Managing chronic periprosthetic infections in patients who have undergone limb-salvage surgery following a malignant bone tumor with megaprosthesis often involves a two-stage revision surgery with the use of a cement-spacer. This paper show details the preparation of a self-made intramedullary metal-stabilized mega-cement spacer for patients needing a two-stage revision surgery due to infection after oncologic bone tumor resection and limb-salvage surgery with megaprosthesis and present two clinical cases treated with this technique. The report provides a practical surgical technique to create a cement hip mega-spacer using readily available tools in most orthopedic surgical settings.
El manejo de las infecciones periprotésicas crónicas en pacientes que han sido sometidos a cirugía de salvamento de extremidades mediante megaprótesis por un tumor maligno óseo a menudo implica una cirugía de revisión en 2 tiempos con el uso de un espaciador de cemento. Este artículo detalla la técnica quirúrgica para la creación de un megaespaciador de cemento con estabilización rígida intramedular y muestra 2 casos clínicos tratados con esta técnica de pacientes que necesitan una cirugía de revisión en 2 tiempos debido a una infección tras la resección del tumor óseo oncológico y cirugía de salvamento de extremidades con megaprótesis. Este texto proporciona una técnica quirúrgica sencilla y accesible para la creación de un megaespaciador de cadera de cemento utilizando herramientas disponibles en la mayoría de los quirófanos de nuestro entorno.
Advancements in the treatment of malignant bone tumors and progress in chemotherapy and radiation therapies have led to an increased demand for limb-salvage surgeries, which often involve the use of a megaprosthesis following surgical resection.1 Megaprosthesis surgical implants exhibit higher complication rates compared to standard hip or hip revision surgeries. Factors such as patient life expectancy, implant design, pathologic soft tissue resection, and accompanying treatments can contribute to infection rates averaging 15–37%.1 Addressing megaprosthesis infection typically involves a multidisciplinary approach, incorporating follow-ups with an infectious disease team, antibiotics, and two-stage revision surgeries with cement spacers.2 Preformed antibiotic spacers have demonstrated improved surgical outcomes in terms of surgery duration, complication rate, and infection eradication in total hip arthroplasty.3 However, such data has not been extensively studied in oncologic patients. Moreover, due to the unique characteristics and relatively infrequent use of megaprosthesis, specific preformed spacers might not always be available or in stock for surgeries that cannot always be delayed.
The objective of this paper is to detail a replicable and accessible technique to assemble a tailored cement-spacer for hip megaprosthesis. This technique can accommodate and adjust to various modular components and bone stock availability, assisting surgical teams with the complex challenge of hip megaprosthesis infections after bone tumor surgery.
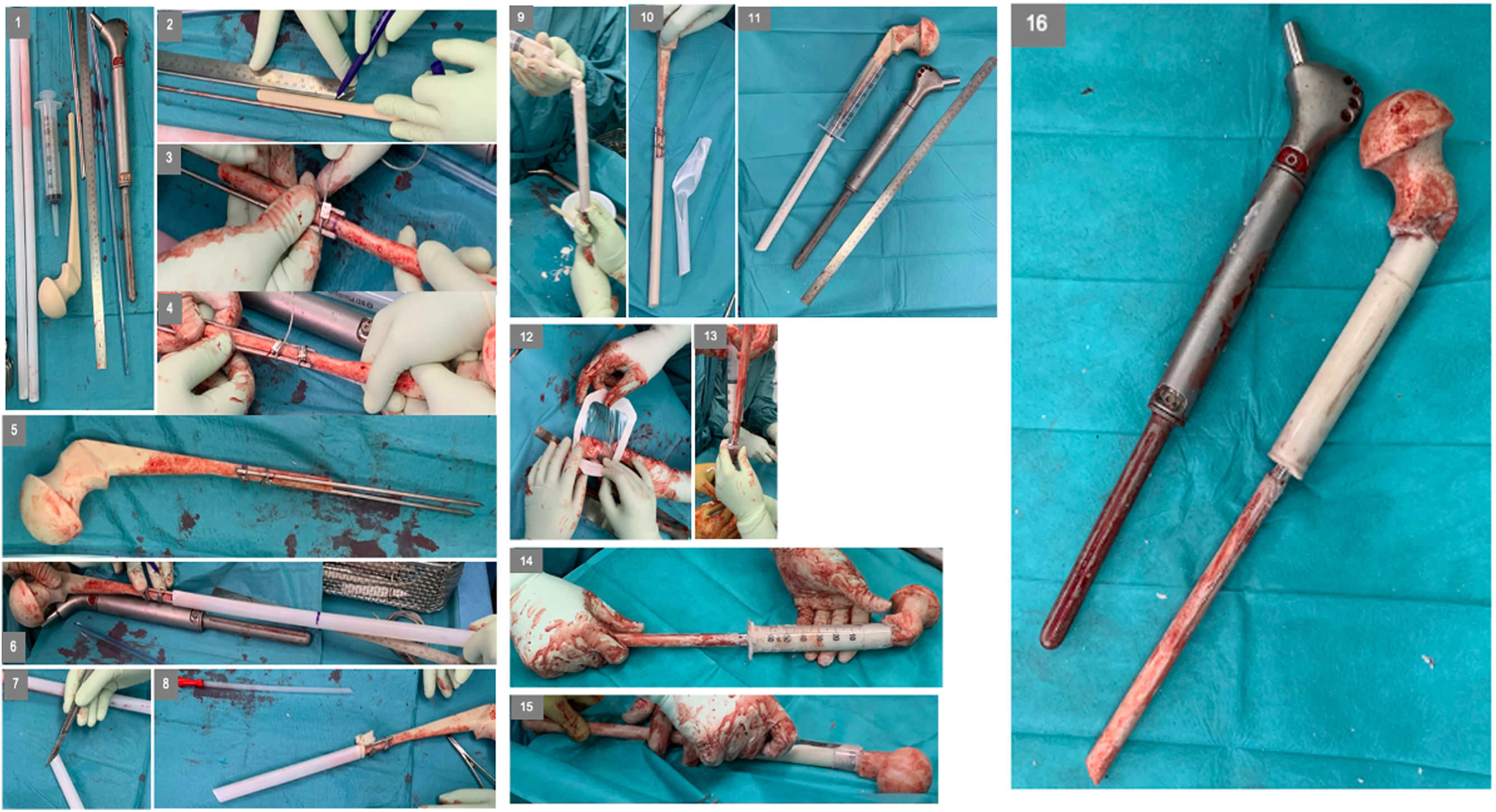
Surgical techniqueTo create the custom cement spacer for a hip megaprosthesis, the following items are needed: (1) explanted stem, (2) specific hip cement spacer, (3) Steinmann's pins, (4) metal banding x2, and (4) plastic tubes (syringe, others).
After surgical explant we will have the available length and width of the hip megaprosthesis stem in the different modular/interface levels.
First, we tried the optimal head measure for the different hip commercial cement spacers available. In our case we used the hip stem cement spacer Vancogenx Space Hip XL Flat System (Tecres SPA).
Then, we measured the distance from the stem tip to the first module-width and from the first module-width to the second module-width interfaces and marked these same distances in the hip cement spacer.
After that, we added two 6mm Steinmann's pins in each side of the cement spacer, with a 10cm superposition with the preformed cement spacer stem and blocked them to the cement stem with two proximal metallic bands.
Once, we used the sterile container tube of a thoracic catheter (Argyle – Thoracic Catheter – Covidien) which has same with as the original distal megaprosthesis stem. However other plastic tubular structures available in the surgery room, such as syringes can be used. This structure can vary depending on required width and available structures but must be wider than new width of the distal stem with added Steinmann. Steinmann can be <6mm in cases with thinner stems.
Afterwards that we introduce the tubular plastic structure until the first marked width-change marked interface from the original megaprosthesis. A transparent dressing or a glove can be used to fix it proximally and avoid leaks. Distal tube is cut according to surgeon preferences and distal bone stock available.
We proceed with the antibiotic-based cementation from the distal tube opening. When cementation process is finished, we remove external plastic cover with a blade.
Then, we used a 60ml-syringe with similar width to the original proximal module of the megaprosthesis, also using a dressing or a glove to fix it proximally and avoid leaks.
Finally, as that diaphysis are usually wider than healthy ones, we proceed again with same cementation process. If needed, a distal cement crown can be hand added in situ to give extra support.
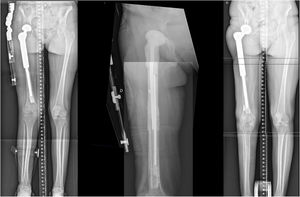
This method facilitates the undertaking of a first-stage megaprosthesis revision surgery in the context of infections specific to hip megaprosthesis (Fig. 1).
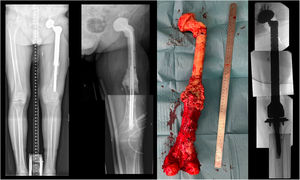
Customized hip cement mega-spacer for a hip megaprosthesis two-stage revision surgery. (1) Material, (2) measure first interphase, (3) Steinman cerclage no. 1, (4) Steinman cerclage no. 2, (5) hip spacer – Steinman construct, (6) measure check, (7) cut according to measurement, (8) glove cement stop, (9) first interphase cementation, (10) plastic tub extraction, (11) preparation of second interphase cementation, (12) dressing cement stop, (13) second interphase cementation, (14) cemented second interphase, (15) syringe tub cut, and (16) final result comparted to explanted megaprosthesis.
Two cases of first-stage septic hip megaprosthesis revision surgery are documented. Both patients suffered periprosthetic infections with a tumor prosthesis initially implanted as a reconstructive measure after a proximal femur resection for limb-sparing surgery of bone sarcoma.
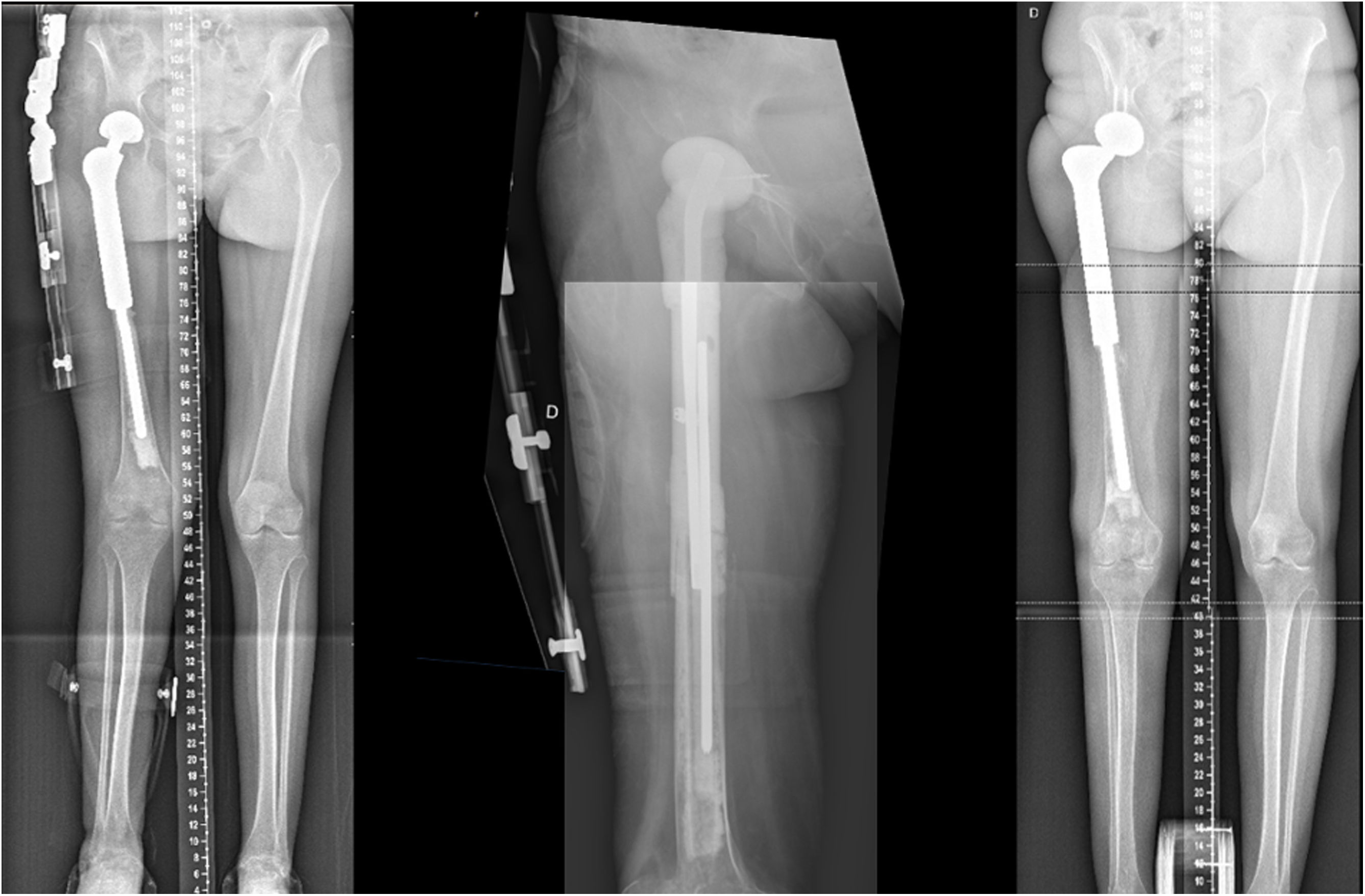
Case report 1Man 62yo, with hypertension and smoking history, choroid melanoma 10 years ago, was diagnosed 6 years ago of a proximal femur II/III chondrosarcoma requiring surgical treatment with marginal resection and a double mobility hip megaprosthesis. Postoperative radiotherapy was done for 6 months with no evidence of periprosthetic infection or tumor recurrence. In the following years, he was diagnosed of loosening requiring revision surgery with negative intraoperative cultures. After 2 months, he presented in the emergency room with tenderness, increased CRP, and he was diagnosed of subacute surgical site infection with negative cultures for Staphylococcus epidermidis requiring two-stage surgical revision. A customized hip antibiotic-loaded cement mega-spacer was used for the first stage of revision surgery as presented. Three months after that, in the 2nd stage revision surgery a revision mega prosthesis was implanted. Follow up, with long term suppressive antibiotic treatment, evolved with no other incidences (Fig. 2).
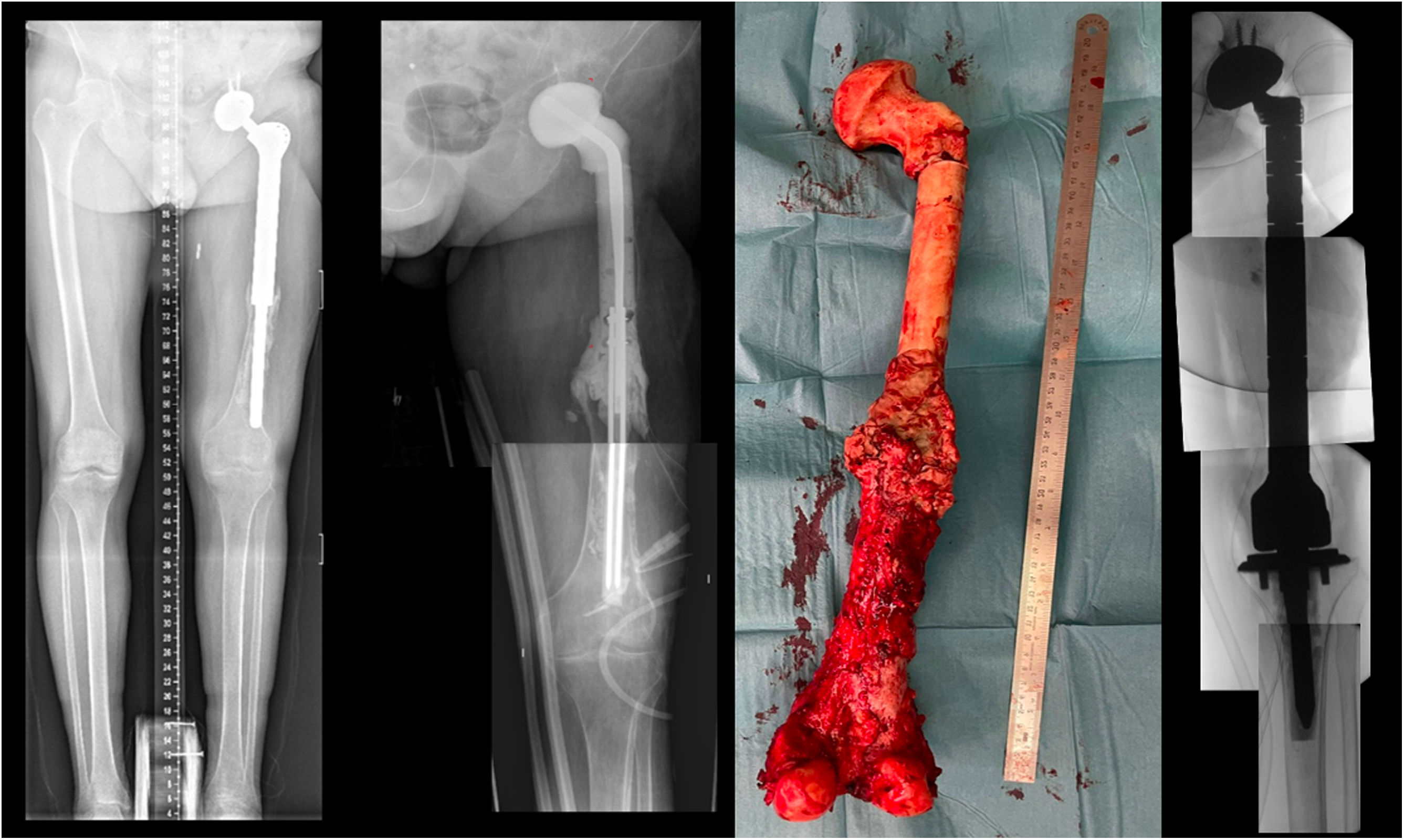
Case report 2Women 21yo, with no previous pathology was diagnosed of a femoral osteogenic osteoblastic sarcoma. Patient started neoadjuvant chemotherapy and required resection tumor surgery with hip mega prosthesis reconstruction. In the immediate postoperative patient required embolization of medial gluteal artery. Postoperatively, surgical wound was associated to drainage, evolving to a fistula with positive cultures to S. epidermidis and starting antibiotherapy. During the following months she presented to emergency room twice with hip megaprosthesis dislocations and was proposed for a two-stage revision surgery. A customized hip antibiotic-loaded cement mega-spacer was used for the first stage of revision surgery as presented (Fig. 3). Three months after that, in the 2nd stage revision surgery a revision mega prosthesis was implanted. Follow up, with long term suppressive antibiotic treatment, evolved with no other incidences.
DiscussionCement spacers play a pivotal role in two-stage revision surgical techniques, with various researchers and literature exploring numerous means to adapt their use in hip and knee periprosthetic infections. Preformed or modeled cement spacers are reported to reduce complications in comparison to non-molded handmade spacers4 and some factors such as spacer design, acetabular and femoral bone loss, offset restoration have been significantly associated with perioperative spacer complications in two-stage revision surgeries.5 However, modeled cement spacers are not always accessible or available.
Previous literature suggested multiple options of customized handmade techniques for hip cement-spacers with good outcomes including: intraoperative articular knee cement spacer for knee revision surgeries,6 acetabular defect cement spacer cover for hip revision surgeries with acetabular defects,7 hip cement spacers made with dental silicone templates for total hip revision surgery8 and reverse hip prefabricated spacer for knee revision surgery with massive bone defects.9 Some authors also proposed alternative techniques including metallic implants combined with cement spacers with good results including: hip arthroplasty revision surgery with Steinmann pins,10 hip arthroplasty revision surgery with reconstruction plates mixed with acetabular cement-ball,11 knee revision surgery with intramedullary stabilized antibiotic spacers in patients with large segmental defects12 and total femur prosthesis cement spacers.13
Despite the promising potential of antibiotic-loaded cement in cement spacers as part of the treatment regimen for periprosthetic joint infections,14 their efficacy against infection remains ambiguous.15 One major complication of two-stage revision surgery due to infections, is THA dislocation, reported in up to 8.9% of cases at 1 year follow up.16 The rate is potentially higher for megaprosthesis, considering the increased soft tissue damage due to pathology and surgery.
Nevertheless, there's a paucity of literature specifically addressing oncologic limb-salvage surgery with megaprosthesis. Most decision-making inputs come from literature on prosthetic revision surgery, experiential insights, or case series reports.
ConclusionThis technique empowers surgical resources of an orthopedic team to conduct a two-stage revision surgery. The straightforward, cost-effective, and accessible surgical technique allows for the creation of an intramedullary metal-stabilized mega-cement spacer for patients who have undergone limb-salvage megaprosthesis surgery following an oncologic bone tumor resection.
Level of evidenceLevel of evidence IV.
FundingThis research has not received specific aid from public sector agencies, commercial sectors or non-profit entities.
Conflicts of interestNone declared.
Orthopedic Oncology Unit, Hospital de la Santa Creu i Sant Pau, Universitat Autònoma de Barcelona, Barcelona, Spain.