Rotator cuff re-ruptures appear in 38–65% of cases. In order to reduce this rate, many studies have been performed using different types of biomaterial for purely mechanical aims (re-inforce the suture) and/or biological agents (growth factor transporterts). The aim of this study is to review 22 cases treated with xenografts and analyse various current alternatives.
Materials and methodsA descriptive and retrospective study was conducted using the variables of age, sex, laterality, time of surgery, involvement in MRI, number of anchors, and final mobility results on the Constant and the University of California Los Angeles (UCLA) validated scales.
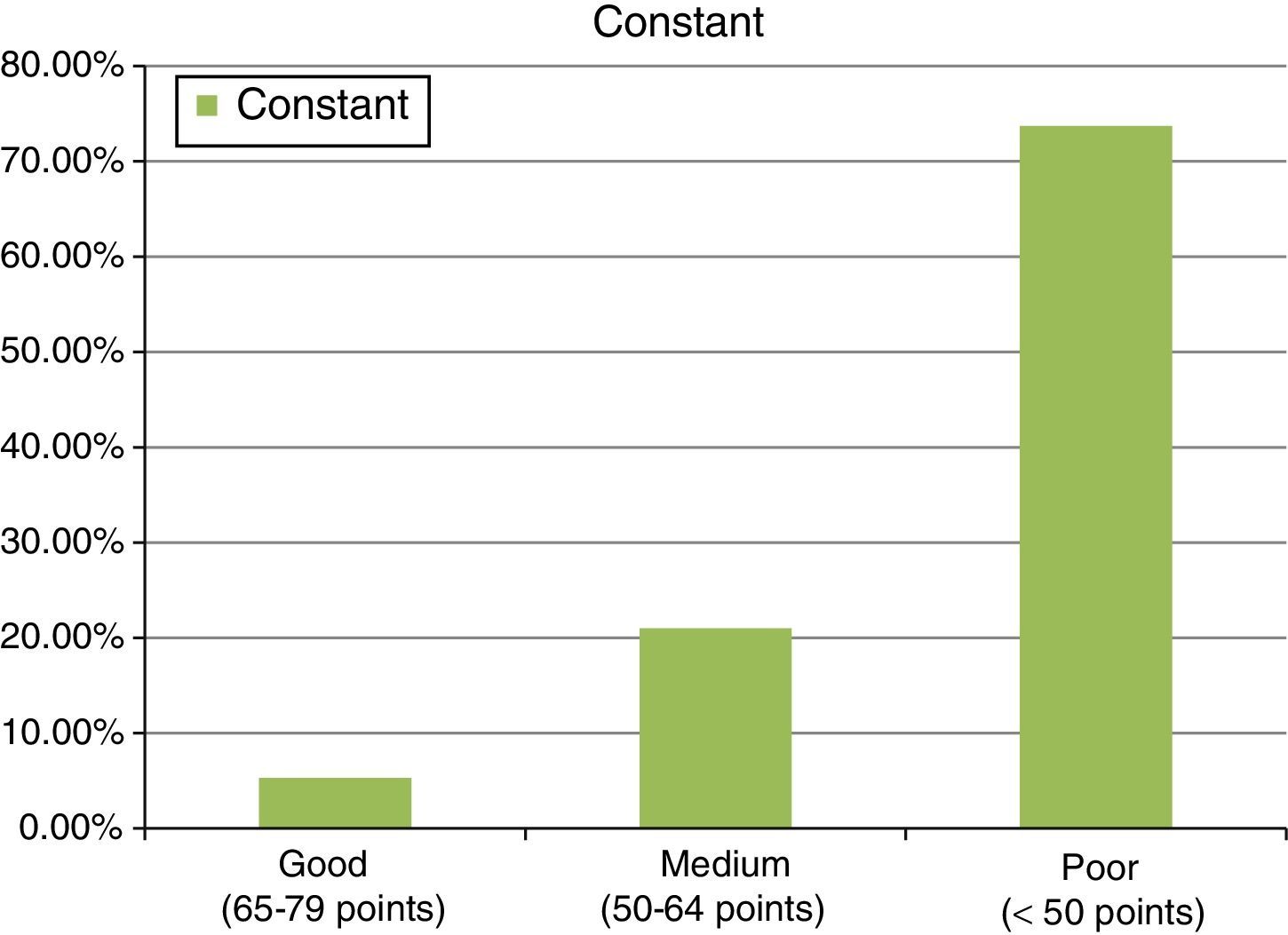
ResultsThe study included 22 patients, with a mean age of 51.7±4.6 years. A mean of 2 anchors were used and 13 patients were treated with a porcine intestinal submucosa implant, 6 with equine pericardium, and 3 with porcine dermis. Final results were: 37.6±13 points for the Constant test, and 16.9±3.9 points on the UCLA scale. The follow up was 36±10.2 months.
ConclusionsIn our experience, xenografts could provide good functional results and they would be a surgical alternative to tendon transfers in cases of massive tears. Further studies should be conducted with other biomaterials.
La rerrotura del manguito rotador aparece en un 38-65% de los casos. Con el fin de disminuir esta tasa se han realizado múltiples estudios que emplean diferentes tipos de biomateriales con fines puramente mecánicos (reforzar la sutura) o biológicos (transportadores de factores de crecimiento). Nuestro objetivo es revisar 22 casos tratados mediante xenoinjertos, así como analizar las diversas alternativas actuales.
Material y métodoEstudio observacional, descriptivo y retrospectivo donde consideramos edad, sexo, lateralidad, tiempos de intervención, afectación en RNM, número de anclajes empleados y resultados de movilidad final en las escalas de Constant y de la Universidad de California Los Ángeles (UCLA).
ResultadosSe observó a 22 pacientes, con una edad media de 51,7±4,6 años, en los que utilizamos una media de 2 anclajes. De ellos, 13 pacientes fueron tratados con parches de submucosa intestinal porcina, 6 con pericardio equino y 3 con dermis porcina. Los resultados finales fueron de 37,6±13 puntos para el test de Constant frente a los 16,9±3,9 puntos para el test UCLA. Seguimiento de 36±10,2 meses.
ConclusionesEn nuestra experiencia, los xenoinjertos proporcionan unos resultados funcionales aceptables para manguitos cuya sutura haya resultado ineficaz y son una alternativa a las transferencias tendinosas en casos de roturas masivas. Se precisan más estudios con otros tipos de biomateriales.
One of the most common complications of rotator cuff surgery is suture rupture, which, according to Schlegel1 occurs in between 38% and 65% of cases. The 300N generated by the supraspinatus in abduction between 0° and 30° might put the suture itself under tension and trigger its failure. According to Derwin,2 the hypovascular nature of the tendon, the degree of rupture, the degree of muscular atrophy, the tendon quality and the postsurgical rehabilitation protocol contribute to this failure. We consider that there is a multifactorial basis for suture failure; essentially a mechanical substrate (influenced by the degree of retraction of the rupture, and starting intensive rehabilitation very early) and a biological substrate (in which the degree of fat atrophy, or the poor vascularisation in the insertional area might contribute towards suture failure). Denard3 described a series of intrinsic causes (such as age, size of tear, number of tendons affected, fatty degeneration, etc.) and extrinsic causes (level of surgical skill, type of suture, anchors or postsurgical rehabilitation protocol), which might influence re-rupture after a previous suture attempt. The patient's type of occupation4 might also affect these injuries.
Currently, biological meshes are a mechanical alternative to relieve suture tension and provide biological support for regeneration of the tendon. In this sense, porcine intestinal submucosa, with 90% collagen, glycosaminoglycans, fibronectin and growth factors, such as the fibroblast (FGF 2), beta transforming (TGF-β) and vasculoendothelial (VEGF) growth factors would be an alternative worth considering. Others, such as equine pericardium or porcine dermis, were used in this study. Placement of this biological support forms “biological scaffolding” on which the damaged rotator cuff can repair itself, particularly useful in cases of degenerated tendon with little capacity for repair, and as interposition material in cases of massive retracted tears.5 These meshes incorporate growth factors, which have been reported as useful in the rotator cuff,6,7 although there are authors8–10 who doubt their benefit. One of the initial descriptions of the use of a biological support mesh in rotator cuff surgery is by Dejardin,11 who published an article in 2001 where he replaced the infraspinatus in dogs with porcine intestinal submucosa, and observed a similar strength to that of the original infraspinatus at 3 and 6 months.
Our objective was to analyse 22 cases of rotator cuff re-rupture treated with resuture and orthobiological reinforcement with porcine intestinal submucosa mesh, equine pericardium and porcine dermis.
Material and methodsA series of clinical cases comprising 22 patients operated in our centre in the past 4 years implanted with reinforcement meshes for rotator cuff repair. Follow-up was from 36±10.2 months.
This study covers patients with tendon re-rupture, after a prior attempt at rotator cuff repair.
The materials used were: in 13 cases, Restore (De Puy-Mitek®) from porcine intestinal submucosa; in 6 cases, OrthoAdapt (Pegasus Biologics®) from equine pericardium; in one case, Conexa (Tornier®) from porcine dermis and in 2 cases, XCM (Synthes®) from porcine dermis. These different meshes were chosen in an attempt to find the mesh that would offer the best technical results. Our choice ranged from porcine intestinal submucosa to porcine dermis, in order to establish which mesh achieved the best result. When we found that there was a case of allergic reaction after placement of a porcine intestinal submucosa mesh, we reconsidered the used of this material. This is how things have developed since 2008 when the first rotator cuff repair meshes were placed in our centre.
In order to establish the indication for reinforcement mesh repair, we used Strauss’ recommendations,12 for patients without glenohumeral arthritis, aged under 65, with a reparable rotator cuff, with Goutallier13 grades 0, 1 or 2, so that the attempt at anatomical repair would not be tension free and would be an alternative to partial repair alone, with margin convergence. We attempted to follow these recommendations in this series. However, we took the recommendations a little further and used them for cases of tendon re-rupture, and therefore we attempted margin convergence because the cuff was not totally reparable, we then we attempted to cover the defect with a mesh. Thirteen point six percent of the patients presented grade 1 fatty atrophy, 81.8% grade 2 atrophy and 4.6% grade 3 atrophy.
The methodology used was an observational, descriptive and retrospective study, where aspects were covered such as age, sex, laterality, surgery times, preoperative findings on MRI (rupture of supraspinatus, infraspinatus, condition of the long portion of the biceps and geodes, amongst other findings); the surgical technique used in the first intervention or initial repair attempt (subacromial decompression, coracoacromial ligament section, double row anchor); the number of anchors used, control MRI (with tendon re-rupture or failure to heal), type of reintervention to treat the tendon re-rupture or failure to heal (open surgery, with or without tenodesis of the long portion of the biceps), surgery time and final mobility outcomes in the scoring systems validated by Constant14 and the University of California, Los Angeles (UCLA).15 The follow-up time in months was also analysed. The patients were informed of the procedure and signed the informed consent form for surgery, giving consent for both the surgical intervention and for participating in our study. There were no ethical conflicts in undertaking this study.
The mean age was 51.7±4.6 years, with a minimum of 43 and maximum of 61. A total of 20 cases (90.9%) were male, and there were 2 female cases (9.1%). Sixty-eight point two percent of the cases were right shoulders and 31.8% left shoulders. Diagnosis by MRI of complete rupture of the supraspinatus was performed on 100% of the cases prior to surgery. Furthermore, the intraspinatus showed complete rupture in 95.5% and tendinitis in 4.5%. All the cases had bursitis. There was no case of advanced arthropathy of the rotator cuff. A degenerative sign in the rotator cuff was found in 63.6% of the cases, since these were cases with re-rupture, with dilaceration of tendon edges, although there were no osteophytes specific to full-blown omarthrosis.
The patients underwent 2 operations:
The first intervention involved an attempt at tendon repair. The mean surgical time was 93.5±19.6min. Some aspects were worthy of note: arthroscopy showed that the long portion of the biceps appeared frayed in 59.1% and normal in 40.9%. Arthroscopic (repair) was performed on 66.7%, and the remaining 33.3% underwent the mini open technique. Acromioplasty was performed on 94.4% of the cases. Four cases underwent tenodesis of the large portion of the biceps and 2 cases, tenotomy. The corocromial ligament was partially resected in 88.9%, with a view to improving the subacromial space.
MRI scanning after the first intervention revealed re-rupture of the sutured tendon or absence of healing in 94.4% of cases. This was not clearly demonstrated in one case, but ultrasound helped to confirm the suspected diagnosis. The time between the first and the second operation was around 4 months. In this time, the tendon re-rupture was assessed by MRI essentially, and the arthrosis by X-ray. MRI scanning was used to assess the fatty atrophy described by Goutalier13 and enabled the degree of retraction to be measured. With regard to arthrosis, Vistosky and Seebauer's16 X-ray classification was useful in chronic rotator cuff arthropathy, the different types being: type 1A, centred stable head, superior humeral head migration, coracoacromial arch acetabularisation; type 1B, centred medialised head, minimal superior head migration, medial glenoid erosion, coracoacromial arch acetabularisation; type 2A, decentred but stable head, superior humeral head migrations, superior medial erosion with significant coracoacromial arch acetabularisation and type 2B, decentred unstable head, humeral head escape, coaracoacromial arch and soft tissues deficient. All these patients started at stage 1A when they were reoperated and the new mesh was placed to prevent progression to a higher stage.
For the second operation, performed to treat tendon re-rupture or absence of healing, the surgical time was 95±20min. In this group, 95.2% of the cases were reoperated using open surgery with the Neer17 approach, Deberyre and Patte's18 tranascromial approach was used in one case. Arthroscopy and subsequent miniopen technique were performed on only one case, in order to place the mesh. In this reoperation, tenodesis of the long portion of the biceps was performed in 5 further cases.
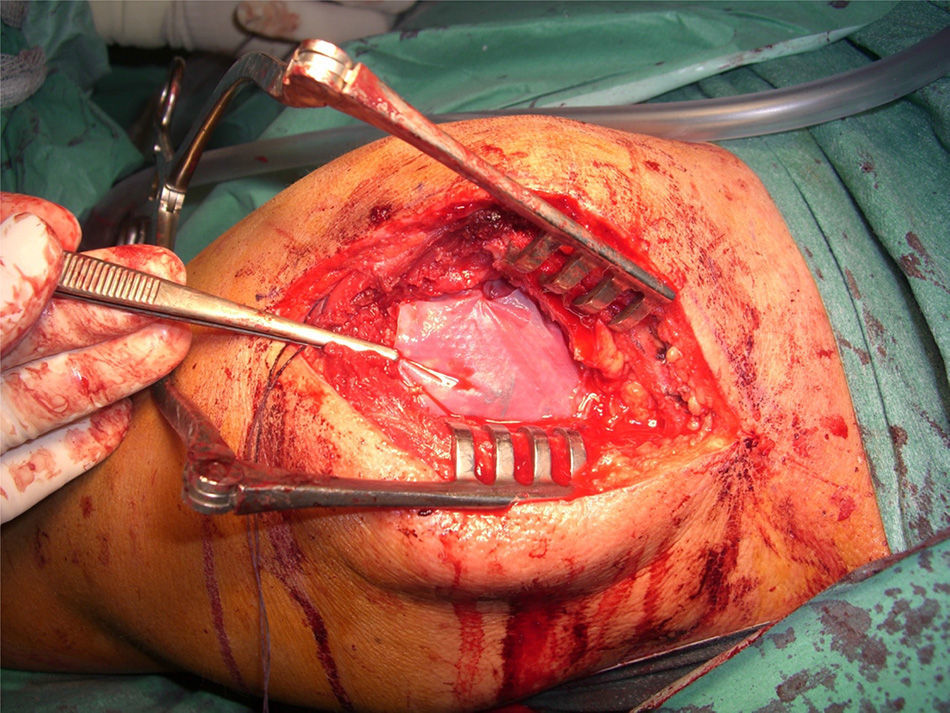
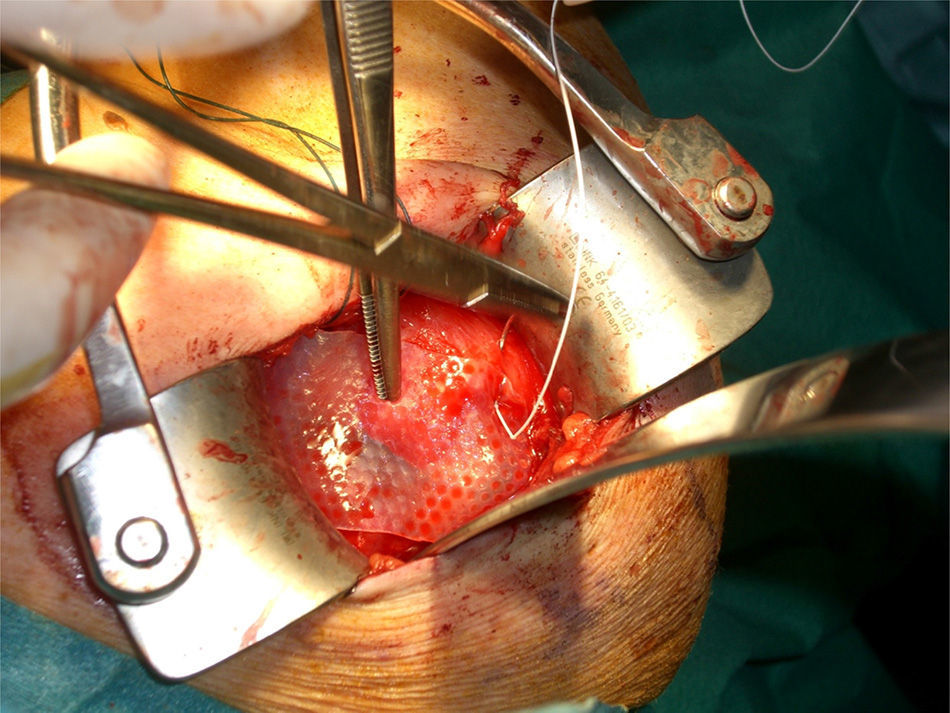
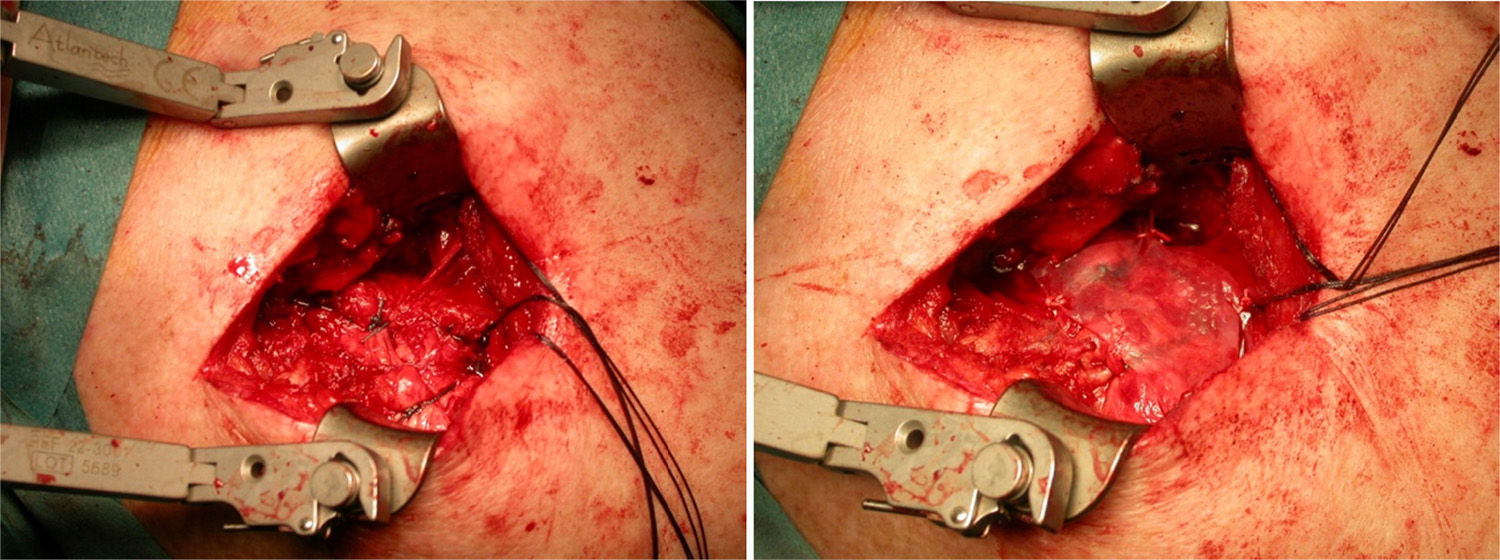
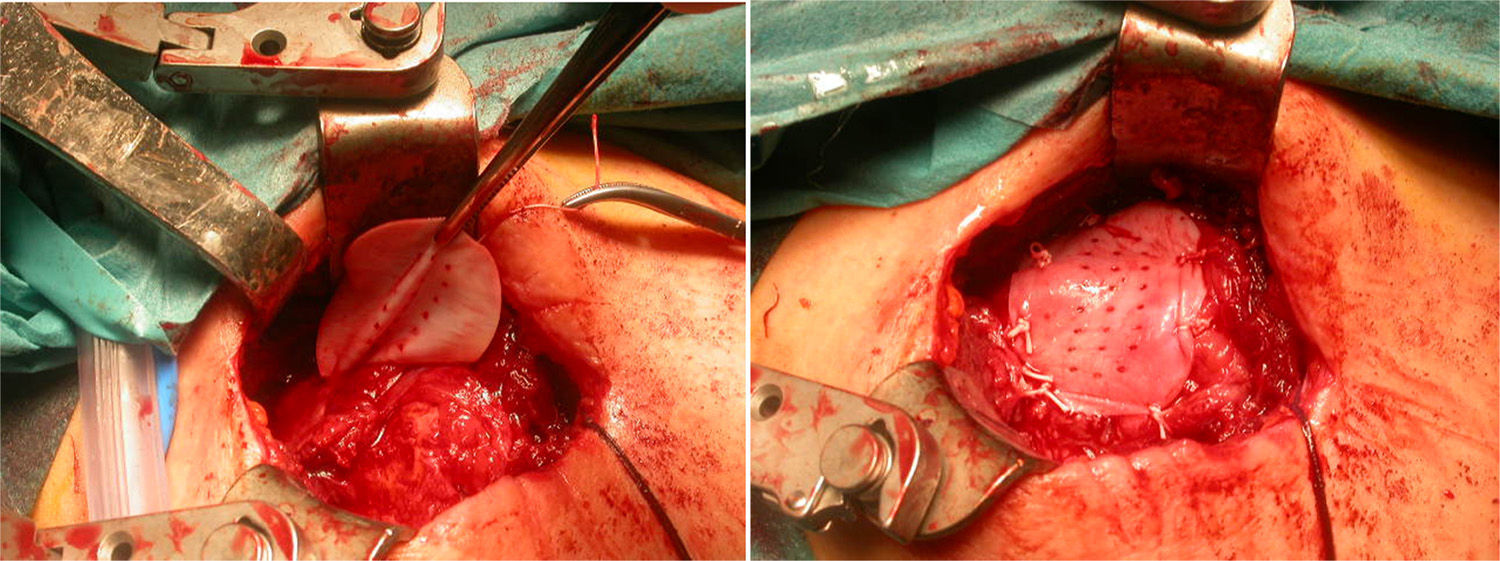
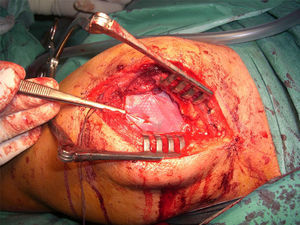
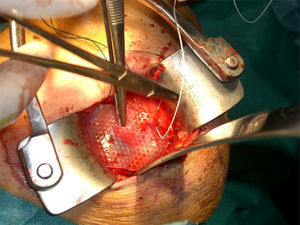
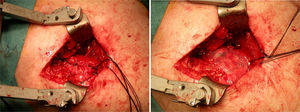
After re-rupture had been established during the surgical procedure, within the technique itself, tendon suture anchors were placed in a single or double row. The reinforcement mesh was placed on top of the sutured area, in order to improve the quality of the repair, rather than as a way of replacing the damaged tendon. The sequence is shown in Figs. 1–5.
An average of 2 anchors was used, with a minimum of 2 and a maximum of 4 anchors. Eighty-five point three percent of the series required 2 anchors, compared to the remaining 14.7%, who required 4 (Fig. 6).
The SPSS software package, version 19.0 was used for the statistical analysis. A descriptive study was performed, with analysis of means, standard deviations, medians and histograms for the numerical variables, as well as analysis by frequency tables for the categorical variables. Subsequently, an analysis of normality was undertaken for the numerical variables using Shapiro Wilk's test, with normal distribution from the Constant test. A non-parametric distribution was observed for the UCLA test. Therefore, the ANOVA test was used for the hypothesis contrast study and specifically, for the Constant test. The Kruskall–Wallis test was used for the UCLA test.
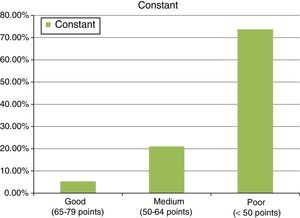
ResultsThe final clinical results, after the second operation, with mesh, in an attempt to repair the tendon re-rupture or absence of healing, were as follows: final abduction achieved was 88.9±33.2°, with a minimum of 90° and a maximum of 150°. Forward flexion achieved was a final mean of around 90±36.5°, with a minimum of 60° and a maximum of 150°. The rotations had limitations, considering 44.2±6° a final result of an external rotation and 8.1±5.3° of active internal rotation, measured in 90° of abduction. Finally, the results showed values of 37.6±13 points for the Constant test (minimum of 22 points and maximum of 68 points). In this test, around 8.6±2.8 points were given for quantifying pain, Courrent mobility limited to around 4.8±1.8 points and power limited to 4.6±3.3 points, which indicates that this surgical procedure contributed towards the patients’ pain recovery, but not towards the recovery of movement or power of the operated limb. A result of 16.9±3.9 points was also observed for the UCLA test (with a minimum of 10 points and maximum of 24) (see Fig. 6).
With regard to the contrast of hypothesis, for the Constant test a p=0.27 was obtained and for the UCLA test a p=0.7. Therefore there were no differences in terms of the clinical results obtained with regard to the type of mesh used. There were no complications, except one inflammatory reaction which resulted in removal of the implanted material.
DiscussionAnley19 summarised the different treatment options for cases of irreparable rotator cuff tear: debridement, partial rotator cuff repair, interval slide, tuberplasty, mesh or graft interposition, supracapsular ablation, superior capsule reconstruction, insertion of a biodegradable spacer, tendon transfer or reverse arthroplasty. Revision surgery of the rotator cuff resulted in improved pain and abduction according to DeOrio.20 In our series, improvement was essentially in pain, not movement. However the best outcomes were achieved in patients whose deltoid origin remained intact.21 Arthroscopic revision might be a good option for cases where the rotator cuff suture has failed. In fact, it also resulted in good outcomes, as described by Lo22 in their study of 14 patients, improving the result of the UCLA test (p<0.00001), anterior flexion to 153.6° (p=0.006) and external rotation to 44.3° (p=0.006). Piasecki23 also performed a study on 54 patients using the same procedure, with improvements in the ASES scale (p=0.0039), VAS (p=0.03) and anterior flexion (p=0.025). Keener24 also demonstrated that arthroscopic revision resulted in significant improvements in anterior flexion, external rotation, ASES scale and essentially, pain (p<0.05).
We have focussed on the revision surgery technique in which the attempt at repair is associated with the placement of a xenograft. Traditional approaches were used for the revision surgery and it was not rearthroscopy.
We used 4 different types of xenograft in our series to repair massive rotator cuff tears. Our series was small, only 22 cases, since we only applied it in cases of re-rupture; this is a limitation to the study. Despite the mean outcomes achieved, these are good considering that these are cases which had previously had poor outcomes, where only a rescue procedure would be suitable, as was the case. We believe that this procedure might be less aggressive than other options, such as tendon transfer.
On analysis of the references, for Mori,25 the application of meshes enabled repair even of cases of low-grade fatty degeneration, since the re-rupture rates with mesh were 8.3 compared to 41.7% when mesh was not used (p=0.015).
In our experience, as these had been previous arthroscopic surgical failures, we decided that we should used the traditional technique (open) for the reconstruction using mesh, as mentioned in the methodology. There are other references, in addition to the abovementioned, which attempt to enlarge on the arthroscopic procedure, such as the recommendations of Mihara,26 Proctor,27 Chalmers28 or Chillemi.29 In this regard, Gilot,30,31 for example, completed a study on 35 patients with arthroscopic cuff repair and mesh, compared to 15 patients without mesh. After follow-up of 26 months, they observed a re-rupture rate of 26% (for the control group) compared to 10% (for the group with mesh) (p=0.048). The ASES score also improved from 62.1 to 72.6 points in the control group compared to the 63.8–88.9 points for the group with mesh (p=0.02).
The use of porcine intestinal submucosa (13 cases in our experience) for example, as an alternative for rotator cuff suture reinforcement for example, has been covered by several authors, such as Dejardin11 who, in their experiment replacing the infraspinatus of dogs with this porcine submucosa, observed that the final strength of the cuffs treated using submucosa grafts was significantly less than that of the original intraspinatus (31±5 compared to 68±21N; p<0.001), although it was similar to it at 3 months (78±33 compared to 93±20N; p>0.005) and at 6 months (85±24 compared to 91±12N; p>0.05) after the intervention, even with integration of the graft in the greater tubercle and in the tendon implant remnants. Other authors such as Schlegel,1 noted that the stiffness of the tissue to the loads generated in rehabilitation was better than for the control group (215±44 vs. 154±63N/mm, respectively; p=0.03). For Badhe,32 it would be an recommended alternative, as they observed an improvement in the Constant test from 41 preoperative points to 62 postoperative points (p=0.003). These studies are supported by animal tests such as those undertaken by Dejardin11 in 2001 on dogs, although Zalavras33 performed experiments on rats and observed neovascularisation and fibroblast growth in the area of the graft, with resistance similar to the normal tendon at 16 weeks. These encouraging findings prompted us to use it in our 22 cases, although the Constant test result was only up to 37.6±13 points, essentially because of improved pain but with little functional improvement.
However, there are opposing literature references. There was an inflammatory response on the edges of the mesh in one case of our series that resulted in reoperating the patient to remove the mesh. In this regard, Walton34 described inflammatory reactions with the Restore® mesh which resulted in its removal, and poorer outcomes for the control group: less strength on raising the arm (28±4 vs. 61±11N; p<0.01), less internal rotation (63±6 vs. 99±11N; p<0.01), abduction (70±7 vs. 100±12N; p<0.05) and greater impingement on external rotation. In our series, internal rotation was limited, and around 18.1±5.3° of active arch abduction was achieved, measured in 90° abduction. It is likely as McDevitt35 acknowledges that these biological responses are due to the fact that the material is immunopositive for TGF-β and that it is biologically active. Cheung,36 like Malcarney,37 also collected inflammatory responses. The latter36 collected up to 16% inflammatory reactions 13 days after the operation, which resulted in the removal of the intestinal submucosa and surgical debridement. The most common adverse event for Lannotti38 was spontaneous drainage from the surgical wound, although with no microbiological growth in the cultures. In our case, the removed mesh did not show any microorganisms in cultures. Other authors such as Zheng39 report inflammatory responses with lymphocytic infiltrates. For Walton34 as well, the patients operated using Restore® had less lift-off strength and less strength on adduction and rotations (p<0.05). They had more pain, more subacromial compromise and less strength on internal rotation, with less sports participation (p<0.05). Even Nicholson40 describes resorption at 9 weeks after the operation, with the presence of ectopic calcifications in the tendon. Furthermore, the biomechanical stability of this mesh has been called into question, with 90.9% re-ruptures according to Sclamberg.41
We attempted to test other therapeutic options, such as pig dermis.
There are varying references regarding porcine dermis, which we used in 3 cases. Badhe,32 despite poor vascularisation and cases of reaction to a foreign body, managed to achieve an improved Constant test result from 41 points preoperatively to 62 at the end of 4.5 years’ follow-up (p=0.0003). Pain, abduction and range of movement also improved significantly after the operation (p<0.05). Chung42 used platelet rich plasma (PRP) and porcine dermis mesh in 80 rabbits. Applying PRP to the mesh was beneficial. Failure load was greater in the group that underwent repair alone compared to the group repaired using mesh (p=0.018); there were differences between the repaired group and the mesh and PRP group (p=0.002) and between the group repaired with mesh and the group repaired with mesh and PRP (p=0.029). In a study in 5 patients, with porcine dermis patches Cho43 described an improvement in VAS score (from 6.8 to 0.8 points; p=0.041), UCLA score (from 15.4 to 31.2 points; p=0.042) and ASES score (from 39.4 to 86.4 points; p=0.043). In our series, the improvements achieved in this group as well, were down to the diminished pain above all, as the defect was “upholstered” in the same way. The functional outcomes in our series were poorer than others described in the references, with an UCLA score, for example, of 16.9±3.9 points.
Fascia is another xenograft. In a study on dogs, Baker44 observed that initially, the cuffs treated with fascia achieved an ultimate resistance of 296±130N (which was 46%±25% more strength than the cuffs that were not treated in this way). However, at 12 weeks, a strength of 192±213N was achieved (15%±16% than the unrepaired cuffs), with no difference in terms of frame stiffness between either group. Ide45 also used dermal patches in rats. In their study they observed greater ultimate force of the tissue than when they were not used (p<0.01). In this study, equine pericardium patches were also used in 6 cases. In our review of the literature we found no references to the cuff, but we did find reference to other tendons, such as the Achilles, with inflammatory reactions, such as that reported by DeCarbo.46
However, we tried to counteract the undesirable effects, after the porcine intestinal submucosa implants especially and occasionally after the porcine dermis implants, by using other synthetic materials.47 Audenaert48 used Mersilene, in a review of 41 patients, and managed to improve the Constant test from 25.7 points preoperatively to 72.1 postoperatively, with p<0.001. Kimura49 performed a study on 31 dogs, with tetrafluoroethylene (Teflon). They observed that the tensile strength generated by the Teflon was 60.84; 172.88 and 306.51N after the surgery, at 6 and 12 weeks, respectively. The stiffness of the felt-bone interface was 9.61; 64.67 and 135.09kN/m after surgery, at 6 and at 12 weeks postoperatively, respectively. Petrie50 describes repair using a LARS patch, with improvements compared to the preoperative situation (p<0.0001). Ciampi,51 in a study on 152 patients, found that the rate of re-rupture was 41% for a group treated with collagen patches and 17% for another group treated with polypropylene patches, at 36 months. Vitali52 also studies reinforcement using polypropylene mesh, with a UCLA test result in the control group (60 patients) of 11.28±1.43 points compared to the result of the group with mesh of 20.85±1.27 points, at 3 months, differences that were maintained at 36 months: 14.7±1.9 compared to 24.6±3.3 points. The re-rupture rates were 40% for the control group compared to 15% for the group with mesh.
Encalada-Díaz53 describes the use of a polycarbonate-polyurethane mesh. This study with 10 achieved improved VAS, SST, ASES scores at 6 and at 12 months (p<0.05 and p<0.01), with a re-rupture rate of 10%. Proctor27 completed another study with poly-l-lactic acid mesh, in 18 patients, and achieved an improved ASES score, from 25 to 71 points, with cure rates and no re-rupture in 78% at 42 weeks after the intervention. Finally Lenart,54 also using poly-l-lactic acid, but in a series of 17 patients, obtained an improved ASES score from 32.8 to 74.2 points, with p=0.0001. We have no experience with these synthetic materials.
However, human dermis, approved by the FDA in the USA, deserves special mention. The data regarding human dermis are the most promising. Adams55 performed a study with human dermis to fill defects created in canine rotator cuffs, observing a histologic structure at 6 months (collagen, elastin and tenocyte-type cells) very similar to the control group, with a tensile strength with no great significant differences (p=0.31). Furthermore, Barber56 studied the strength of repairs of cadaveric supraspinatus with human dermis patches, and achieved strengths before the tear of 313.7 compared to 219N of the control group (p<0.05). However, replacements in human beings performed by Bond et al.,57 operating on 16 patients with massive tears of the rotator cuff, obtained significant differences in UCLA test scores pre and postoperatively (18.4 compared to 30.4 points; p=0.001) and Constant score (53.8 compared to 84.0 points; p=0.001). Nicholson40 recently demonstrated that the mean failure load of dermis grafts in ovine cuffs at 9 (weeks) after the repair was 182±63 compared to 137±16lb of using intestinal submucosa. At 24 (weeks) there was vascular and fibroblastic invasion into the dermal patch, compared to the porcine intestinal submucosa, which presented heterotopic ossification. For Derwin58 the GraftJacket® human dermis patch is the thickest of all the currently used orthobiological materials (1.58±0.15mm thick). For Barber,56 the strength of the repair was augmented with this human dermis mesh to 325±74 compared to 273±116N without the mesh. For Snyder,59 there was no inflammation, no calcification, and no inflammatory response at 3 months. In fact, the collagen was well aligned and there was vascular growth. In Wong's60 review of 13 patients treated with arthroscopic reconstruction using cadaveric human dermis patches, an improved UCLA test result was achieved from 18.4 to 27.5 points (p=0.0001). The Constant test improved from 53.8 to 87.3 points (p=0.0001). In this regard, specifically, there were improvements with regard to pain, forward flexion and external rotation. Bond,57 in a similar study on 16 patients treated arthroscopically with GraftJacket® patches, improved the UCLA test score from 18.4 to 30.4 points, with p=0.0001. The Constant test improved from 53.8 to 84 points, with p=0.001. The pain improved from 4.6 to 9.8 points, with p=0.0001, as did forward flexion from 106 to 142°, with p=0.0001. Elevation strength also improved from 2.5 to 4.2N, with p=0.0001, as did external rotation from 2.6 to 4.4N, with p=0.001.
Acevedo61 described the placement of these meshes, on top of the repaired tendon area (onlay technique), suture bridging, squeezing the cuff like a sandwich, or reconstructing the upper capsule. In our series, they were placed on top; we attempted to close the defect and avoid rubbing between the acromion and proximal humerus.
Finally, no great benefits are reported for biological patches with human donor materials. As early as 1988 Nasca48 described reactions to a foreign body and in 2006, Moore62 described the use of cadaveric Achilles or quadriceps with 12.1–26.1 points in the UCLA test, although in a series of 32 patients. It is worth noting that there were 2 cases of infection.
Advantages of this studyWe understand that this study offers a review of an alternative for rotator cuff repair, where the initial repair suture has failed, with re-rupture or failure to heal. It enables a review of the various materials that are currently available on the market.
Not all rotator cuff re-ruptures are irreparable, or massive. There are partial or small re-ruptures and favourable outcomes in terms of pain diminishment and moderate outcomes in terms of function have been demonstrated. Despite the outcomes of this series, none of which were excellent, the improved Constant test result was largely down to pain relief, and therefore we consider the use of grafts justifiable.
Limitations of this studyWe consider the heterogeneous nature of the study, in examining different materials, to be a limitation of the study. As we mention in the section on methodology, these different meshes were used in an attempt to find the graft that would offer us the best technical outcomes. This has been the development since the first meshes were implanted in rotator cuffs in our centre in 2008. We believe that the description of the different alternatives in this study could be valuable, despite their heterogeneity.
These meshes have been used in cases of re-rupture that are not completely reparable, where an attempt has been made at a new repair with convergence of margins, and subsequently to cover the defect with a mesh. We consider that using this procedure on patients with fatty atrophy (in this series, predominantly grade 2), perhaps contributed towards functional outcomes which were not as favourable as we would have hoped.
We believe that new studies with more case studies, with less heterogeneous material should be considered. We believe that comparative studies between reoperated patients, with and without mesh, could be undertaken to observe the outcomes in both groups.
ConclusionsWe consider that this study is of interest, given the current preoccupation with finding tissue substitutes for rotator cuff repair. In our experience, xenografts provide biological support for cuffs where the suture has been ineffective. We believe that more studies should be undertaken, using human dermis fundamentally for use in humans, since we consider this a powerful alternative to intestinal submucosa, for example, because of its weak immunogenic effect as endorsed by the references.
Level of evidenceLevel of evidence IV.
Ethical responsibilitiesProtection of people and animals subjectsThe authors declare that research was carried out complied with the Ethics Committee research regulations and of the World Medical Association and of the Helsinki Declaration.
Confidentiality of dataThe authors declare that no patients’ data appear in this article.
Right to privacy and informed consentThe authors declare that no patients’ data appear in this article.
FundingThe authors have received no funding of any type to undertake this study.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors have not received financial help of any kind in undertaking this study. Neither have we signed any agreement for which we will receive fees or honoraria from a commercial entity. Furthermore, no commercial entity has paid, or will pay, foundations, educational institutions or other not-for-profit organisations to which we are affiliated.
We would like to thank Sr Antonio Muñoz, Sr Rafael Lora, Sr José Villanueva and Sra. Lucía Buzón, members of the surgical staff at our hospital, for the intraoperative photography in this study.
Please cite this article as: Jiménez-Martín A, Santos-Yubero FJ, Najarro-Cid FJ, Navarro-Martínez S, Zurera-Carmona M, Pérez-Hidalgo S. Utilización de injertos en la reparación de rerroturas del manguito rotador. Rev Esp Cir Ortop Traumatol. 2016;60:286–295.