To determine the effects of applying vancomycin powder within the surgical wound on the risk of surgical infections, pseudo-arthrosis and adverse events, in patients undergoing spinal surgery.
Material and methodsA meta-analysis was carried out, including controlled studies that evaluated the risk of postoperative infections and/or pseudo-arthrosis in patients undergoing spinal surgery in which vancomycin powder was applied within the surgical wound.
Results were presented as pooled relative risks, with its 95% confidence intervals. Additionally, the frequency of complications attributable to vancomycin was also assessed.
ResultsA total of six controlled studies (3379 subjects) were included. Pooled relative risks were: surgical site infection, 0.11 (95% CI: 0.05–0.25; P<.00001), and pseudo-arthrosis, 0.87 (95% CI; 0.34–2.21; P=.77). No statistically significant heterogeneity was found in both analyses. In 1437 patients treated with vancomycin, there were no recorded vancomycin-related adverse events.
ConclusionsApplication of vancomycin powder into the wound was associated with a significantly reduced risk of surgical site infections, without increasing pseudo-arthrosis or adverse events. However, randomized controlled trials are needed, in order to confirm the present results and make recommendations with more certainty.
Determinar los efectos de la aplicación de la vancomicina en polvo dentro de la herida quirúrgica, sobre el riesgo de infecciones postoperatorias, pseudoartrosis y efectos adversos en pacientes sometidos a cirugías de columna.
Material y métodosSe realizó un metaanálisis incluyendo los estudios controlados que evaluaron el riesgo de infecciones postoperatorias y/o pseudoartrosis en pacientes sometidos a cirugía de columna a quienes les fue aplicada vancomicina en polvo en la herida quirúrgica.
Los resultados se presentaron como riesgos relativos combinados, con sus intervalos de confianza del 95%. Adicionalmente, se evaluó la frecuencia de complicaciones atribuibles al tratamiento.
ResultadosSe incluyeron 6estudios controlados (3.379sujetos). Los riesgos relativos combinados fueron: infección del sitio quirúrgico, 0,11 (IC95%: 0,05-0,25; p<0,00001), y pseudoartrosis, 0,87 (IC95%: 0,34-2,21; p=0,77). No se encontró heterogeneidad estadísticamente significativa en ninguno de los análisis. En 1.437pacientes tratados no se reportaron complicaciones asociadas al uso de la vancomicina.
ConclusiónLa aplicación de vancomicina en polvo dentro de la herida se asoció con una reducción significativa del riesgo de infecciones del sitio quirúrgico, sin incrementar el de pseudoartrosis o de efectos adversos. Sin embargo, se requieren estudios controlados y aleatorizados, con el fin de confirmar los presentes resultados y realizar recomendaciones más certeras.
Surgical site infections (SSI) are one of the most common and devastating complications in spinal surgery. Their incidence varies according to several factors and it is estimated that between 2.8% and 11.9% of patients undergoing spinal surgery will suffer SSI, despite the application of conventional prevention strategies.1,2
Patients affected by these infections present prolonged hospitalization times and incapacity for work, reduced quality of life indices and, in general, notably unfavorable outcomes compared to patients who do not suffer these complications.1 Additionally, the treatment of SSI requires considerable expenditure stemming from the prolonged hospitalization time, the use of diagnostic aids, reinterventions and intravenous antibiotic therapy, among others.2,3 For these reasons, several measures focused on reducing their incidence to the minimum level possible have been investigated.1,4
The administration of intravenous antibiotics is perhaps the most widely used strategy for the prophylaxis of SSI. The most recent clinical guidelines from the Antibiotic Prophylaxis Work Group of the North American Spine Society5 promote the systematic administration of intravenous prophylaxis. However, it has been demonstrated that the magnitude of SSI reduction is relatively low, thus leading to the search for other alternatives.6
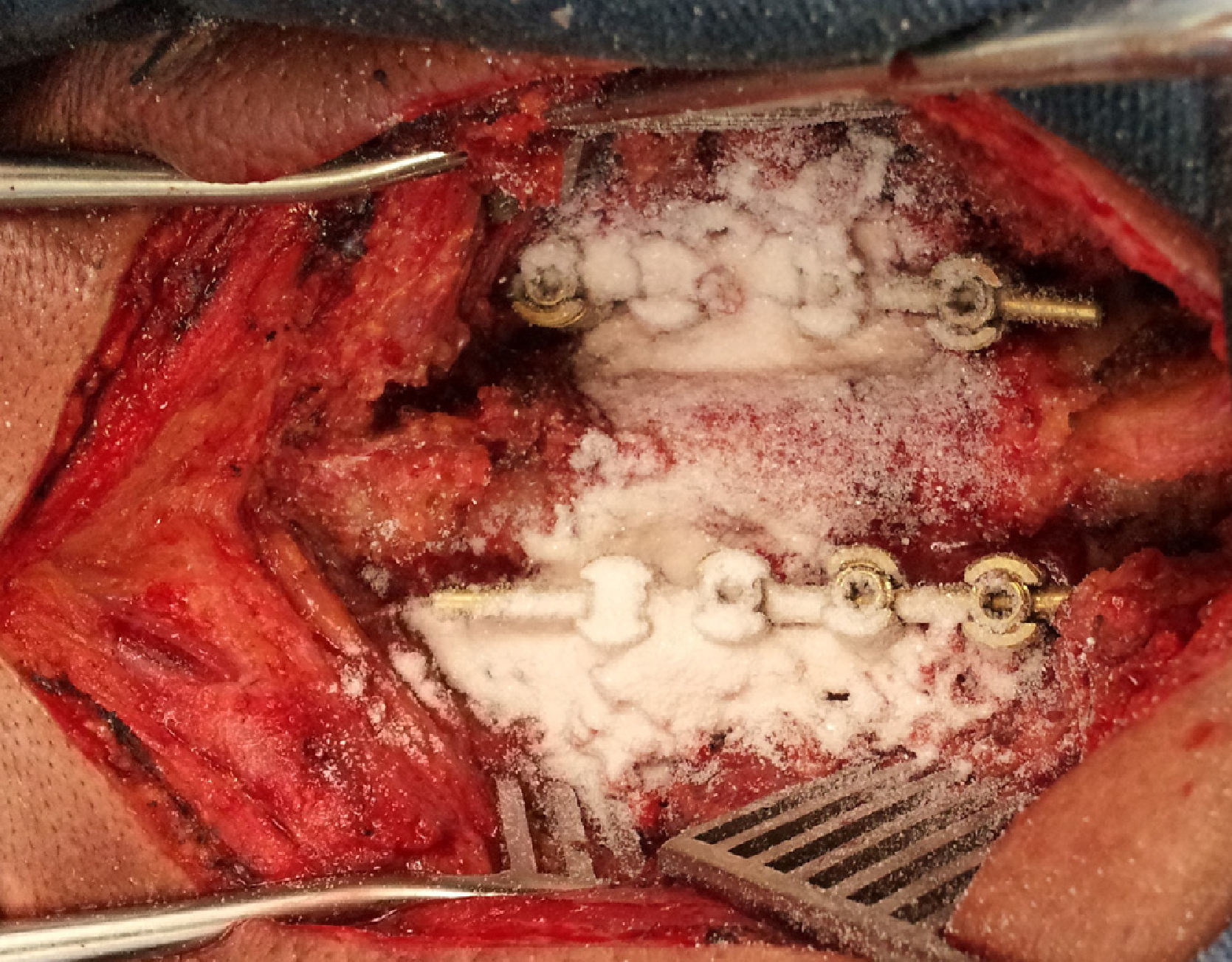
The application of vancomycin in the form of unreconstituted powder within the surgical site represents an innovative trend for the prevention of SSI and is increasingly gaining supporters among spinal surgeons due to its low cost, extensive availability, ease of application, good safety profile and perception of effectiveness.7–9 Sweet et al.10 determined that the application of vancomycin under the muscle fascia can lead to concentrations within the surgical site up to 1000 times greater than the mean inhibitory concentration required to destroy methicillin-resistant Staphylococcus aureus, one of the most frequently isolated germs in spinal surgery infections. Moreover, its microbicide spectrum also includes other Gram-positive bacteria, such as Staphylococcus epidermidis and Enterococcus spp., which can also cause postoperative spinal infections.3,11
Although the pharmacokinetic properties of vancomycin applied within the surgical site make it a very attractive method for prophylaxis, its potential adverse effects represent a significant drawback. Intravenous administration has been associated with anaphylactic reactions, arterial hypotension, renal toxicity, otological toxicity and induction of antibiotic resistance. Nevertheless, its safety profile when applied topically is still not fully known.12,13
Furthermore, some studies describe mechanisms through which vancomycin could interfere with the maturation and functioning of osteoblasts, which would alter the biological pathways involved in bone fusion. For this reason, several authors maintain that high local vancomycin concentrations within the surgical site could be associated to a higher risk of pseudoarthrosis (or nonunion).14–16
Due to its recent use in clinical practice, several studies examining the effects of powdered vancomycin within the surgical site during spinal surgery have been published in the last decade. However, these studies have not been previously assessed through a metaanalysis.
The objective of the current metaanalysis is to determine the effects of applying vancomycin within the surgical site on SSI and pseudoarthrosis among patients undergoing spinal surgery, as well as the potential adverse effects of this practice.
Material and methodsA systematic literature review was conducted following the protocol described in the declaration Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA),17 and the metaanalysis was conducted applying the recommendations from the Quality of Reporting of Meta-analyses (QUORUM) Conference.18
Literature searchThe literature was identified and classified independently by 2 reviewers, using the following search engines: Medline/Pubmed, EMBASE, Google Scholar and Cochrane Controlled Trials Registry. The selection of studies took place on July 2013, with no restrictions regarding language and publication date.
The combinations of terms employed were:
- •
“VANCOMYCIN” and “SPINAL SURGERY”
- •
“VANCOMYCIN” and “SPINE SURGERY”
- •
“VANCOMYCIN POWDER”
Type of studies:
- •
Observational controlled studies or randomized clinical trials.
- •
Prospective or retrospective data collection.
Type of participants:
- •
Studies including adult and pediatric patients.
- •
Studies including only patients undergoing operations by posterior approaches, including minimally invasive techniques for intervertebral decompression or fusion.
- •
Studies including cervical, thoracic, lumbar or lumbosacral interventions.
Type of intervention:
- •
Local application of unreconstituted vancomycin powder within the surgical site at the end of the procedure, prior to closure.
- •
Minimum local dose of 500mg.
Type of evaluation of outcomes: including studies which explicitly reported the percentage frequency or raw data of any of the following variables:
- •
SSI.
- •
Pseudoarthrosis.
- •
Adverse effects and/or complications associated to the use of vancomycin.
- •
Studies of case series and population registries.
- •
Studies including less than 10 patients in each group.
- •
Studies in patients intervened through anterior approaches.
- •
SSI.
- •
Pseudoarthrosis.
- •
Adverse effects attributable to the application of vancomycin within the surgical site.
- •
SSI. This included superficial infections (involvement of skin and subcutaneous tissues) and deep infections relative to the muscle fascia (including instrumentation material). We excluded infections of simultaneous incisions (e.g. to take autologous bone grafts or combined anterior approaches).
- •
Pseudoarthrosis. Clinical and radiographic documentation of bone fusion or consolidation failures, or the need for reintervention for a new fusion at the same level intervened previously.
- •
Adverse effects attributable to the application of vancomycin within the surgical site. Incidences and complications described as potential adverse effects of the intravenous administration of vancomycin.3
This process was initially conducted by reviewing the titles of articles and their corresponding summaries. The full text of potentially relevant articles was also examined. Following the application of the inclusion and exclusion criteria mentioned above, we selected articles for qualitative and quantitative review. Discrepancies in the selection were resolved by consensus among the authors who reviewed the literature.
Extraction of dataTwo independent reviewers extracted the data regarding the design and methodology of the study, total population, kind of pathology treated (degenerative, trauma, tumoral, inflammatory or mixed), spinal segment treated (cervical or thoracolumbar), vancomycin dose administered, postoperative follow-up period and frequency of primary and secondary outcomes.
Assessment of methodological qualityControlled and randomized studies were assessed using the Jadad scale,19 which assigns scores based on 3 criteria: description of randomization (2 points), blindness of researchers (2 points) and explanation of limitations (maximum 3 stars), with 0 being the minimum score and 5 the maximum.19
The Newcastle-Ottawa scale was used to assess the quality of observational studies. This is the scoring system recommended by the Cochrane Non-Randomized Studies Methods Working Group.20 The classification is established based on 3 criteria: selection (maximum 4 stars), comparability (maximum 2 stars) and outcomes (maximum 3 stars). Those studies with scores between 7 and 9 were considered to have a high methodological quality, those with scores between 4 and 6 were moderate, and scores below 4 reflected low quality.20
Data analysisThe raw data were entered into a Microsoft Office Excel 2010 spreadsheet, and from there were exported to the Cochrane Collaboration RevMan 5.0 (Cochrane Collaboration, Oxford, UK) statistical software package for their combined analysis.
The effects of local application of vancomycin on each of the dichotomous outcomes were estimated by calculating the combined relative risk (RR), with its corresponding 95% confidence interval.
In order to select the method of combined RR estimation, we first calculated the statistical heterogeneity indices. This was done by simultaneously applying the Chi square test and measuring the I2 index. Statistically significant heterogeneity was considered when the Chi square test returned values of P<.1 or the I2 index was over 50%, and the random effects model was used to asses the combined RR. Conversely, when the results were not statistically significant we employed the Mantel–Haenszel fixed effects model. The estimations derived from observational studies were obtained through the random effects model.20
The detection of potential sources of statistical heterogeneity was carried out through a visual inspection of forest plots and analysis of the subgroups according to the anatomical site of surgery (cervical versus thoracolumbar) and the use of instrumentation. We also used funnel plots to detect any possible publication bias.
ResultsSearch resultsWe identified a total of 233 articles which coincided with the search terms. Following assessment of the summary and the full text and application of the inclusion and exclusion criteria, we selected 6 studies for qualitative and quantitative assessment, which included a total of 3379 subjects.2,10,21–24 The results of the literature review are shown in Fig. 1.
All the studies were observational and had a cohort design; 1 was prospective and the other 5 were retrospective.2,10,22–25 All were carried out in the US and published after the year 2011.2,10,21–24 We found no clinical trials evaluating the primary outcome of this metaanalysis (SSI).
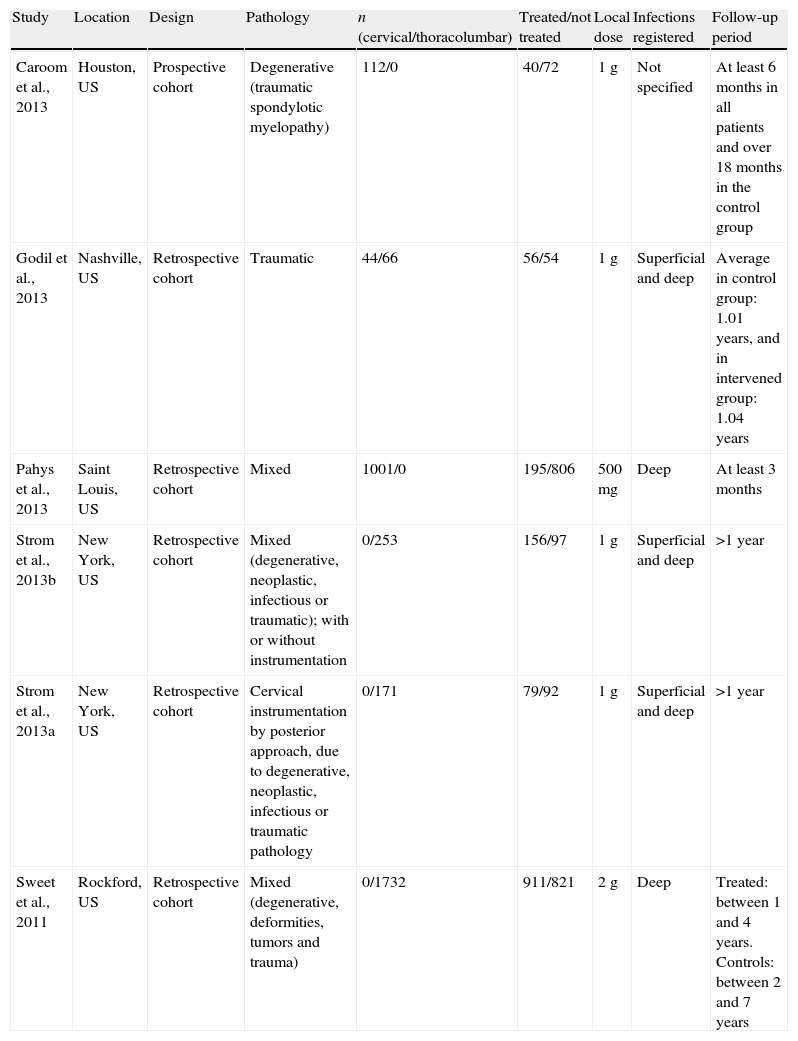
A total of 4 studies were conducted on mixed populations, involving cases of traumatic, degenerative, neoplastic and infectious pathologies. Two studies involved patients with a preexisting infectious pathology (2 cases in the 2 studies), but none of these cases presented SSI.23,24 The study by Caroom et al.21 exclusively analyzed patients intervened due to cervical spondyloarthritic myelopathy and treated by instrumented fusion and decompression. The 6 studies referred to patients intervened through posterior approaches and no studies were found which referred to surgical interventions conducted by anterior approaches. A total of 5 studies evaluated the incidence of infections in patients who required instrumentation,2,10,21,22,24 and only the study by Strom et al.23 analyzed the infection rate among patients undergoing lumbar decompressive and fusion surgery, with or without instrumentation. The general characteristics of each of the studies included are presented in Table 1.
General characteristics of each of the included studies.
| Study | Location | Design | Pathology | n (cervical/thoracolumbar) | Treated/not treated | Local dose | Infections registered | Follow-up period |
| Caroom et al., 2013 | Houston, US | Prospective cohort | Degenerative (traumatic spondylotic myelopathy) | 112/0 | 40/72 | 1g | Not specified | At least 6 months in all patients and over 18 months in the control group |
| Godil et al., 2013 | Nashville, US | Retrospective cohort | Traumatic | 44/66 | 56/54 | 1g | Superficial and deep | Average in control group: 1.01 years, and in intervened group: 1.04 years |
| Pahys et al., 2013 | Saint Louis, US | Retrospective cohort | Mixed | 1001/0 | 195/806 | 500mg | Deep | At least 3 months |
| Strom et al., 2013b | New York, US | Retrospective cohort | Mixed (degenerative, neoplastic, infectious or traumatic); with or without instrumentation | 0/253 | 156/97 | 1g | Superficial and deep | >1 year |
| Strom et al., 2013a | New York, US | Retrospective cohort | Cervical instrumentation by posterior approach, due to degenerative, neoplastic, infectious or traumatic pathology | 0/171 | 79/92 | 1g | Superficial and deep | >1 year |
| Sweet et al., 2011 | Rockford, US | Retrospective cohort | Mixed (degenerative, deformities, tumors and trauma) | 0/1732 | 911/821 | 2g | Deep | Treated: between 1 and 4 years. Controls: between 2 and 7 years |
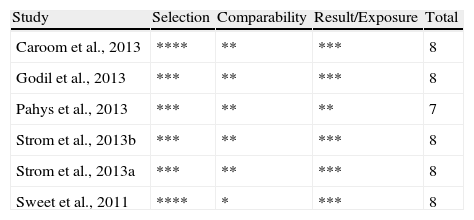
All the studies were considered to have a high methodological quality, according to the score obtained in the Newcastle-Ottawa scale for cohort studies. The detailed results of each study are presented in Table 2.
Assessment of the quality of included studies through the Newcastle-Ottawa scale for cohort studies.
| Study | Selection | Comparability | Result/Exposure | Total |
| Caroom et al., 2013 | **** | ** | *** | 8 |
| Godil et al., 2013 | *** | ** | *** | 8 |
| Pahys et al., 2013 | *** | ** | ** | 7 |
| Strom et al., 2013b | *** | ** | *** | 8 |
| Strom et al., 2013a | *** | ** | *** | 8 |
| Sweet et al., 2011 | **** | * | *** | 8 |
Six studies assessed the risk of SSI, comprising a total of 3379 subjects.2,10,21–24 At the end of the monitoring period, the incidence among treated patients was 0.28%, whilst in the control group it was 3.70%. The combined RR of the studies evaluating this outcome was 0.11 (95% CI: 0.05–0.25), complying with the preestablished criterion for statistical significance (P<.00001). The I2 indices and the value of P did not display significant heterogeneity (0% and 0.78, respectively) (Fig. 2).
The beneficial effect of treatment was also observed in most subgroups analyzed, except for patients whose surgical interventions did not include instrumentation. The subgroup of patients undergoing cervical spinal surgery comprised 1328 subjects, from 4 studies.2,21,22,24 The combined RR for SSI was 0.19 (95% CI: 0.06–0.57; P=.003). The analysis of the I2 index showed that heterogeneity was not statistically significant (0%). The subgroup of patients undergoing surgery in thoracolumbar segments comprised a total of 2163 subjects, from 4 studies.2,10,21,23 The application of powdered vancomycin was also associated with a reduced risk of SSI, since the combined RR was 0.07 (95% CI: 0.02–0.21; P<.00001), and the indices did not display statistically significant heterogeneity (I2=0%) (Fig. 3).
In the subgroup of 2290 instrumented surgical interventions, assessed in 5 studies,2,10,21,23,24 the risk of SSI was lower with the application of vancomycin (RR=0.11; 95% CI: 0.05–0.26; P<.00001) (Fig. 3). Patients in the subgroup which did not undergo surgery with instrumentation were exclusively obtained from the study by Strom et al.23 so it was not possible to calculate the combined RR.
PseudoarthrosisThis outcome was assessed by 3 studies, including a total of 2156 subjects.10,23,24 The incidence of pseudoarthrosis was of 0.7% among treated patients, and of 0.9% among the control group. The analysis showed that the combined RR of pseudoarthrosis was 0.87 (95% CI: 0.34–2.21), which did not reach statistical significance (P=.77). The Chi square test and the value of the I2 index did not display statistically significant heterogeneity among the studies (P=.84 and 0%, respectively) (Fig. 2).
Complications attributable to the use of powdered vancomycinNo incidences or complications related to the application of vancomycin within he surgical site were registered in any of the 1437 subjects thus treated, from the 6 observational studies.2,21,23–25 Therefore, it was not possible to carry out comparative analyses.
Risk of bias in the studiesObservational studies included were considered to have a low risk of bias due to their high methodological quality. The detection of publication bias risks through funnel plots did not reveal asymmetries in any of the outcomes (Fig. 4).
DiscussionThe present metaanalysis describes the effect of applying vancomycin within the surgical site in patients undergoing spinal surgery, showing a statistically significant reduction of the risk of SSI. The findings offer a scientific basis for this practice, which is gaining support among spinal surgeons worldwide. According to a study by Glotzbecker et al.7 with 277 spinal surgeons from the Pediatric Orthopedic Society of North America/Scoliosis Research Society, 24% of them reported routinely using powdered vancomycin administered directly within the surgical site or mixed with the bone graft used during intervertebral fusions (Fig. 5). Furthermore, based on the emerging evidence, it seems likely that this percentage will increase.
The incidence of SSI varies in relation to the anatomical region of the spine which is intervened. It has been proven that procedures conducted on the cervical spine through a posterior approach entail a greater risk, compared to thoracolumbar interventions.23,24 It is for this reason, that we decided to conduct an analysis of these 2 subgroups, which showed that the effects are applicable in both spinal segments.
Additionally, we also considered the increased risk of infection entailed by the use of spinal instrumentation. Nevertheless, the preventive effects were also observed in patients requiring instrumented surgery, whilst it was not possible to determine them in procedures which did not use instrumentation, since we only obtained data from a subgroup of 77 patients studied by Strom et al.23
Despite theories pointing to a potential toxic effect on osteoblasts of high concentrations of vancomycin within the surgical site, the analysis of the combined RR indicated that there was no statistically significant difference in the risk of pseudoarthrosis, which is probably related to the rapid repopulation of these cells in the fusion site, as soon as the vancomycin concentrations within the surgical site decrease.10
We planned on conducting the analysis of the complications related to the application of vancomycin by estimating the combined RR, but this was not possible because no adverse effects were recorded in 1437 patients treated, which highlights the safety profile of this mode of administration. Two case series have also described similar findings.10,11 In a retrospective study of 1512 patients intervened for 6 consecutive years, Molinari et al.11 did not find higher rates of complications related to the use of vancomycin at a center with a high standard of trauma care. This series only reported 1 patient who developed postoperative renal failure after elective surgery (0.07% of all cases). Nevertheless, it should also be noted that renal failure is one of the potential complications in patients undergoing spinal surgery, with an estimated incidence of 1.6%.26,27
Although in the past nephrotoxicity was recognized as one of the major complications derived from the use of intravenous vancomycin, the consensus review published in 2010 by the American Society of Infectious Diseases, Pharmacologists of the Healthcare System and Infectious Disease Pharmacologists recognized that there was scarce evidence of this association when used as monotherapy, although it did note that it could enhance renal failure induced by other drugs, like aminoglycosides.28 There have also been cases of nephrotoxicity with vancomycin doses exceeding 4g per day or plasma concentrations exceeding 28mg/l, both of which exceed by several tens of times the blood concentrations obtained through local application.13,28
The series by Molinari et al.11 contained 2 cases (0.13%) of transient hearing loss, which recovered during the early postoperative period. Otological toxicity, like renal toxicity, has not been well established in patients receiving treatment with vancomycin. However, it is believed that it could also be related to plasma concentrations above therapeutic ranges.28 Another potential adverse effect of the topical application of vancomycin is circulatory collapse, although the only evidence for this complication comes from 1 anecdotal case reported recently by Mariappan et al.12 and no similar complications have been documented.
LimitationsThe interpretation of the findings of this metaanalysis should take into account several limitations inherent to its methodology and the studies that were included. Perhaps the most important relates to the design of the studies analyzed, since all were observational.2,10,21–24 Although the results were consistent, as evidenced by the low levels of heterogeneity (I2=0% in all analyses), and the magnitude of the beneficial effect was statistically significant, the recommendations of the Cochrane Collaboration emphasize that metaanalyses of randomized controlled studies are best suited to evaluate the effects of an intervention on health, as they enable us to overcome the potential selection bias that may affect the results of observational studies.20 It is for this reason that the recommendations on the use of vancomycin should be updated as soon as clinical trials become available.
Another potential limitation of this metaanalysis was the assessment of possible publication bias risks. This procedure was performed by visual inspection of funnel plots, which displayed no asymmetries. However, due to the low number of studies included, the certainty of this interpretation may be altered.28
Additionally, it is worth noting that in the study by Pahys et al.22 the control group was heterogeneous, since in some patients preoperative antisepsis was performed with alcoholic preparations, which was associated with a decreased risk of SSI, and this would represent a risk of selection bias in the control group, thus decreasing the magnitude of the treatment effect.
Another consideration that must be taken into account regarding the routine use of vancomycin within the surgical site is the development of bacterial resistance to antimicrobials, especially the emergence of vancomycin-resistant Enterococcus spp. and Staphylococcus aureus. One of the main factors associated with the development of these strains is underdosing, since microorganisms are exposed to concentrations which do not eradicate them for relatively long periods of time.28
A prospective study conducted in 87 pediatric patients by Gans et al.13 showed that applying 500mg of powdered vancomycin within the surgical site did not produce increases in systemic concentration, which remained undetectable (<2.0μg/ml) in 86 of the 87 patients from the first postoperative day. This suggests that the risk of inducing bacterial resistance might be low. Nevertheless, there are still no studies focused specifically on resolving this uncertainty.
ConclusionThe application of powdered vancomycin within the surgical site was associated with a statistically significant reduction in the risk of SSI, without increasing the risk of pseudoarthrosis or adverse effects related to vancomycin. However, randomized controlled studies are required in order to confirm these results and to make recommendations with greater certainty.
Level of evidenceLevel of evidence II.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation did not require experiments on humans or animals.
Confidentiality of dataThe authors declare that this work does not reflect any patient data.
Right to privacy and informed consentThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
FinancingThis study was financed through a grant from the Neuroscience and Healthcare Science Research Group (CISNEURO).
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Alcalá-Cerra G, Paternina-Caicedo A, Moscote-Salazar L, Gutiérrez-Paternina J, Niño-Hernández L. Aplicación de vancomicina en polvo dentro de la herida quirúrgica durante cirugías de columna: revisión sistemática y metaanálisis. 2014;58:182–191.