The piriformis syndrome is one of the etiologies of pelvic pain due to the sciatic nerve's entrapment by the piriformis muscle. Nowadays this syndrome might be difficult to be diagnosed. The aim of this study is to know the prevalence of anatomic variations in our population that may contribute to the appearance of piriformis syndrome. Furthermore, anthropometric measurements of the piriformis muscle and the sciatic nerve procedures are studied for a possible application in the gluteal region.
Materials and methodsThe study was carried out in 59 pelvis of 32 cryopreserved bodies. The anatomical variations of piriformis and sciatic nerve founded were described following the Beaton and Anson's classification. Anthropometric measurements of both structures with reference to the greater trochanter of the femur were performed.
ResultsThe sciatic nerve and the piriformis had an anatomical variation in a 28.13%. The most frequent variation found was tipus II (21.64%) and tipus III (6.49%).Insertion most frequently observed was an independent piriformis tendon inserted into the trochanteric fossa with 53.85%.
ConclusionsThe anatomic variations' incidence in the population studied indicates that those have to be evaluated as a differential diagnosis of gluteal region pain due to the symptoms and signs resemblance with the vertebral disc pathology involving nerve root injury. In addition, anatomical knowledge of this region can be useful for the interpretation of imaging techniques, especially when ultrasound-guided injections are performed.
El síndrome piriforme constituye una de las causas de dolor pélvico debido al atrapamiento del nervio ciático por el músculo piriforme. En la actualidad es un síndrome de difícil diagnóstico. El objetivo de este estudio es conocer la prevalencia de variaciones anatómicas en nuestra población que puedan contribuir a la aparición del síndrome piriforme. También se estudian medidas antropométricas del músculo piriforme y el nervio ciático para su posible aplicación en procedimientos de la región glútea.
Material y métodoEl estudio se realizó en 59 pelvis de 32 cadáveres criopreservados. Las variaciones anatómicas del músculo piriforme y nervio ciático encontradas se describieron según la clasificación de Beaton y Anson. Se realizaron mediciones antropométricas de ambas estructuras con referencia al trocánter mayor del fémur.
ResultadosEl nervio ciático y el músculo piriforme presentaban una variación anatómica en un 28,13%. La variación mas frecuente fue la de tipo II (presente en 21.64%) y la de tipo III (6.49%). La inserción observada con mayor frecuencia fue un tendón independiente del músculo piriforme insertado en la fosa trocantérea con un 53,85%.
ConclusiónLa incidencia de variaciones anatómicas en la población estudiada indica que son importantes como diagnóstico diferencial de posibles dolores en la región glútea ya que los síntomas y signos del síndrome piriforme se asemejan a la patología discal vertebral con afectación radicular. Además el conocimiento anatómico de esta región puede ser útil en la interpretación con técnicas de imagen, especialmente cuando se realizan inyecciones guiadas por ecografía.
The most common causes of sciatic nerve entrapment are traumas (hip joint dislocations and proximal femur fractures), but lesions from intramuscular injections or even those caused by surgery in this area have been reported.1 However, the aetiology of sciatic nerve compression, which according to some researchers is the most common cause of deep gluteal syndrome,2 corresponds to piriformis syndrome or to the recently suggested anomaly of the piriformis and sciatic nerves.3 This occurs as a consequence of an anatomical course or variation as the sciatic nerve descends through or inferiorly to the piriformis muscle.4,5 Normally initiation of piriformis syndrome are primary causes, for example a piomiositis, myositis ossificans or an irritation of the sacroiliac joint originating from piriformis contracture and the consequent pressure on the sciatic nerve. Notwithstanding, the most acknowledged cause as the origin of the síndrome is the anatomical variant of the piriformis muscle.6,7
The piriformis muscle originates from the anterior part of the sacrum and laterally from the anterior sacral holes but without covering them. It enters the pelvis laterally through the greater sciatic foramen. On exiting it divides this orifice into 2 orifices: superiorally the suprapiriform foramen and inferiorally the infrapiriform foramen.8,9 In the gluteal region the piriformis muscle passes behind the hip joint to enter into the ridge of the upper edge of the greater trochanter3,8–10 or the trochanteric fossa of the greater trochanter of the femur.3,10 This muscle is innervated by the nerve of the piriformis muscle which originates from the anterior division of the second sacral spinal nerve (S2). Its function is to rotate the femur externally when it is extended and abduct it when it is flexed. The main relationships of this muscle are superiorally with the vascular or nervous structures of the upper buttock and inferiorally with the vascular or nervous structures of the lower buttock, the sciatic nerves, the posterior femoral cutaneous nerve, the pudendal nerve and the internal pudendal vessels.4,9
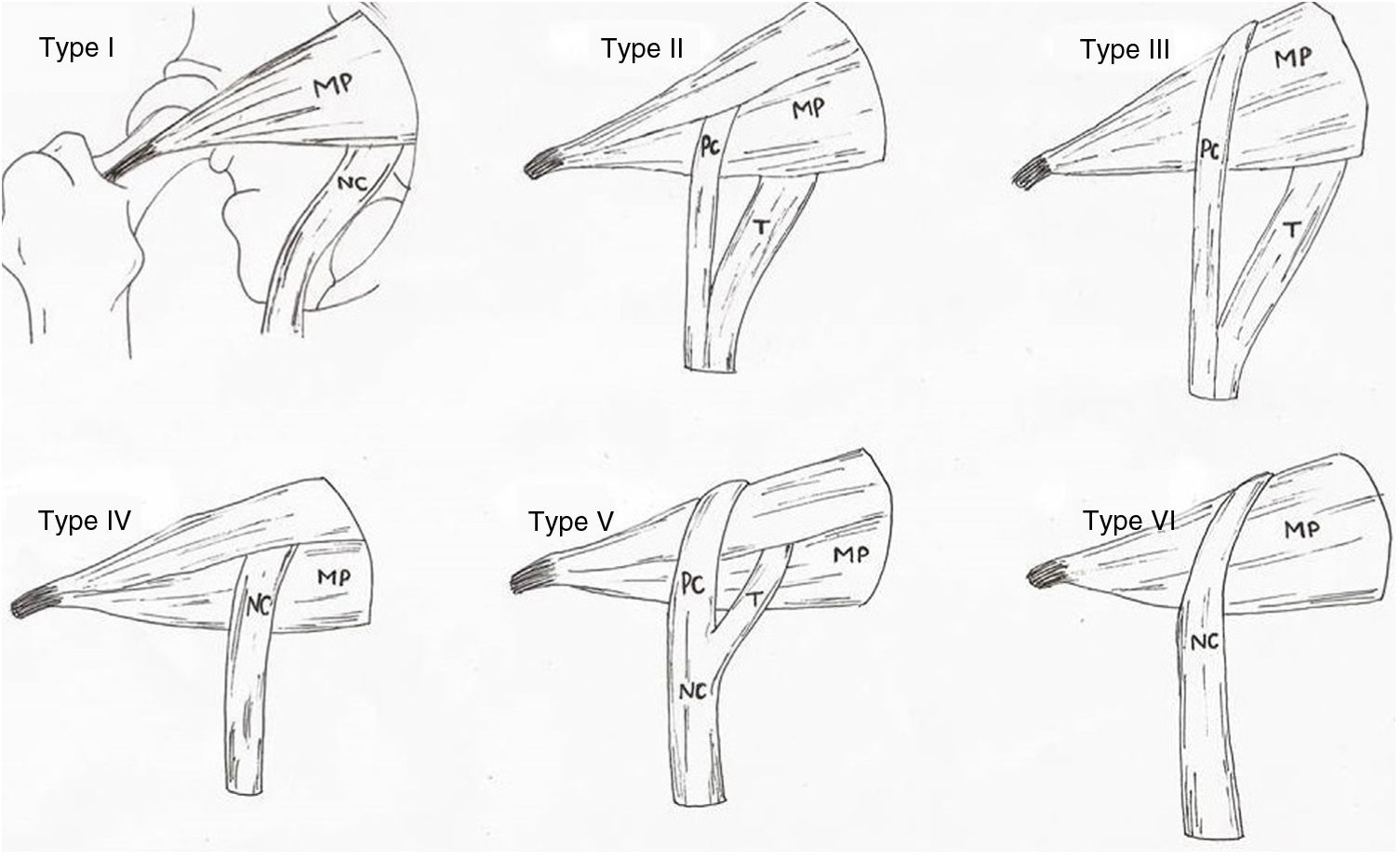
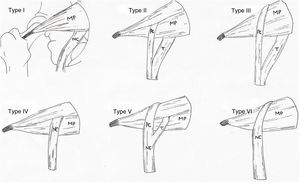
The anatomical variations of the piriformis muscle and the sciatic nerve were already studied by Beaton and Anson in 1937,3,11–15 who described 6 variants (Fig. 1). These were described throughout the 19th, 20th and 21st centuries, from dissection of cadvers of different races, ages and sexes, determining incidence, topography and medical features of these variations. However, in 1947 Robinson was the first author to come up with the term piriformis syndrome.16,17
Types of anatomical variations of the piriformis muscle and sciatic nerve, according to Beaton and Anson’s classification (1937).
CP: common peroneal nerve; T: tibial nerve.
Source: illustration by Carla Marco following said classification. PM: piriformis muscle; SN: sciatic nerve.
Piriformis syndrome is a cause of low back pain and as a result should be considered in the differential diagnosis of lumbalgias (between .3 and 5%).18 It usually presents as a low back pain, which is more intense in the gluteal area and which gets worse with prolonged sitting down. During physical examination the pain in the piriformis area increases on internal rotation of the thigh and on abduction (and may on occasions be combined with dyspareunia).19
Firm diagnosis is established with electroneurophysiology with imaging studies being required to rule out disc or spinal pathology. Electromyogram (EMG) picks up changes in the gluteus maximum and normally in the gluteus medius and minimus. The paraspinal muscles must preserve their innervations and the posterior branches must remain intact.
Conservative treatment must be sufficient but in highly exceptional cases surgical sectioning of the piriformis and neurolysis of the sciatic nerve may be performed.5,20 Only with an intraoperative EMG record (demonstrating the lowering of strength through the piriformis area and its improvement after sectioning of the muscle) can a firm diagnosis be reached that this infrequent entrapment syndrome is the cause of the clinical picture.
The aim of this study was to describe the anatomical variations found in the population, through an ultrasound and anatomical study, which may lead to piriformis syndrome. Moreover, the anthropometric measurements of the piriformis muscle and of the sciatic nerve are studied and compared for possible application in procedures in the gluteal area, including ultrasound-guided puncture of the sciatic nerve or posterior surgical interventions of the hip.
Material and methodThe study was carried out in 59 pelvis (29 right and 30 left) of 16 women (14 right and 15 left) and 16 men (15 right and 15 left) of a total of 32 cadavers cryopreserved at −20°C, whose age range was between 65 and 92 years. No scars were observed in any of the cadavers studied since those pelvises which presented with a scar in the gluteal area were not included in the study. One of the cadavers was injected with latex (Latex Compound Española S.A., Barcelona, Spain) which was black in colour, in the internal iliac artery to observe the blood vessels in the gluteal region.
The dissection of each cadaver was performed in prone position in accordance with standard procedure. Firstly a vertical incision at mid-line sacrum level was made and another 2 horizontal incisions, one at the height of the iliac crest and the other below the ischial tuberosity. The skin and subcutaneous tissue were then parted to each side to expose the deep fascia and the gluteus maximus muscle. A further vertical and medial incision was made of the gluteus maximus at its source and was withdrawn in medial to lateral direction and from proximal to distal direction to expose the gluteus medius, the piriformis muscle and the sciatic nerve.
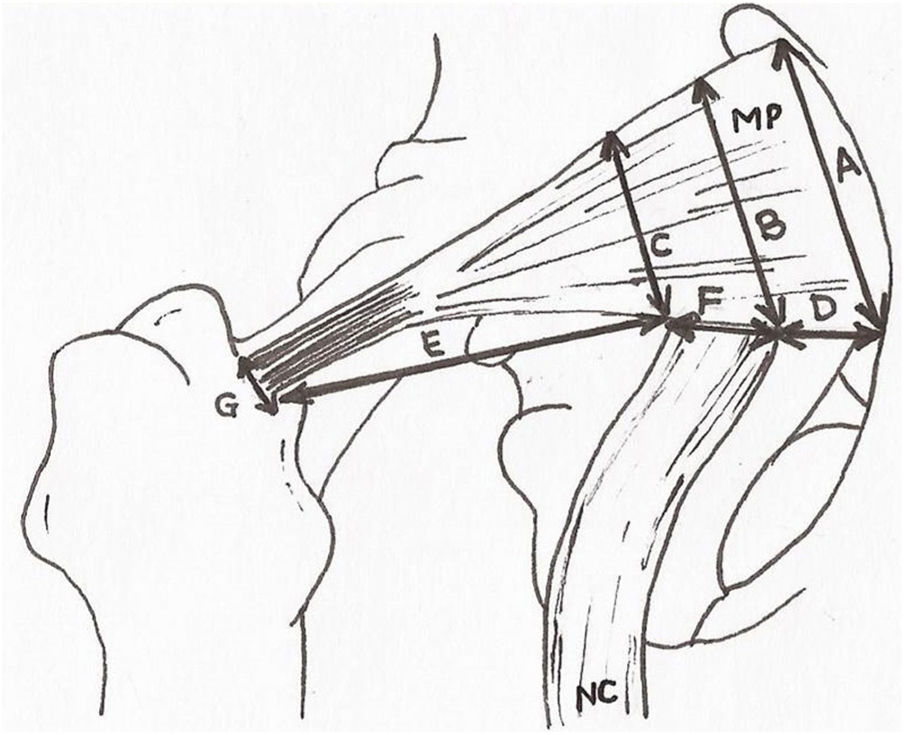
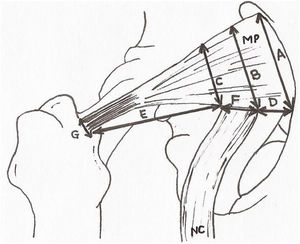
Once the area had been dissected the sciatic nerve was studied and its relationship with the piriformis muscle. Normality was assessed at the exit of the same nerve and its anatomical variations with respect to the piriformis muscle in accordance with the classification by Beaton and Anson (1937)17 to describe the anatomical variations found. The upper and lower gluteal vascular and nervous structures were discarded. After this, measurements of the distances contained in Fig. 2 were made. These measurements were obtained by 2 different observers aimed at minimising any individual measurement errors. Qualitative variables were presented in the form of percentages and the 95% confidence interval and statistical differences were calculated through comparison of the respective confidence intervals. Quantitative variables were presented as a mean and standard deviation and the 95% confidence interval. Normality and homogeneity of variances were studied with the Kolmogorov–Smirnov and Lavene tests, respectively. Comparison between quantitative variables with normal distribution was performed using the student’s t-test or the Mann–Whitney U test. The software used was Microsoft Excel® and IBM SPSS.17® for Windows.
Anthropometric measurements. A: extension of the piriformis muscle in its origin. B and C: extension of the piriformis muscle at medial and lateral level of the sciatic nerve exit. D: distance between the origin of the piriformis muscle and the exit of the sciatic nerve. E: distance between the sciatic nerve and the greater trochanter of the femur. F: diameter of the sciatic nerve at its exit. G: diameter of the piriformis muscle of the tendon prior to insertion. PM: piriformis muscle; SN: sciatic nerve.
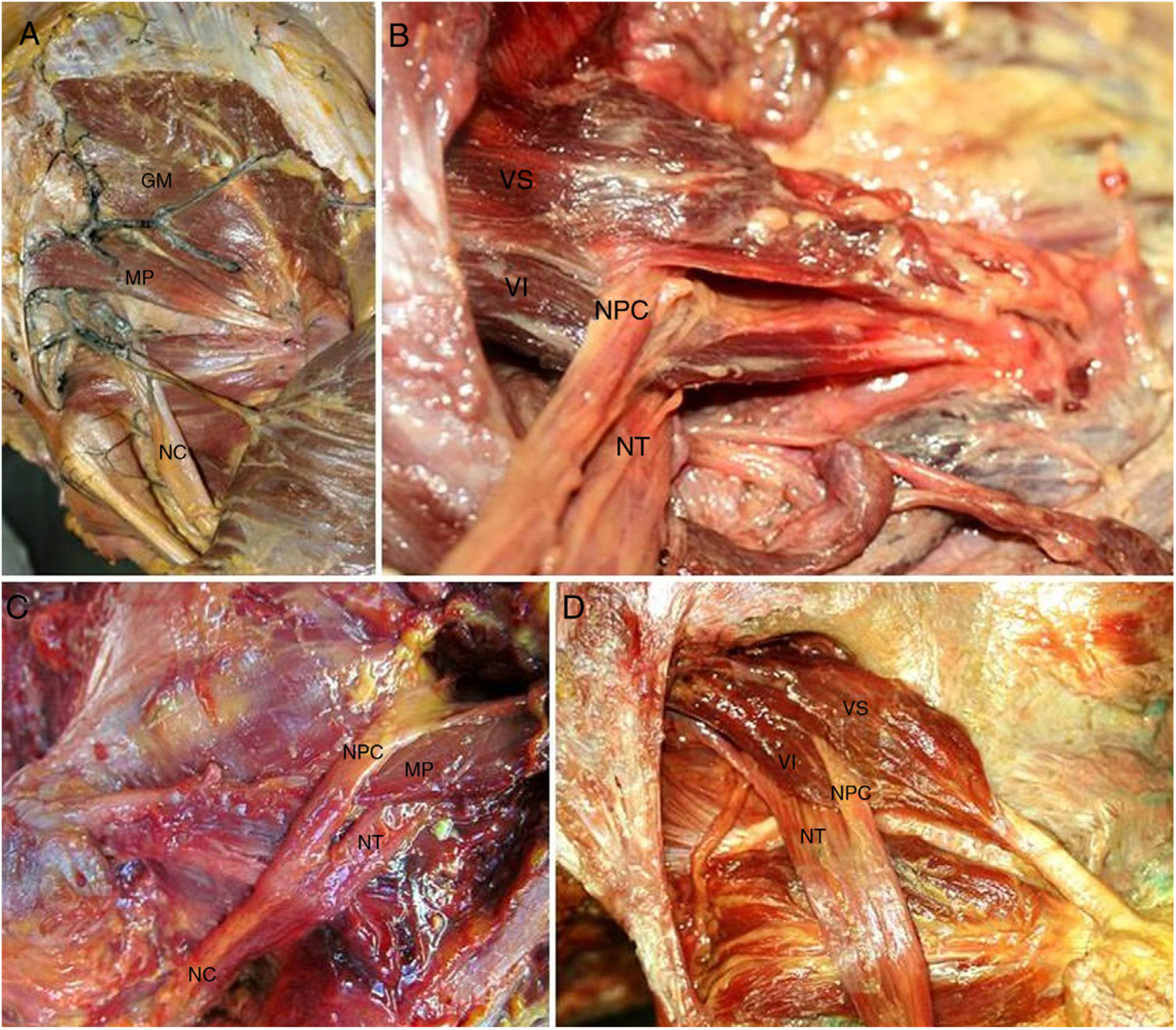
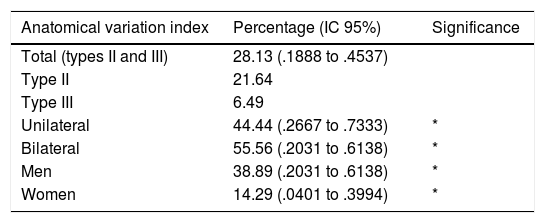
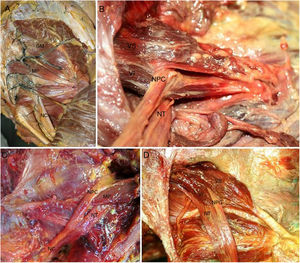
The anatomical study showed an incidence of anatomical variations in 28.13% of cases (Table 1), with a higher frequency at bilateral level (55.56%) and in men (38.89%), without the existence of any significant differences. According to the classification by Beaton and Anson (1937), the type I pelvis was found in 71.87% (Fig. 3A) of cases, whilst the rate of type II and III anatomical variations were 21.64 and 6.49%, respectively.
Anatomical variation index.
A) Normal anatomy of the gluteal region. Injected gluteal blood vessels. Corresponds to type I anatomical variation. B) Type II anatomical variation. Common peroneal nerve crossing piriformis muscle. C) Type II anatomical variation. D) Type II anatomical variation (left and right). In the figure on the right we observe 2 muscle heads of the piriformis muscle. The lower one has a tendinous origin. GM: gluteus medius; PM: piriformis muscle; SN: sciatic nerve; CPN: common peroneal nerve; TN: tibial nerve; LH: lower head of the piriformis muscle; UH: upper head of the piriformis muscle.
Type II anatomical variation consists of a piriformis muscle formed by 2 muscle heads relating to a division of the sciatic nerve. The branch which forms the common peroneal nerve exits between the 2 muscle heads whilst the nerve branch corresponding to the tibial nerve exits below the inferior muscular head (Fig. 3B). Type III variation consists of a single piriformis muscle and a sciatic nerve divided where the common peroneal nerve exits above the piriformis muscle whilst the tibial nerve does so below it. (Fig. 3C).
Of all the piriformis muscles studied it is worth noting the presence of a piriformis muscle with 2 muscular heads of independent origin, the lower muscle head of which presented with a more tendinous origin compared with the upper one. These muscle heads join up laterally to form a single tendon in the trochanter fossa (Fig. 3D).
Regarding the exit of the sciatic nerve, of note in type II and type III variations is that the sciatic nerve already exits the pelvis divided into common peroneal nerve and tibial nerve. The common peroneal nerve always exits laterally crossing over the piriformis muscle (type II) or superiorally to this (type III), whilst the tibial nerve always exits inferiorally to the piriformis muscle (Fig. 3B–D).
With regard to insertion of the piriformis muscle, in 53.85% of cases this was located in the trochanter fossa. This insertion is variable and may present with anatomical variations with other pelvi-trochanteric muscles. Table 2 summarises the different insertions found.
Piriformis muscle insertions (%).
| Independent in TF | JT with GM in GT | JT with UC and IO in FT | JT with UC, LC and UO in TF | JT with OI in TF | JT with Gm in GT | JT with Gm and OI in TF | TC with Gm, UC and OI in TF | |
|---|---|---|---|---|---|---|---|---|
| % of cadaver | 53.85 | 15.38 | 2.56 | 10.26 | 7.69 | 5.13 | 2.56 | 2.56 |
TF: trochanteric fossa; LC: lower calf; GM: gluteus medius; Gm: gluteus minimus; UC: upper calf; IO: internal obturador; JT: joint tendon; GT: greater trochanter.
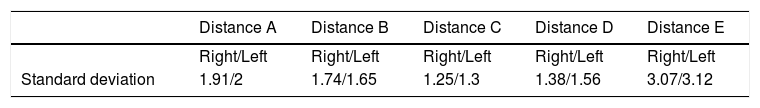
Lastly, the anthropometric measurements obtained for the piriformis muscle and the sciatic nerve (Fig. 2) are summarized in Table 3.
The other measurements correspond to the diameter of the sciatic nerve, with a mean of 2.11 and standard deviation of .34. The length of the piriformis muscle was 10.85, with a standard deviation of 1.44, and diameter of its tendon was 1.05, with a standard deviation of .9. There were no significant differences regarding sex and laterality.
DiscussionPiriformis syndrome is considered an entrapment neuropathy, in this case the sciatic nerve by the piriformis muscle.3,8,12,13,20 In accordance with other authors,4,13,14,21 the presence of anatomical variations of the piriformis muscle and/or the exiting of the sciatic nerve through this are believed to contribute to the presence of this syndrome.
The rate of anatomical variations found in the population studied which may explain piriformis syndrome was 28.13%, being most common in men (38.89% [95% CI: .2031 to 0.6138]) than in women (14.29% [95% CI: .0401 to .3994]) with no significant differences. However, these data differ from those found by other authors who describe a similar frequency in both sexes.22,23 This frequency is higher than that observed by other authors: 10.2%,12 16.9%,3 20.9%,24 but is in keeping with the incidence interval described in other studies (1.5 and 35.8%).3,12–15 However, this interval is highly extensive and non specific.
Different classifications exist to describe the different variations,25,26 the most recent being the one which includes 7 different types.26 However, the anatomical variations found in this study were described according to the anatomical classification of Beaton and Anson (1937), which is the original. In this classification and in others3,26 the type I variation is regarded as normal anatomy, and this would be better reconsidered since cadavers without any anatomical variation should be excluded from the classification.
According to the results obtained and in accordance with other autos, the type II anatomical variation is the one with the most probabilities of presenting itself, with a rate of 21.64%, followed by type III variation with a rate of 6.49%.12–14,24,27 However, in the cases studied no type IV, V and VI anatomical variations were found, as described in other studies.12–14,21,24 These types of variations were only documented with a frequency of 1.5% for type V and 2.9% for type VI.12
The possibility exists also that other unclassified anatomical variations may occur intraoperatively, for example transverse fibrous bands inferior to the piriformis muscle with compression of the sciatic nerve.28 These anatomical structures were not found in this study. However, all these variations described or others imply the importance that, on intervention, not only should the known variations be sought, but any other causes which may involve sciatic nerve compression symptoms. For this reason, decompression of this nerve may be insufficient when these anatomical variations exist or there are other causes of nerve compression.12
Type II, IV and V variations describe the piriformis muscle with double muscle head. This anatomy suggests that the exit of the sciatic nerve or of its branches between both muscle heads may be more easily changed and facilitate the presentation of a piriformis syndrome.
The sciatic nerve is divided into the common peroneal nerve and the tibial nerve in the popliteal region. Thus division above this area is already in itself a variation. Type II, III and V variations describe the different exits of these nerves with the peroneal nerve being the most commonly affected and according to some authors,13,14 the clinical symptoms of the piriformis syndrome will reflect the pathway of the compressed nerve. Furthermore, knowledge of the exit and division of the sciatic nerve is also relevant for preventing nerve damage during procedures in the gluteal region.29
With regard to the insertion of this muscle, great variability was found in our study. The most common (53.85%) was the insertion with its tendon independently from the trochanteric fossa in keeping with other authors.3 However, a certain frequency of insertion was also observed together with other adjacent muscles, highlighting the union with the medius gluteus muscle, unlike another anatomical study (29%) where fusion with the tendon of the internal obturator muscle and upper calf were described.3
The measurements obtained from the sciatic nerve inferiorially to the piriformis muscle presented a mean of 2.11cm. This data is concurrent with those obtained by other authors, with a diameter of 1.7±.37cm.13,27 The measurements of the piriformis muscle and sciatic nerve compared with the greater trochanter of the femur were 3.1cm, but we were not able to be compare this to other gluteal region studies. These may be useful for maintaining safety margins of the nerve exit in surgical interventions, in anaesthetic techniques or in imaging interpretations of the gluteal region.
Lastly, it should be noted that this study cannot relate the anatomical variations found with confirmation of piriformis síndrome diagnosis because the study was performed on cadvers with no previous medical history information. However, study may be conducted to relate the mechanism of sciatic nerve compression with the piriformis syndrome clinical picture, studying anatomical changes occurring in static situations and during the stretching test of the FAIR manoeuvre (flexion, adduction and internal rotation).4,13,30
ConclusionThe rate of anatomical variations in the population studied indicates that possible pain in the gluteal region should be assessed as differential diagnoses because the signs and symptoms of piriformis síndrome are similar to vertebral disc pathology with radiculer involvement. Also, anatomical knowledge of this region may be useful for the interpretation of imaging techniques, particularly when ultrasound-guided injections are performed.
Level of evidenceLevel of evidence iv.
FinancingThere was no source of financing.
Conflict of interestsThe authors have no conflict of interests to declare.
Our thanks to all the cadaver donors who voluntarily contributed to a greater knowledge of anatomy, teaching and research.
These authors contributed equally to this work.
Please cite this article as: Marco C, Miguel-Pérez M, Pérez-Bellmunt A, Ortiz-Sagristà JC, Martinoli C, Möller I, et al. Causas anatómicas de compresión del nervio ciático en la pelvis. Síndrome piriforme. Rev Esp Cir Ortop Traumatol. 2019;63:424–430.