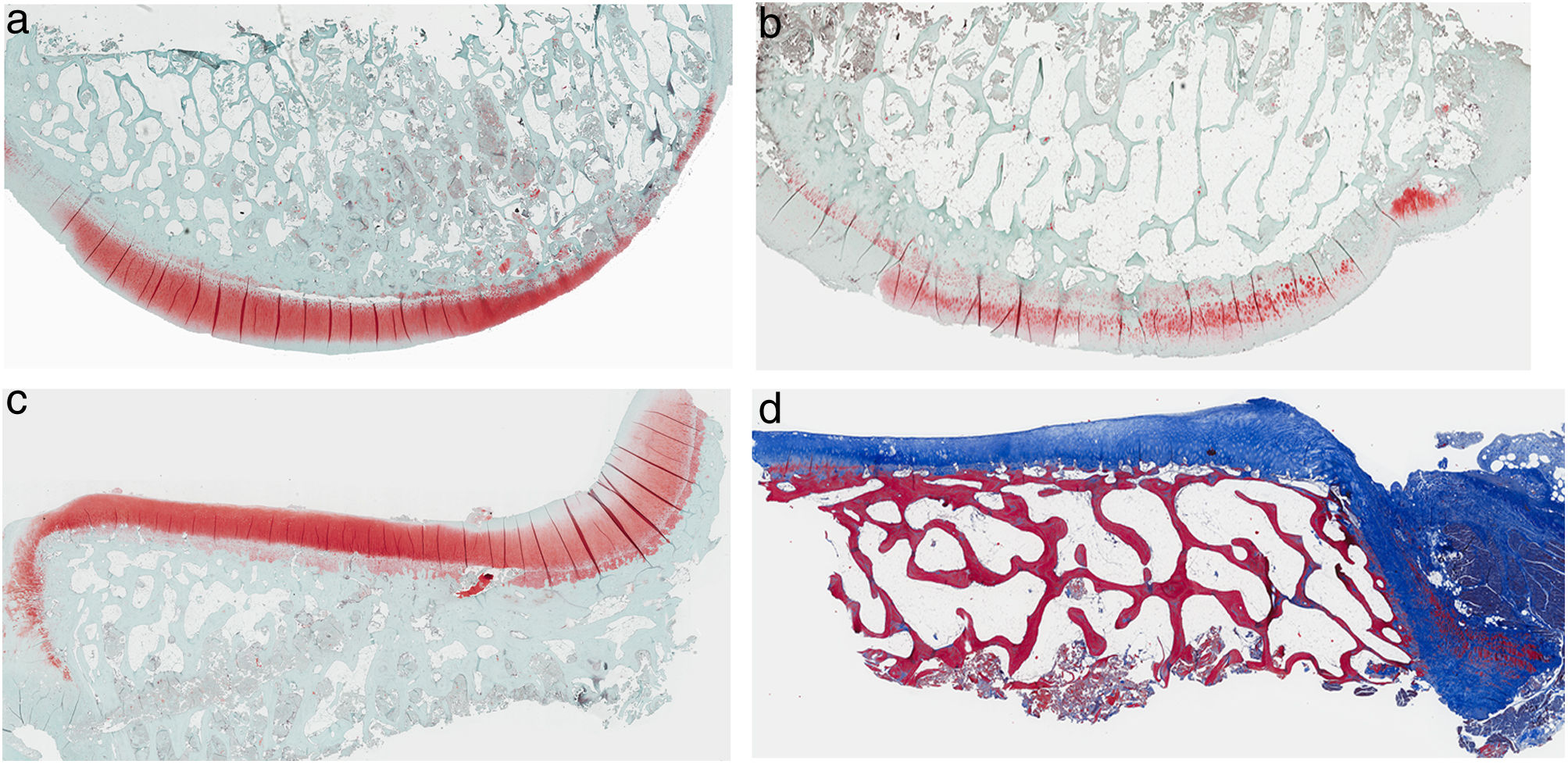
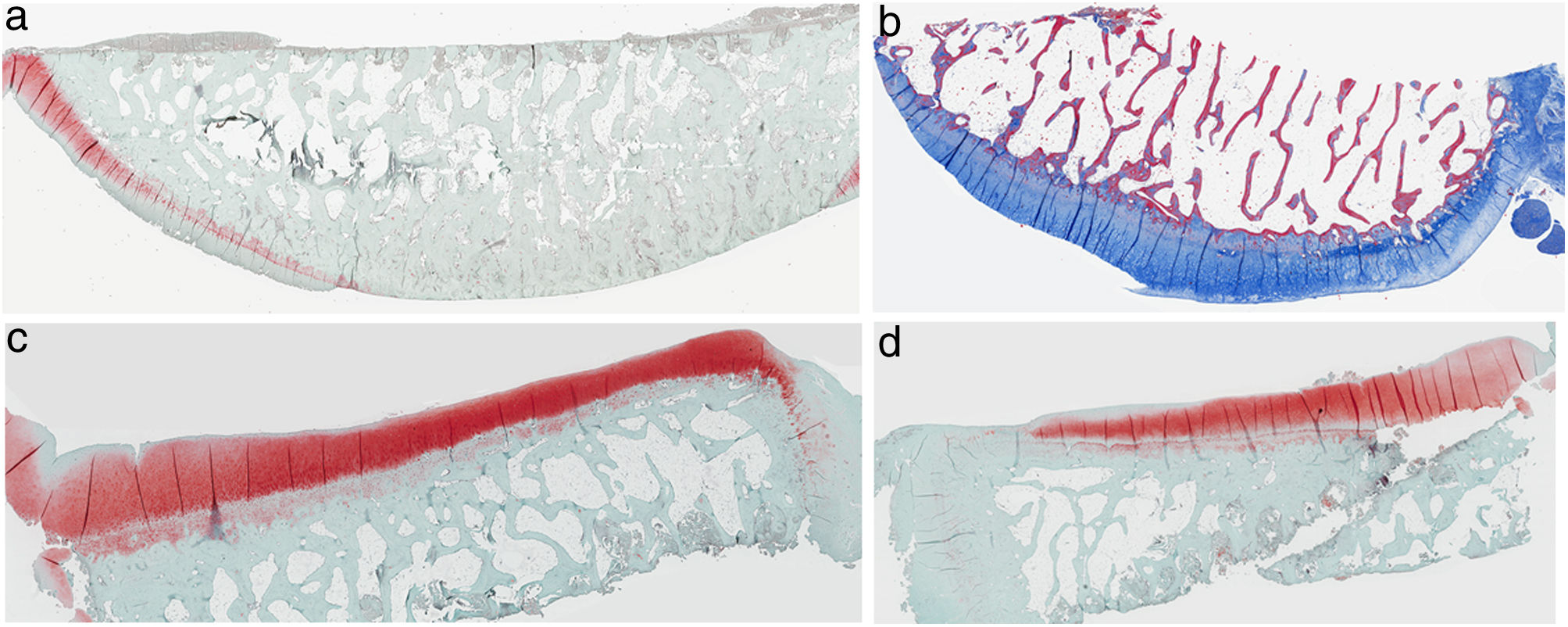
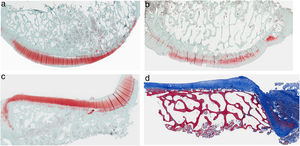
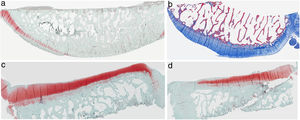
ResultsHistologyMasson's trichrome staining revealed the differences between both groups. The tibial cartilage samples exhibited no obvious morphological differences. Differences were evident in the femoral cartilage with a tendency towards greater deterioration in the model that had Hoffa's fat removed; specifically, more diffuse hypocellularity and greater irregularities on the articular surface. Safranin staining revealed greater loss of proteoglycan content in the knees without IFP, displaying large unstained areas (Figs. 2 and 3).
Histology of the group without Hoffa's fat resection, (a) #1 femoral condyle with normal structure and maintenance of proteoglycans; (b) #5 femoral condyle with loss of tissue structure and decreased proteoglycans; (c) #2 tibial condyle with normal structure; (d) #3 tibial condyle with isogenic clusters, destructured cartilage tissue, and decreased proteoglycans.
Histology of the group with Hoffa's fat resection, (a) #1 femoral condyle with missing articular cartilage; (b) #2 femoral condyle with normal structure and hypocellularity and isogenic groups; (c) #4 tibial condyle with missing cartilage; (d) #5 tibial condyle cartilage maintains tissue structure and displays slight proteoglycan loss.
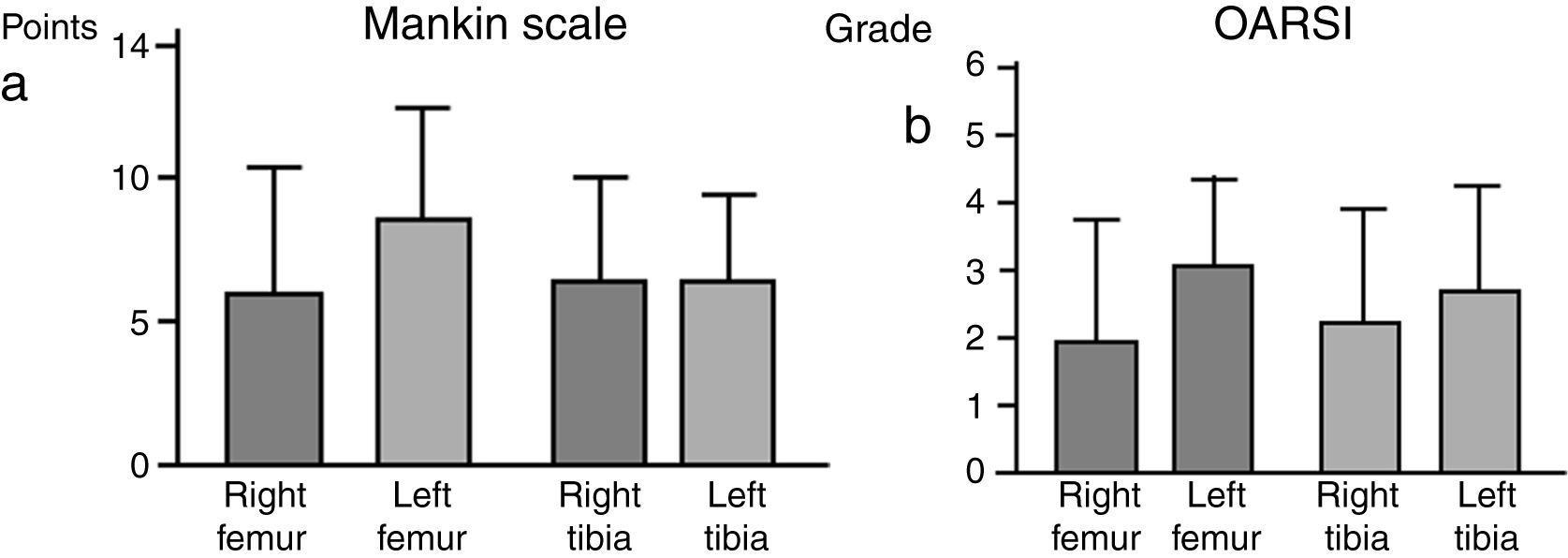
There were no significant intergroup differences on the Oarsi scale, with the values obtained in the tibia being very similar in both knees. The Mankin scale also exhibited no differences between the two groups; the values obtained for the tibia were similar on both sides, despite a tendency towards a higher value on the scale for the left femur, the group without IFP (Fig. 4).
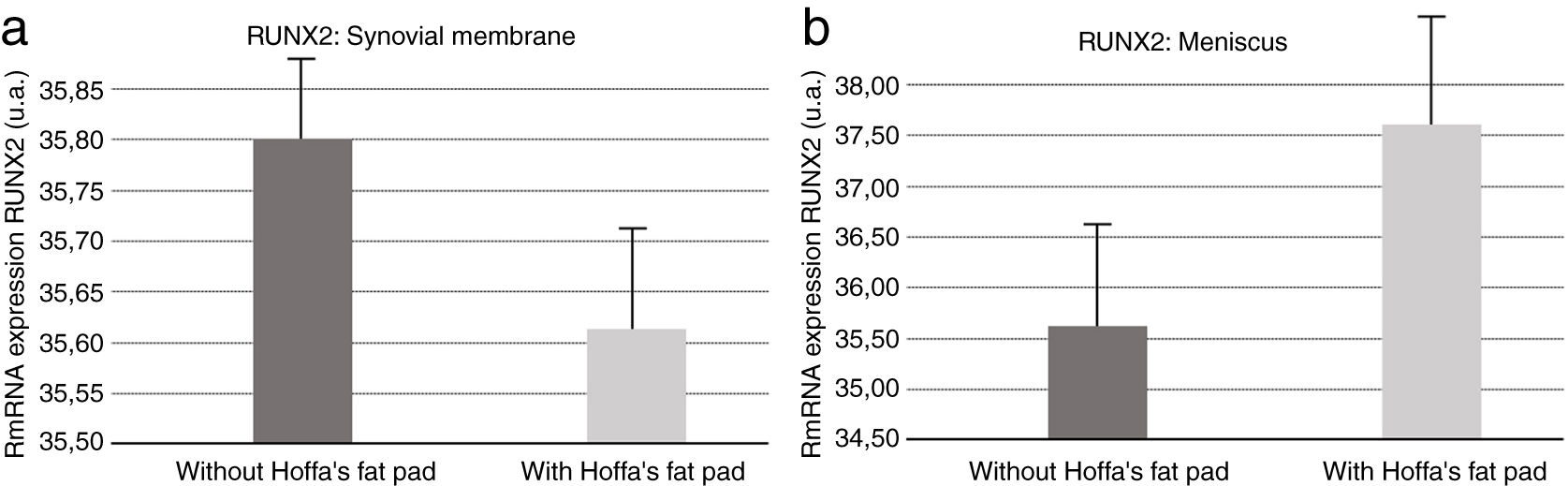
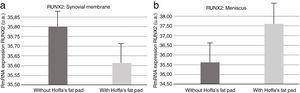
Molecular biologyOur results revealed (Fig. 5) that RUNX2 expression was increased in the synovial membranes of the resected IFP fat group as quantified by PCR. As for the meniscus samples, RUNX2 expression was elevated in the group in which the fat had not been removed (Fig. 5).
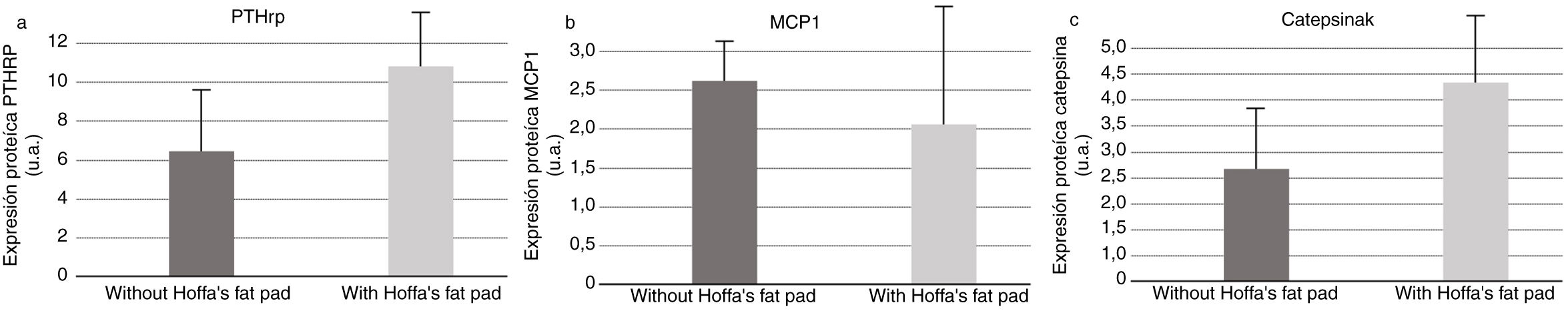
As for PTHrP quantification by Western blot in synovial fluid samples, we found higher secretion of PTHrP in synovial fluid in the group in which the IFP had been excised (Fig. 6). Measurement of MCP1 in the synovial fluid samples by Western blot revealed greater secretion of MCP1 in the synovial fluid in the samples collected from the group having an intact IFP (Fig. 6). Similarly, Western blot measurement of cathepsin K in synovial fluid samples also demonstrated increased secretion of cathepsin K in synovial fluid in the group in which the IFP had been excised (Fig. 6).
DiscussionThe relationship between osteoarthritis and obesity has been raised and weight gain is thought to lead to increased joint load bearing and therefore, increased joint wear and tear as a result of the activation of molecular processes and the endocrine function of adipose tissue.15,16 White adipose tissue produces substances similar, but not identical, to endocrine hormones, known as adipocytokines, which intervene in the breakdown of cartilage, synovium, and bone.17 Jedrzeczyk et al.18 documented increased adipocyte numbers and lymphocyte infiltration in the IFP of individuals having a high body mass index, which has also been associated with lower knee cartilage volume and thickness, increased tibial surface area, and more chondral damage.19 We know that diet and exercise are effective for weight loss, reducing knee pain, improving joint function, and decreasing plasma levels of Il-6.20
Two types of osteoarthritis have been hypothesised, one related to obesity and metabolic syndrome and the other as an effect of physical activity and joint overload. Fat in obese people contains activated macrophages that cause low-grade systemic inflammation, and inflammatory cytokines produced by these macrophages and by adipokines generated by adipocytes might be responsible for initiating the process of degeneration.17,21–23 Moreover, obesity affects joint fat as adipose tissue is less oxygenated and hypoxia stimulates the spread of pro-inflammatory adipokines.24
Not all studies demonstrate that IFP contributes to a catabolic inflammatory joint environment. IFP extracted during knee replacement surgery exhibits lower glycosaminoglycan release and lower MMP expression.25 Maculé et al.26 found marked inflammatory cell infiltration in 36% of IFP samples obtained from patients undergoing knee replacement for osteoarthritis and contrast-enhanced MRI evinced a positive correlation between changes in IFP perfusion and severity of osteoarthritic symptomatology.27 It has been reported that immune cells can infiltrate the IFP as a consequence of osteoarthritis, triggering the release of proinflammatory cytokines from adipocytes,21,28,29 with a catabolic effect on cartilage, initiating a vicious cycle.21 However, Wang et al.30 found no relationship between IFP and osteoarthritis or that fat changes observed on MRI are a consequence of osteoarthritis.
For Cao9 RUNX2 levels go up in the presence of joint injury or inflammation, contributing to the degeneration process of the entire area, especially the cartilage. Our results indicate that there is an increase in RUNX2 in meniscus and synovial membrane samples when IFP was preserved. This supports the notion that IFP fat is involved in the degenerative process in both tissue types.
PTHRP analysis found that more PTHRP was secreted in the synovial fluid of the models without IFP; given that this receptor is elevated in osteoarthritic inflammatory processes,10 we deduce that IFP is directly involved in the inflammatory process.
With respect to the analysis of cathepsin K in the synovial fluid, we should bear in mind that this enzyme is involved in the process of bone remodelling; it is used as a clinical biomarker for osteoarthritis activity, and its presence is indicative of a response to inflammatory activity.11 In our study, the protein is also secreted in higher amounts in animals without IFP, which lends further support to the involvement of Hoffa's fat in the inflammatory and degenerative process of osteoarthritis.
The animals with intact fat displayed more MCP1. Adipokines are known to induce MCP1, intervening in the regulation of monocyte migration and infiltration,20 thus participating as a pro-inflammatory cytokine.
One limitation of this study is the limited number of animals and the lack of analysis of other cytokines or growth factors that would enable us to visualise the overall behaviour of fat mediators.
Preserving Hoffa's fat pad during the initial process of osteoarthritis does not modify the degenerative structural changes that take place following ACL section. The IFP is an important part of the knee joint and, like all other structures, plays a role in osteoarthritis. Nevertheless, we have observed that the IFP fat is involved in the inflammatory process and has an effect on cartilage degeneration. In advanced osteoarthritic processes, the fat of the infrapatellar bundle, by itself, is incapable of halting an already established degenerative process or of reversing this process.
The molecular results indicate that Hoffa's fat is involved in the inflammatory process and in the progression of joint degeneration, inasmuch as the resection of Hoffa's fat affects the values of the inflammatory molecules analysed, while intact fat increases the pro-inflammatory marker MCP1 in synovial fluid, which suggests that fat is an active part of the inflammatory microenvironment that accompanies the progression of osteoarthritis.
Level of evidenceLevel of evidence iii.
FundingThis project was funded by the SECOT Foundation as part of the research project grants awarded in 2018.
Conflict of interestsThe authors have no conflict of interests to declare.