Steroid injections are effective in the treatment of trigger digits but the pain during the injection is an always-present accompanying effect. The aim of this study was to assess the effectiveness and perceived pain during an out-of-sheath corticosteroid injection through the dorsal webspace in the treatment of trigger digits.
Material and methodA total of 126 consecutive patients were included. A subcutaneous (out-of-sheath) corticosteroid injection was performed through the dorsal webspace in all digits. In cases where signs or symptoms persisted, a second injection was offered. Visual analog scale for pain during the injection, DASH questionnaire, success rate and complications were collected.
ResultsThey were 86 women and 40 men with a mean age of 61 years. The mean VAS for pain during the injection was 3.8. Twelve patients were lost to follow-up. The overall success was 68% and success after a single injection was 54%. The best result was achieved on the ring finger. Patients who were not previously operated on carpal tunnel syndrome responded better. No complications were noted.
ConclusionsThe extra-sheath corticosteroid injection through the dorsal webspace is effective and safe. It seems to be less painful than the reported scores for the palmar midline technique although it should be assessed in a comparative study.
Las infiltraciones de corticoides son efectivas en el tratamiento de los dedos en gatillo, pero el dolor percibido por el paciente durante la inyección es un efecto acompañante siempre presente. El objetivo de este estudio fue evaluar la efectividad y el dolor percibido durante una infiltración corticoidea depositada fuera de la vaina tendinosa utilizando la técnica comisural dorsal.
Material y métodoSe incluyeron 126 pacientes consecutivos. Se realizó una infiltración esteroidea subcutánea (fuera de la vaina) a través de la comisura dorsal. En los casos en que los signos o síntomas persistieron, se ofreció una segunda infiltración. Se registró el dolor percibido durante la infiltración mediante la escala visual analógica, el cuestionario DASH antes del tratamiento y al final de seguimiento, la tasa de éxito y las complicaciones.
ResultadosFueron 86 mujeres y 40 varones con una edad media de 61 años. La puntuación media del dolor durante la infiltración fue de 3,8. Doce pacientes se perdieron durante el seguimiento. El éxito global fue del 68% y el éxito tras una única inyección fue del 54%. El mejor resultado se obtuvo en el dedo anular. Los pacientes que no habían sido operados previamente del síndrome del túnel carpiano respondieron mejor. No se objetivaron complicaciones.
ConclusionesLa infiltración esteroidea fuera de la vaina tendinosa y utilizando la técnica comisural dorsal es efectiva y segura en el tratamiento de los dedos en resorte. Parece ser menos dolorosa que los resultados publicados para la técnica palmar sobre la línea media, aunque esto debe evaluarse en un estudio diseñado para ello.
Stenosing tenosynovitis, trigger finger or spring finger is one of the most common causes of hand pain and disability, affecting 2.6% of non-diabetic adults and up to 10% of the diabetic population.1,2 The goal of treatment is to restore smooth, painless gliding of the tendons and full range of motion in the affected finger.
Trigger fingers can be effectively and efficiently treated by steroid injections,3–8 but pain experienced by the patient is an ever-present side effect. Few studies have assessed patient-perceived pain during trigger finger injections, but the score seems to vary depending on the injection technique.9–13
The palmar technique over the midline and depositing the injection into the tendon sheath is probably the most commonly used technique, but others have been described, such as the distal palmar technique, the mid-axial technique or an intra-vain technique through the dorsal webspace.1,8–10,14
The volar region of the hand is more innervated than the dorsal region and therefore injection through the dorsum may theoretically be less painful than injection through the palm. This claim has recently been questioned in some studies conducted to evaluate the least painful method of performing a digital block, in which volunteers prefer a single injection through the palm rather than 2 through the dorsum. In the treatment of trigger fingers, only one dorsal injection would be performed and not 2, so the best technique for injection remains a controversial issue.6,9,15–19
Injection within the tendon sheath has been associated with an increase in perceived pain compared to a subcutaneous technique.20 Some studies have shown that subcutaneous palmar injection is as effective as the intra-sheath technique, reducing the risk of flexor tendon and pulley injury; therefore, depositing the injection within the tendon sheath appears to be unnecessary.1,21,22
The aim of this study was to evaluate the effectiveness and perceived pain during subcutaneous corticosteroid injection using the dorsal commissural technique in the treatment of trigger fingers.
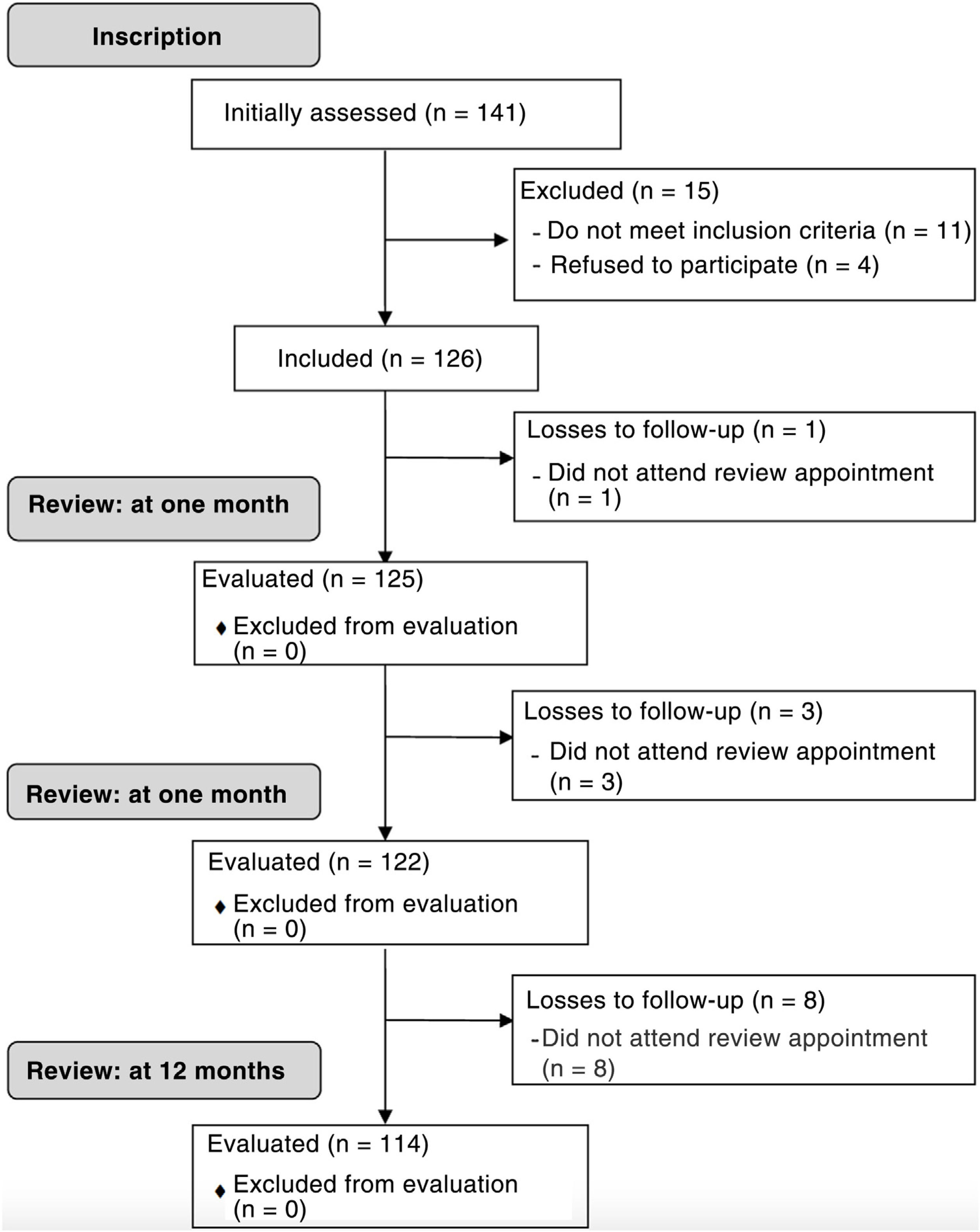
Material and methodStudy population and designFollowing approval by the clinical research committee of our centre (CEIC-CHUIMI-2015/789) and after evaluating the safety and potential efficacy of the dorsal web space technique,23 all patients diagnosed with spring finger by one of 3 orthopaedic surgeons and traumatologists during a one-year period were invited to participate in this prospective cohort study (Fig. 1). Written informed consent was obtained from all subjects prior to the study. All procedures followed conformed to the ethical standards of the committee responsible for human experimentation (institutional and national) and to the Helsinki Declaration of 1975, revised in 2008.
The diagnosis was made based on the patient's symptoms and signs, such as painful springing or locking of the involved finger when the patient flexes and extends it.
Inclusion criteria: any adult patient diagnosed with trigger finger who agreed to participate. Exclusion criteria: (1) involvement of more than one finger; (2) contraindication for treatment according to the product package insert, such as known allergy to the injection components or uncontrolled diabetes mellitus (glycosylated haemoglobin>8%); (3) inflammatory or autoimmune arthritis; (4) previous injection or surgery on the affected finger; and (5) pregnancy or breastfeeding.
The minimum follow-up was 12 months.
Injection techniqueIn all cases a 2ml syringe loaded with 1ml betamethasone 6mg/1ml (Celestone Cronodose®, Merck Sharp & Dohme Laboratories, Spain) and 1ml mepivacaine 2% (Normon Laboratories, Spain) was used. Puncture was performed using a 25G needle (SurGuard2, Terumo®, Terumo Medical Corporation, USA).
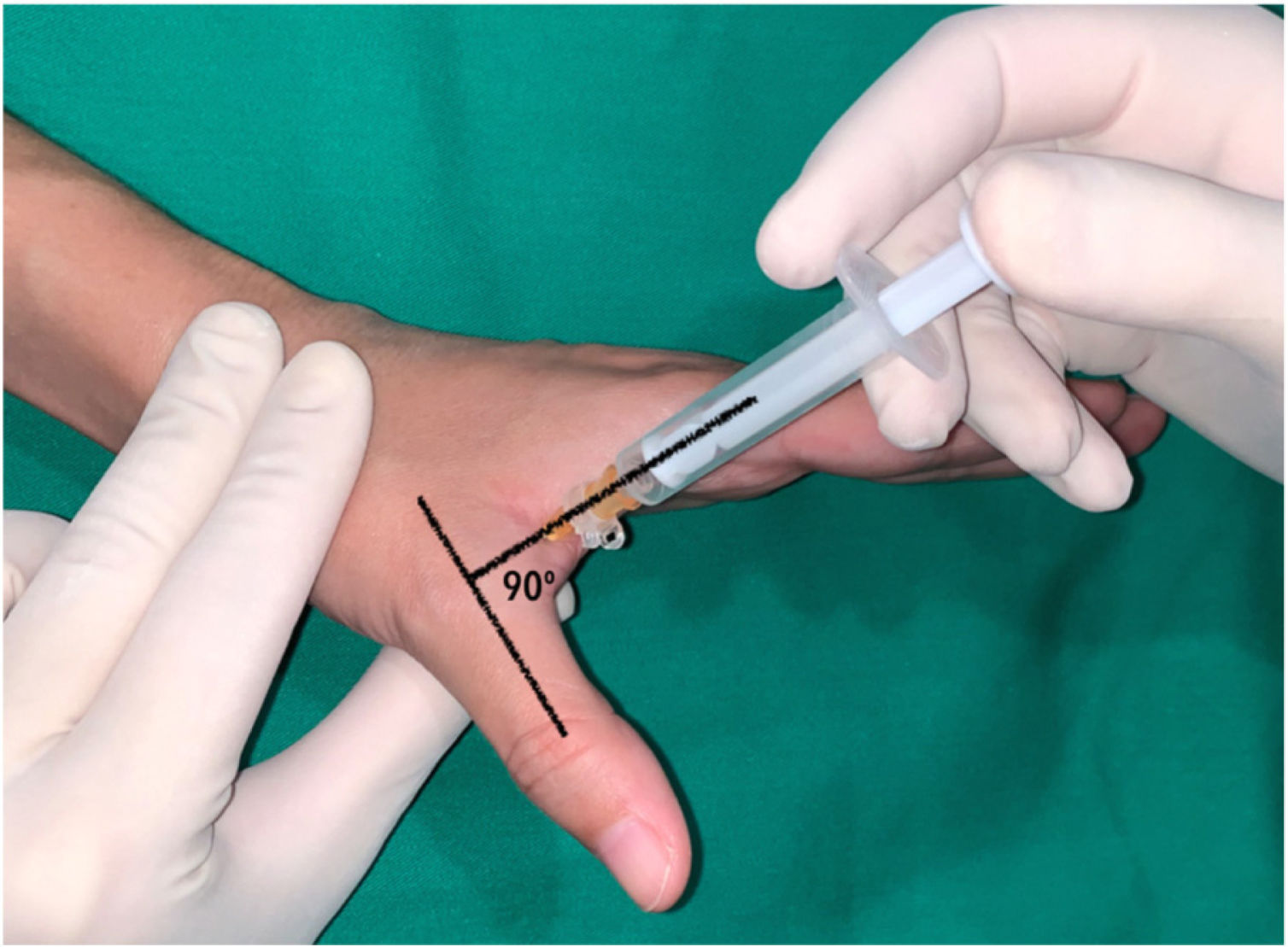
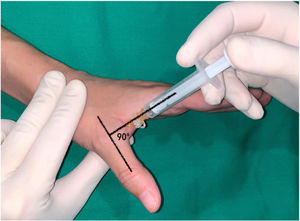
In the thumb, a dorsal-ulnar injection was performed at the level of the metacarpophalangeal joint, keeping the thumb in maximum abduction and directing the needle into the subcutaneous cellular tissue at the level of the head of the first metacarpal at a 90° angle to the axis of the thumb (Fig. 2).
Dorsal-ulnar injection performed on the thumb at the level of the metacarpophalangeal joint keeping the thumb in maximum abduction, directing the needle towards the subcutaneous cellular tissue at the level of the head of the first metacarpal at an angle of 90 with respect to the axis of the thumb.
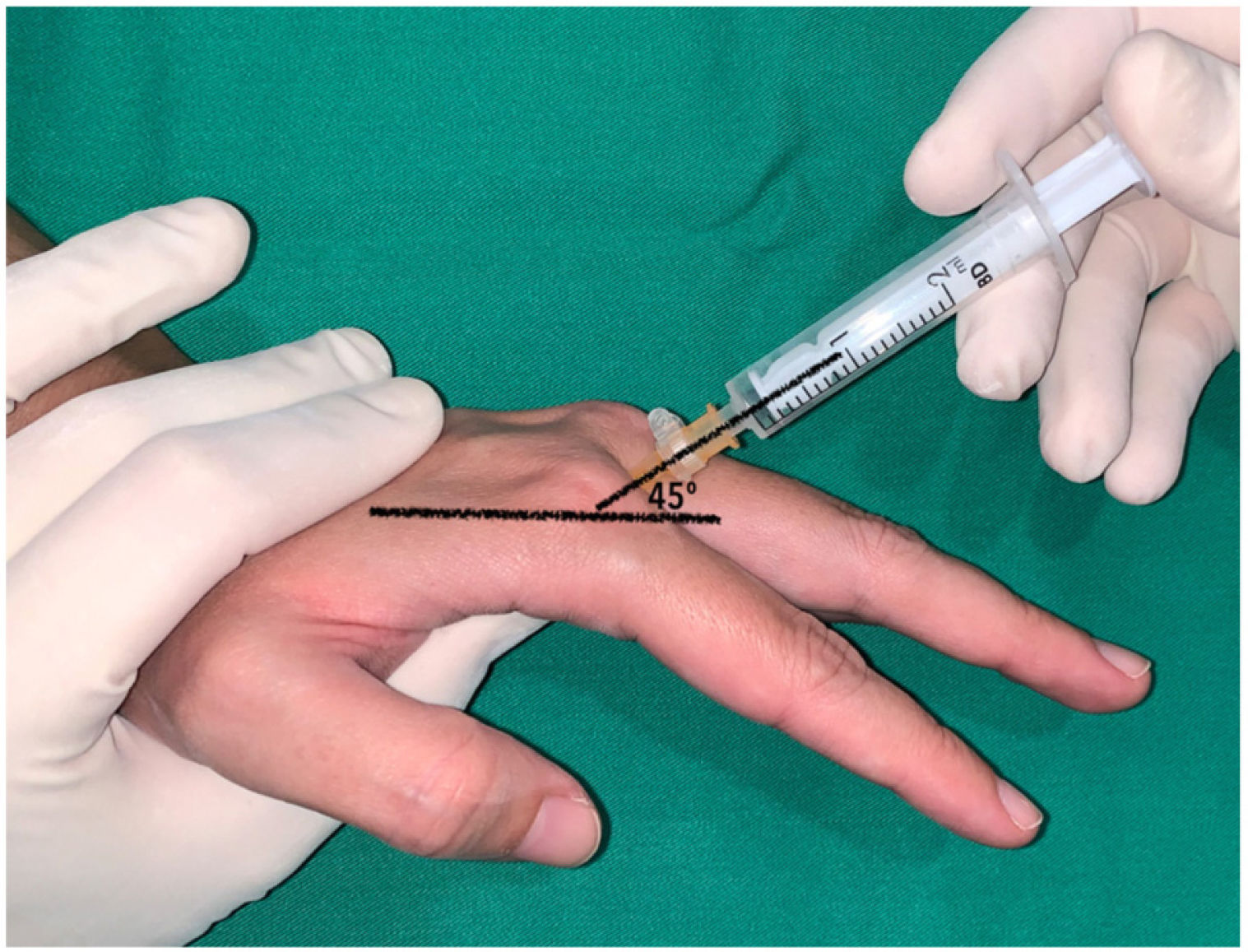
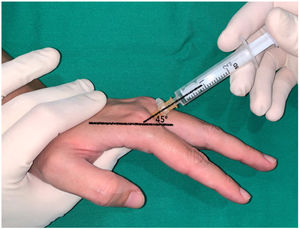
In the long fingers, the puncture was performed keeping the metacarpophalangeal joint slightly flexed (approximately 30) and directing the needle into the subcutaneous cellular tissue at the level of the metacarpal head at an angle of 45 with respect to the axis of the finger (Fig. 3). In the index finger, a dorsal-ulnar injection was performed (through the second web space). The technique was not performed through the first web space because it is wider and deeper, which, in our experience, makes it difficult to guide the needle. In the middle and ring fingers, dorsoradial or dorsal-ulnar injection was performed according to the investigator's preference. Finally, the little finger was infiltrated dorsoradially.
The location of the needle tip outside the tendon sheath was confirmed by asking the patient to slightly flex the finger after inserting the needle and verifying that the needle did not move with flexor tendon movement.
All patients were reviewed in the clinic at 1 month, 3 months and 12 months. Those patients in whom signs or symptoms persisted at the 1-month or 3-month visit were offered a second injection.
Tools and measurementsDemographics; comorbidities; time from symptom onset to diagnosis; Green's classification (Table 1)1; Disabilities of the Arm, Shoulder and Hand (DASH; 0–100 points) before injection and at final follow-up24; pain during injection measured by visual analogue scale (VAS; 0–10 points); number of injections; success rate and complications were recorded. During and after injection, patients were specifically asked about the presence of paraesthesia or numbness of the finger.
Green's classification for trigger fingers.
| Grade | Clinical findings |
|---|---|
| I | Pain on pulley A1 |
| II | Trigger. The patient can actively extend the finger |
| III | Blocked. The patient passively extends the finger. |
| IV | Fixed stiffness in flexion |
Success was defined as complete resolution of symptoms with no recurrence of pain or trigger finger during the 12 months of follow-up.
Recurrence was defined as the appearance of symptoms (pain) or signs (trigger or block) after an asymptomatic period of at least one month.
Pain score was measured after injection in an adjoining room using a 10cm line (from 0=no pain to 10=the worst pain experienced), where patients marked a score.
Statistical analysisAll data were analysed using Microsoft Excel 2011 (version 14.6.0, 2010; Microsoft Corporation, USA), StatPlus: mac (version V6 2016; AnalystSoft Inc., USA) and MatLab (version R2019a; The MathWorks Inc., USA). Statistical analysis of the data was performed using Student's t-test for paired samples, the Chi-square test and Fisher's exact test. A value of p<.05 was considered statistically significant.
ResultsOne hundred and twenty six of the 141 consecutive patients were included. Demographic characteristics of the patients and the distribution according to the affected finger are shown in Table 2.
Demographic data and digital distribution.
| Age (years), mean (range) | 61 (42–82) |
| Sex, n | |
| Woman | 86 |
| Man | 40 |
| Diabetes mellitus, n (%) | |
| Yes | 14 (11) |
| No | 112 (89) |
| Previous carpal tunnel surgery, n (%) | |
| Yes | 18 (14) |
| No | 108 (86) |
| Time since onset of symptoms (months), mean (range) | 7 (1–24) |
| Affected finger, n (%) | |
| Thumb | 38 (30) |
| Index | 12 (10) |
| Middle finger | 48 (38) |
| Ring finger | 24 (19) |
| Little finger | 4 (3) |
| Grade according to Green, n | |
| I | 6 |
| II | 28 |
| III | 86 |
| IV | 6 |
The mean VAS score for pain during injection was 3.8 points (range 0–10; standard deviation [SD]: 2.3; 95% confidence interval [95% CI95%]: 3.4–4.2) and was considered as no pain (VAS for pain=0) in 4 patients; mild pain (VAS for pain=1–4) in 78, moderate pain (VAS for pain=5–7) in 36 patients and severe pain (VAS for pain=8–10) in 8 cases. These categories were arbitrarily created to stratify patients.
We found no statistically significant differences in the VAS score for pain according to the affected finger.
The mean DASH questionnaire score at diagnosis was 48 points (range 0–91; SD: 25; 95% CI: 44–52). The mean DASH questionnaire score at the end of follow-up was 8.6 points (range 0–50; SD: 16; 95% CI: 5.8–11) (p<.05).
Twelve patients were lost to follow-up. In the 114 patients available at the end of follow-up, the overall success rate was 78/114 (68%).
The success rate after one injection was 62/114 (54%). Twenty-eight patients refused a second injection and were referred to surgery. The success rate of the second injection was 16/24 (66%).
Success rates according to affected finger and according to Green's classification are shown in Table 3.
Success rate according to the affected finger and Green's classification.
| Affected finger | n | Resolved | Unresolved | % |
|---|---|---|---|---|
| Thumb | 34 | 18 | 16 | 53 |
| Index | 8 | 2 | 6 | 25 |
| Middle | 44 | 37 | 7 | 81 |
| Ring | 24 | 20 | 4 | 83 |
| Little | 4 | 1 | 3 | 33 |
| Green's grade | n | Resolved | Unresolved | % |
|---|---|---|---|---|
| I | 6 | 6 | 0 | 100 |
| II | 24 | 18 | 6 | 75 |
| III | 78 | 49 | 29 | 63 |
| IV | 6 | 5 | 1 | 83 |
| Total | 114 | 78 | 36 | 68 |
There were 4 recurrences between the 3-month and 12-month review which were considered and analysed as treatment failures.
No neurological or other complications were observed.
We found no statistically significant difference in success rate between the diabetic and non-diabetic population (10/12 and 68/102, respectively; p=.33).
There was a statistically significant difference in the success rate between patients who had previously undergone surgery for carpal tunnel syndrome and those who had not (8/18 and 70/96, respectively; p<.05).
DiscussionThe results obtained in this cohort study support the use of steroid injection deposited outside the tendon sheath and performed using the dorsal commissural technique in the treatment of trigger fingers as an effective, safe and potentially less painful technique.
Corticosteroid injection is a useful and safe treatment in stenosing tenosynovitis of the flexor tendons1,3,7 but pain perceived during injection is always present. Among the different injection techniques described, using the medioaxial technique, a mean pain score of 4 has been published; using the palmar over midline technique, 5.4 (ranging from 3.3 to 6.6); using the distal palmar technique, 6 (only in 12 fingers), and finally, using the dorsal intra-axial technique, the mean VAS score was 6.8 (only in 5 fingers) (Table 4).9–13 In our series, using a dorsal commissural extra-valvular technique, the mean VAS score was 3.8.
Published pain score for the different techniques described.
| Author (year) | Technique | Cases | Mean score for VAS pain (0–10) |
|---|---|---|---|
| Jianmongkol et al. (2007)9 | Mid-axial | 53 | 4.0 |
| Palmar mid-line | 48 | 4.8 | |
| Park et al. (2014)12 | Palmar mid-line | 30 | 5.6 |
| Palmar mid-line+vibration | 30 | 5.6 | |
| Palmar mid-line +false vibration | 30 | 6.3 | |
| Earp et al. (2017)11,aRosenbaum et al. (2018)10 | Palmar mid-line | 29 | 4.6 |
| Palmar distal | 12 | 6.0 | |
| Palmar mid-line | 22 | 6.6 | |
| Dorsal intra-sheath | 5 | 6.8 | |
| Patel et al. (2019)13,a | Palmar mid-line | 15 | 4.9 |
| Our series | Dorsal out-of-sheath web space | 126 | 3.8 |
In our study population, resolution of symptoms was obtained after a single injection in 54% of cases, a result similar to previously published data for other techniques, which range from 44% to 66%. The overall success rate at the end of follow-up was 68%, also similar to previously published results using the palmar midline technique.2–7,25
In our cohort, we found no differences in the success rate between the diabetic and non-diabetic population, in contrast to what has been previously published in other studies.3,26,27 We considered and analysed as diabetic those patients who reported being diabetic, but the rest of the patients did not undergo blood glucose testing. This may have underestimated the number of diabetic patients in the sample and should therefore be considered a limitation of the study.
In our study population, patients who had not previously undergone surgery for carpal tunnel syndrome responded better to injection. In vitro and in vivo studies have shown that the flexor tendons are displaced palmarly after opening of the transverse carpal ligament and this modifies the angle of entry of the flexor tendons into the A1 pulley, leading to increased friction.28,29 Our results could be explained by the fact that this increased friction between the flexor tendons and the pulley entry would be more difficult to counteract by the anti-inflammatory effect of corticosteroids.
We observed a significant decrease in the score on the DASH questionnaire, although the final score was not zero, as would be expected after resolution of the condition. This result should be interpreted with caution, as other factors may influence the score on self-perceived questionnaires and disability questionnaires. This observation is in line with other studies showing that the DASH questionnaire score is largely determined by psychosocial factors that may not change even if the objective disease state under study does.30
The strengths of our work are: the functional status of the patients was assessed prospectively before and after treatment. The rate of follow-up loss was under 10%.
This study has limitations. Assuming that the needle tip was located outside the tendon sheath due to the absence of movement of the needle during flexion-extension of the finger could lead to an error, as strictly speaking, this method would only confirm that the needle tip was not inserted into the flexor tendon. The use of ultrasound could have been helpful in this respect. This is a prospective, non-comparative cohort and therefore lacks a control group. The sample size was small and this may have influenced the results of the subgroup analysis.
According to the results of this cohort, steroid injection deposited outside the tendon sheath and performed using the dorsal commissural technique in the treatment of trigger fingers is effective and safe. This technique appears to be less painful and at least as effective as previously published results using the palmar midline technique, although this aspect needs to be evaluated in a comparative study designed for this purpose.
Level of evidenceLevel of evidence ii.
Authors’ contributionIJ and JM conceived the study, conducted the literature search and wrote the first draft of the manuscript. IJ, GG and AMG were involved in protocol development and obtaining ethical approval. IJ, AMG and JM participated in patient selection. IJ and GG were involved in data analysis. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
FinancingThis research was partially funded by the Foundation of the Colegio Oficial de Médicos de Las Palmas (Grant: XXIV, 2019 I+E Grand Edition).
Conflict of interestsThe authors have no possible conflict of interests to declare with respect to the research, authorship and/or publication of this paper.
Our thanks to A. Samuel A. Jiménez, BEng., MSc, for their help with the statistical analysis of this investigation.
The preliminary results of this study were presented orally at the annual congress of the French Hand Surgery Society in 2019, the abstract of which was published in their journal (Hand Surg Rehabil. 2019;38:419. doi:10.1016/j.hansur.2019.10.079).