To establish a simple, reproducible and safe experimental model, for the development of ischemic vascular necrosis of the hip in the lamb.
Material and methodsWe used 15 lambs (10 males and 5 females) aged four weeks, divided into a control group (7 animals) and an experimental group (8 animals) producing ischemia in the proximal femur. Standard radiography and MRI were performed. The animals were euthanised at the 4th, 8th and 12th weeks after surgery. The femoral heads were extracted and measured and a histological analysis was performed with hematoxylin–eosin staining.
ResultsDecreased height and increased width of the femoral head was observed in the X-rays, particularly after the 4th week. We did not observe any changes in the height of the lateral pillar or trochanteric distance. The experimental group showed macroscopical hypertrophy and progressive flattening of the head. At 4 weeks necrotic areas in articular cartilage were observed, bone marrow was dense and the growth cartilage height was lower. The vessels were thickened by proliferation of the medial and adventitia layers. At 8 weeks, we found fibrosis in the subchondral bone with thinned and devitalized angiogenesis fat areas. The articular cartilage showed irregularities. At 12 weeks the closure of the physis was noted, as well as chondral areas in the trabecular bone and fat cells in the methaphysis.
ConclusionAlthough the histological changes are consistent with necrosis of the femoral head, the images obtained did not resemble Perthes disease, so we do not advise this experimental model for the study of this disease.
Establecer un modelo experimental sencillo, reproducible y seguro para conocer el desarrollo de la necrosis vascular isquémica de la cadera en el cordero.
Material y metodologíaUtilizamos 15 corderos (10 machos y 5 hembras) de 4 semanas de edad, divididos en un grupo control (7 animales) y otro grupo experimental (8 animales), a los que se provocó la isquemia de la extremidad proximal del fémur. Se efectuaron radiografía convencional y resonancia nuclear magnética. Tras el sacrificio de los animales, a la 4.a, 8.a y 12.a semanas poscirugía, extrajimos y medimos la cabeza femoral. Una vez fijada la pieza obtuvimos cortes histológicos de diferentes zonas que se tiñeron con hematoxilina-eosina.
ResultadosRadiográficamente disminuyó la altura y aumentó la anchura de la cabeza femoral, más evidente a partir de la 4.a semana. No objetivamos cambios en la altura del pilar lateral ni en la distancia artículo-trocantérea. El grupo experimental macroscópicamente demostró hipertrofia y aplanamiento progresivo de la cabeza. A las 4 semanas de la cirugía aparecieron zonas de necrosis en el cartílago articular, una médula ósea más densa y menor altura de la fisis. Los vasos estaban engrosados por proliferación de la capa media y de la adventicia. A las 8 semanas encontramos una fibrosis subcondral, con un cartílago articular irregular, adelgazado y desvitalizado, y áreas de angiogénesis con grasa en el hueso subcondral. A las 12 semanas apreciamos el cierre de la fisis, áreas condrales en las trabéculas óseas y células adiposas en la médula diafisaria.
ConclusiónAunque los cambios histológicos son compatibles con necrosis de la cabeza femoral, las pruebas de imagen obtenidas no se asemejan a la enfermedad de Perthes, por lo que desaconsejamos este modelo experimental para el estudio de esta entidad.
The clinical relevance of Leg-Calvé-Perthes disease (LCPD) is based on the progressive deformity of the immature femoral head, which causes premature degeneration of the joint.1 Several works have discussed the etiopathogenesis of idiopathic avascular necrosis in humans and the different etiopathological mechanisms involved, such as ischemia secondary to fatty microemboli from the bone marrow,2 intravascular coagulation3 and retrograde embolization of bone marrow fat.4 The theory of accumulated cellular pressure as the cause of ischemic phenomena5 has also been reported. This theory postulates that the cells would be exposed to multiple pressures and aggressions which would result in cell death. Thus, osteonecrosis would be caused by a disease of bone cells or mesenchymal stem cells (MSCs), suggesting that the cause is not only vascular. In support of this theory, it has been observed that the degree of activity and the number of MSCs in patients with osteonecrosis of the femoral head is below normal,6 as is also the case with the proliferation capacity of osteoblastic cells.7
Necrotic lesions are characterized by the apoptosis of osteocytes and lining cells of the trabecular bone in the femoral head, which can distantly affect the proximal femur bone.5 Kim8 showed the mechanism of cell death secondary to the process of ischemic injury of the femoral head in pigs.
The regenerative process which follows ischemic necrosis takes place by deposition of new bone layers from the necrotic bone itself. Studies conducted on human femoral heads6,9,10 and an experimental study on rabbits11 have shown that tissue regeneration takes place through the penetration of MSCs and capillaries into the necrotic bone from areas which are not affected by ischemia. These studies also observed that MSCs located near the surface of the necrotic bone were differentiated into osteoblasts, which, in turn, formed bone by apposition on the surface of the trabecular necrotic bone and eventually coated the surface of the femoral head. Subsequently, the central necrotic area was resorbed by the osteoclasts and replaced by newly formed bone. This explains the changes observed during necrosis of the femoral head, such as the widening of the trabeculae, increase of bone mass per volume and density increase in the areas being repaired. The newly formed bone has less mechanical rigidity and plasticity, so the axial load and repeated trauma cause a progressive flattening and deformity of the femoral head. Koob et al.12 observed that the alteration of the mechanical properties of epiphyseal bone and cartilage were associated with pathogenesis of the deformity of the femoral head.
The etiopathogenic mechanism of LCPD is unknown, and its anatomopathological evolution is difficult to assess clinically. Despite employing four-legged animals which do not much resemble humans, experimental models are very useful. Various animal models, such as rabbits,11 dogs,13,14 goats,15,16 and, particularly, pigs,14,17–22 have been used for the experimental study of LCPD. However, the macroscopic and radiographic results obtained have not been able to explain the pathogenesis of femoral head deformity. Works using lamb models are scarce despite being simple to obtain, work with and manage. Although it has been used in works studying the development of femoral head pathology, these works have never been conducted by isolated alteration of vascularization.23–25 Our hypothesis is that the alteration of isolated vascularization causes an alteration in the proximal end of the femur similar to LCPD. The objective of this work was to establish a simple, reproducible and safe experimental lamb model enabling us to understand the development of ischemic vascular necrosis in lamb hips. This is the first time that a lamb experimental model has been used in the study of osteonecrosis of the femoral head in immature skeletons by devascularization, in an attempt to reproduce the conditions associated with Perthes disease.
Material and methodologyWe conducted a comparative experimental study, assessing morphological and imaging tests in lambs. The study was approved by the Ethics Committee of the Foundation for Biomedical Research of our hospital. We used 15 lambs (10 males and 5 females) of the Manchego breed. We established 2 groups: a control group with 7 animals of 4 weeks of age in which no experimental techniques were performed, and an experimental group with 8 animals of 4 weeks of age in which we caused ischemia of the proximal end of the femur through a surgical intervention. The animals were sacrificed sequentially, 2 by 2 (at 4, 8 and 12 weeks).
Surgical techniqueThe surgery was performed under general anesthesia and antibiotic prophylaxis. The animals were premedicated with diazepam (0.5mg/kg), whilst anesthetic induction employed ketamine (2mg/kg) and propofol (0.5mg/kg). Anesthesia was maintained using isoflurane in 100% oxygen. The animals were placed in the left lateral decubitus position in order to carry out a posterolateral approach to the right hip by a 10cm longitudinal incision. The greater trochanter was located and the rotator and gluteal musculature were disinserted so as to access the hip joint capsule. Partial capsulotomy was performed, with cerclage of the femoral neck by double ligation (PremiCron No. 2, Aesculap, Tuttlingen, Germany). Next, the round ligament was sectioned, subluxating the femoral head. Lastly, the capsule was closed and the musculature was reinserted with layered transosseous sutures. The mean duration of surgery was 22min (range: 15–30min).
PostoperativeNo immobilization was performed after the intervention and the animals were allowed to walk freely within their boxes with ad libitum feeding. Some animals suffered complications: ischemic necrosis areas in the periarticular musculature, 1 superficial abscess in the surgical wound that extended into the joint capsule and, due to the section of the round ligament, dislocation of the hip in 60% of the animals.
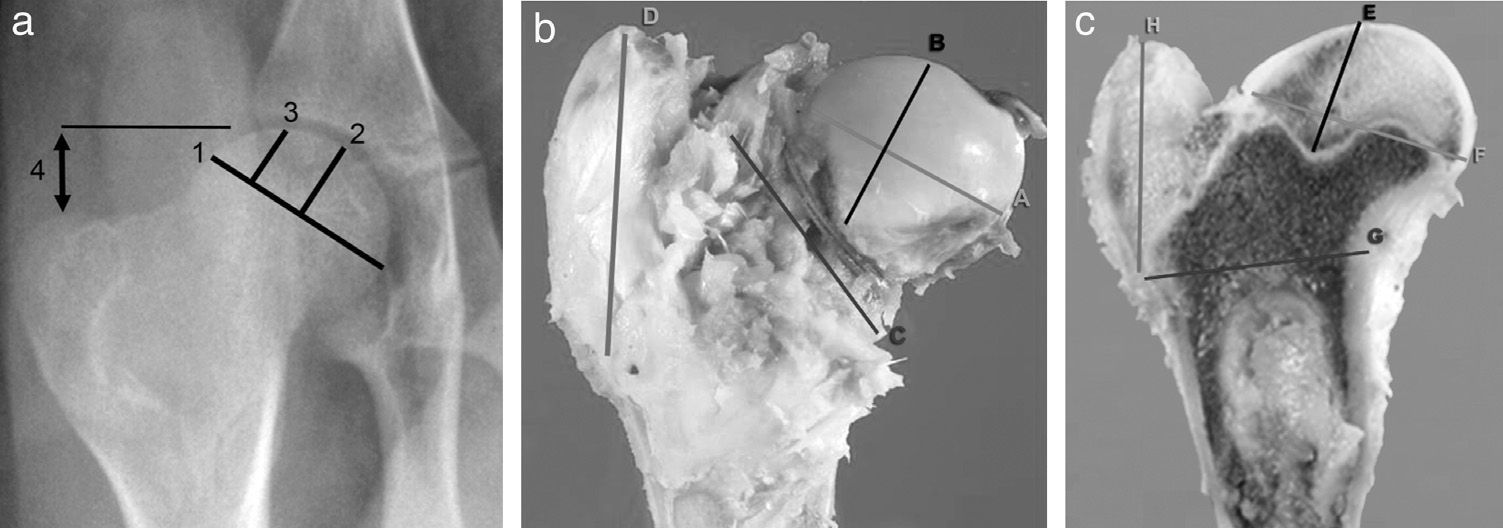
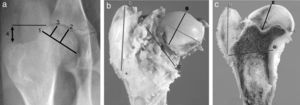
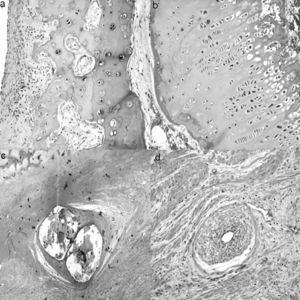
Imaging testsImaging tests of all animals were performed serially each week. We employed conventional computed radiography (35cm×45cm Agfa CR 30-X, AGFA Healthcare, Barcelona), with 2 radiographic projections being obtained (anteroposterior and axial of both hips). The following radiographic measurements were assessed by comparison with the contralateral hip (Fig. 1a):
- 1.
Width of the femoral head: obtained by measuring the length of the greater diameter of the femoral head joining the ends defined by the growth physis.
- 2.
Central pillar height: measurement of the perpendicular line to the growth physis in its middle area up to the upper edge of the head.
- 3.
Lateral pillar height: measurement of the perpendicular line to the growth physis at the junction of the middle third with the lateral third up to the upper edge of the head.
- 4.
Joint-trochanter distance: measurement of the vertical line connecting the proximal part of the epiphysis and the tip of the greater trochanter.26 A greater growth of the greater trochanter is considered when the distance is reduced by 20% or more compared to the contralateral side.
- 5.
Subluxation of the femoral head: assessed by the acetabulum-head index.27
We also conducted a study using magnetic resonance imaging (MRI) (Panorama 0.23 T, Philips Medical Systems, Eindhoven, Netherlands) for an accurate assessment of the bone edema and necrosis phenomena. No measurements were recorded in this study, as it was only used for morphological assessment.
SacrificeSacrifices were performed sequentially at 4, 8 and 12 weeks after surgery, following induction of sodium pentobarbital and subsequent intracardiac injection of 10 cc of T61 (Intervet Schering-Plough Animal Health, Kenilworth, NJ). The animals underwent a complete and ordered necropsy to determine the possible complications, accurately extracting the proximal section of the intervened femur, which was analyzed macroscopically, measured and sectioned sagittally. In order to standardize the study, we conducted 4 measurements of the head and the greater trochanter with a caliper, noting: (A) width of the femoral head by measuring the major diameter, joining the ends of the physis; (B) height of the femoral head by measuring the line perpendicular to the physis in its middle area; (C) femoral neck diameter, obtaining femoral neck diameter at its widest part, and (D) trochanter height, measuring the distance from the proximal physis of the metaphysis to the tip of the trochanter (Fig. 1b).
Once the part was fixed, we sectioned the femur head sagittally to record 4 measurements with the caliper, noting: (E) the distance between the most proximal part of the head and the deepest area of the growth cartilage; (F) the distance between the most cranial and most caudal points of the physis; (G) the distance from the most caudal point of the physis and the inner cortex above the lesser trochanter, and (H) the perpendicular distance between the most caudal point of the physis and the most cranial point of the greater trochanter (Fig. 1c). To carry out the histopathological study of the samples, the medial section of the femoral head was fixed in commercial 10% buffered formalin (Panreac©, Barcelona) and stabilized with methanol at pH=7 for 12–24h, at room temperature.
Next, the femoral head was divided in half and 1 part was decalcified for 5–7 days with Osteodec (Bioptica Milano Spa. 05-M03005), whilst the other part was preserved in 10% formalin.
We obtained a section of the head and trochanter of the femur which were included in synthetic paraffin (Casa Alvarez Histo-comp, ref. CA-09-5658), with a melting point of 56°C, using a Leica© ASP 300 automatic tissue processor with an automatic program to exchange alcohols of increasing graduation and histo-clear. The blocks were formed in a Leica© EG1140H block-forming unit and a Leica© EG1130 cold plate. We obtained 3–4μm thick sections with a rotary microtome (Leica© RM2155 model). The sections were stained with hematoxylin–eosin in a Leica© SP4040 automatic stainer.
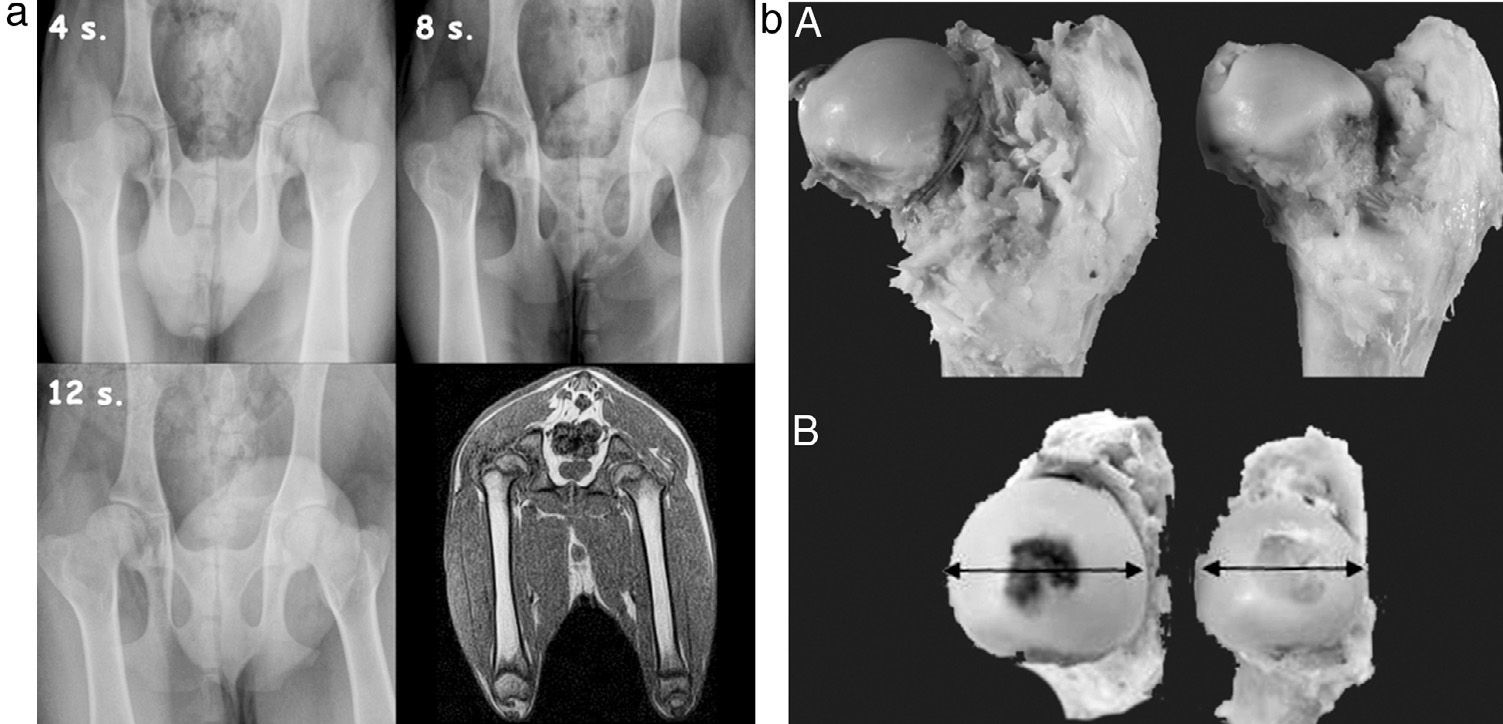
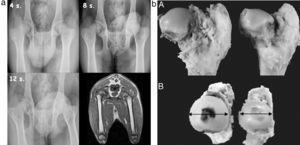
ResultsImaging testsConventional radiography indicated an increase in the density of the femoral head from the second postoperative week, compatible with necrosis phenomena (Fig. 2a). We also observed a decrease in the height of the central pillar and an increase in the width of the femoral head, which was more evident from the 4th week. We did not observe any changes in the height of the lateral pillar and the joint-trochanteric distance (Fig. 2b).
(a) Sequential radiographic study at 4, 8 and 12 postoperative weeks. An enlargement and flattening of the femoral head can be observed, becoming more evident at 12 weeks. The MRI scan shows a flattening and lateral subluxation of the femoral head. (b) Macroscopically, we observed hypertrophy and a progressive flattening of the femoral head in the experimental group relative to the control group, from week 4 to week 12 after the intervention.
MRI studies showed a flattening of the head and widening of the neck. In addition, a lateral subluxation of the femoral head was also observed.
Pathological anatomyMacroscopically, in the experimental group we observed hypertrophy (measurement B) and a progressive flattening (measurement A) of the femoral head relative to the control group from the 4th to the 12th postoperative weeks (Fig. 2b). In the sagittal section we appreciated a loss of thickness of the joint cartilage, an irregular physeal growth line and a change in the reddish color of the trabecular bone due to a whitish fibrous tissue with internal ossification nuclei.
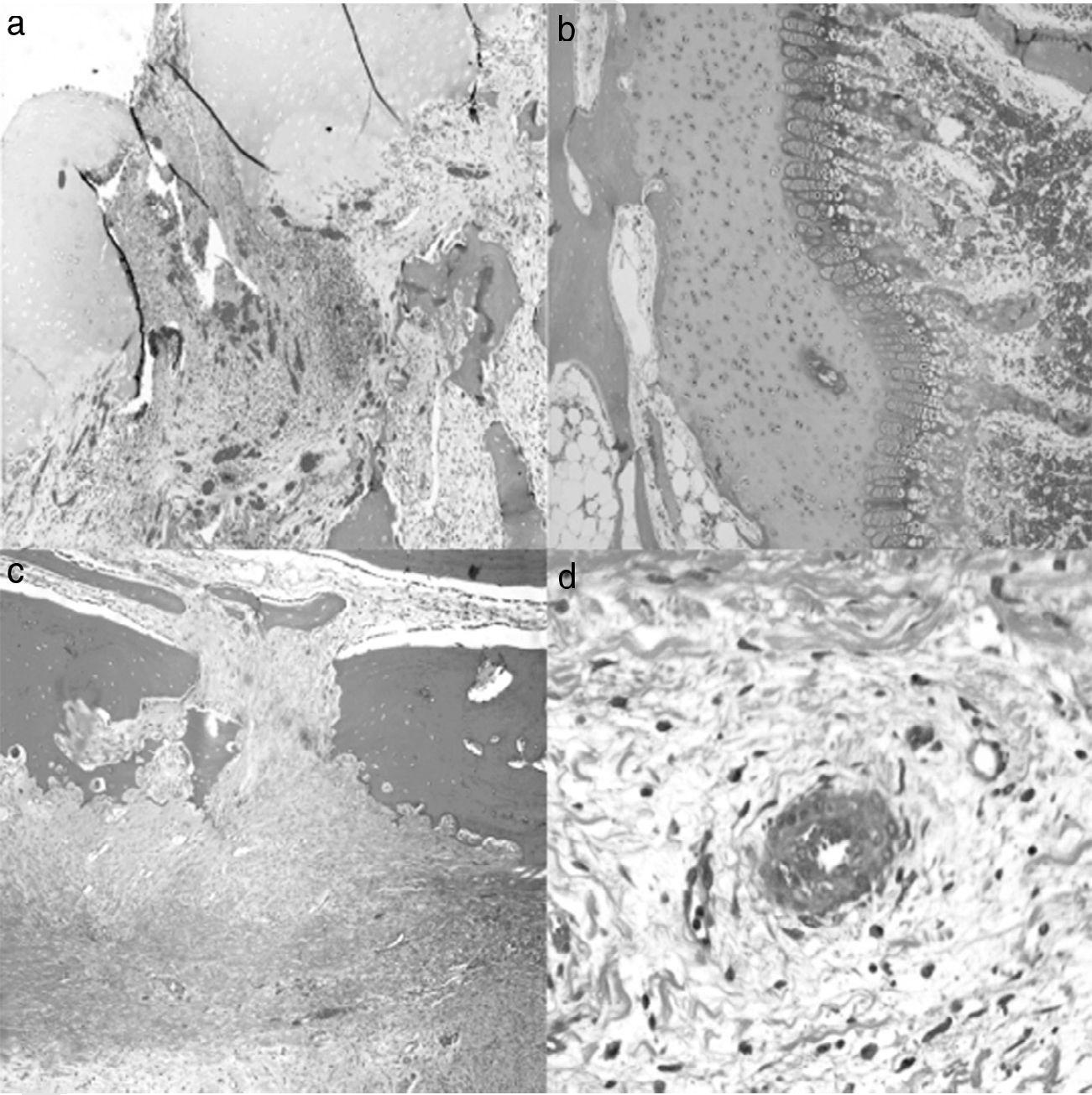
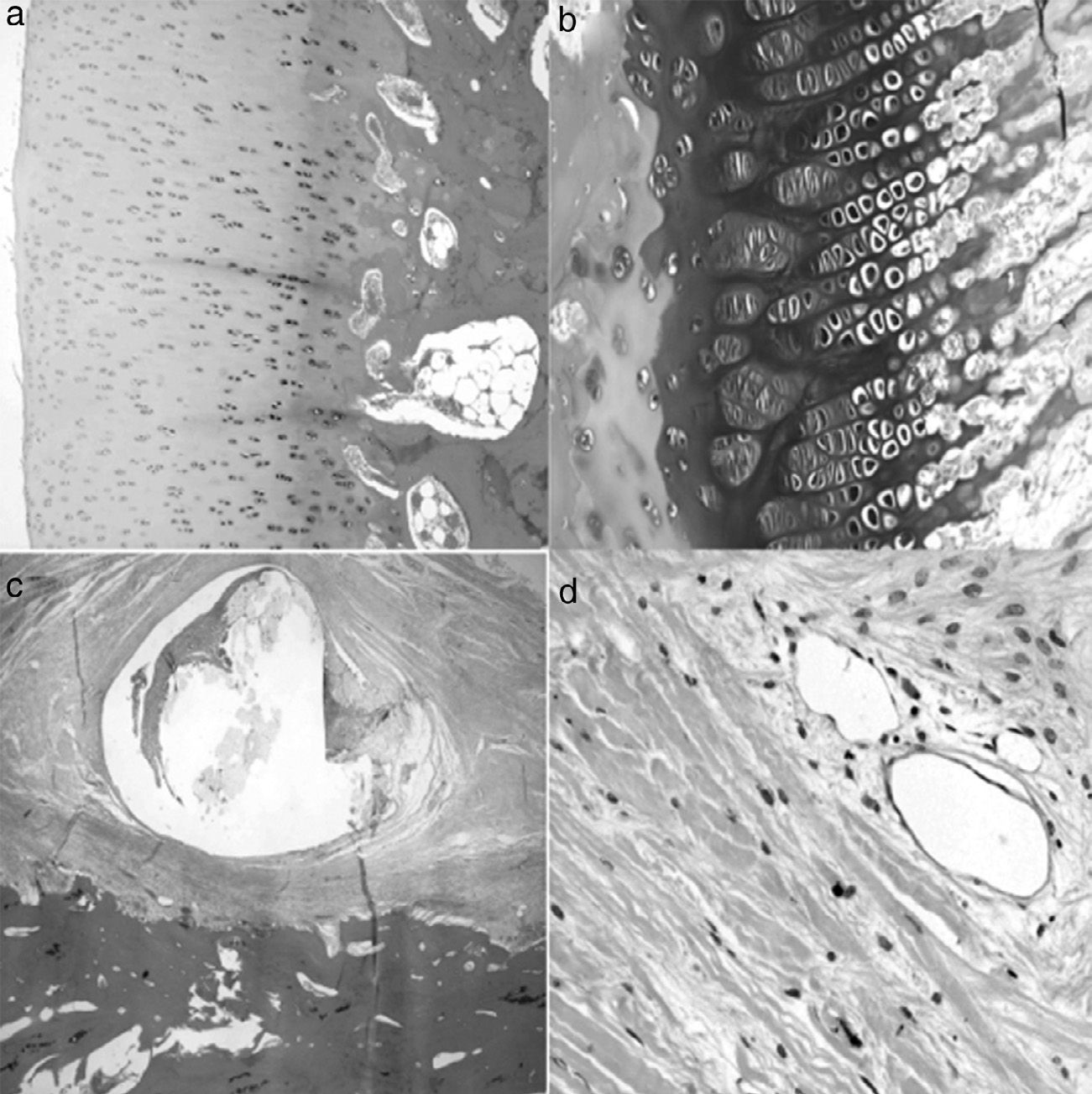
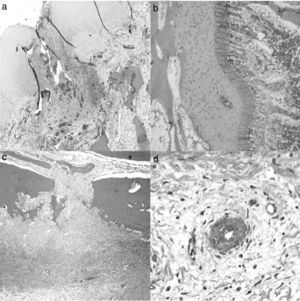
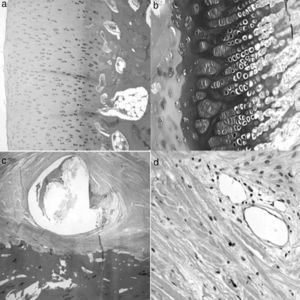
A histological examination of the parts obtained after 4 weeks of surgery revealed small areas of necrosis in the articular cartilage, as well as a denser bone marrow and shorter physeal cartilage. We also observed a periosteal reaction with greater osteoclastic activity in the cortical space and presence of bridges between the periosteum and diaphysis which crossed the cortex. We also observed neovascularization processes in the ligation area. The vessels presented a marked thickening characterized by a proliferation of the middle and adventitial layers compatible with external plexiform hyperplasia. The trochanter of the physeal cartilage presented areas of premature ossification (Fig. 3).
Histology at 4 weeks after surgery. (a) The superficial articular cartilage showed small foci of necrosis with an associated inflammatory reaction (H&E, ×2). (b) The physis appeared slightly increased in relation to the control animals and with a narrower area of serial cartilage (H&E, ×4). (c) Marked periosteal reaction with more osteoclastic activity in the cortical border with bone remodeling. Bony bridges between the periosteal and endosteal zones traversing the cortical area of the bone (H&E, ×4). (d) Neovascularization can be observed associated to the ligation; the vessels show thickening of the wall by external plexiform hyperplasia (H&E, ×10).
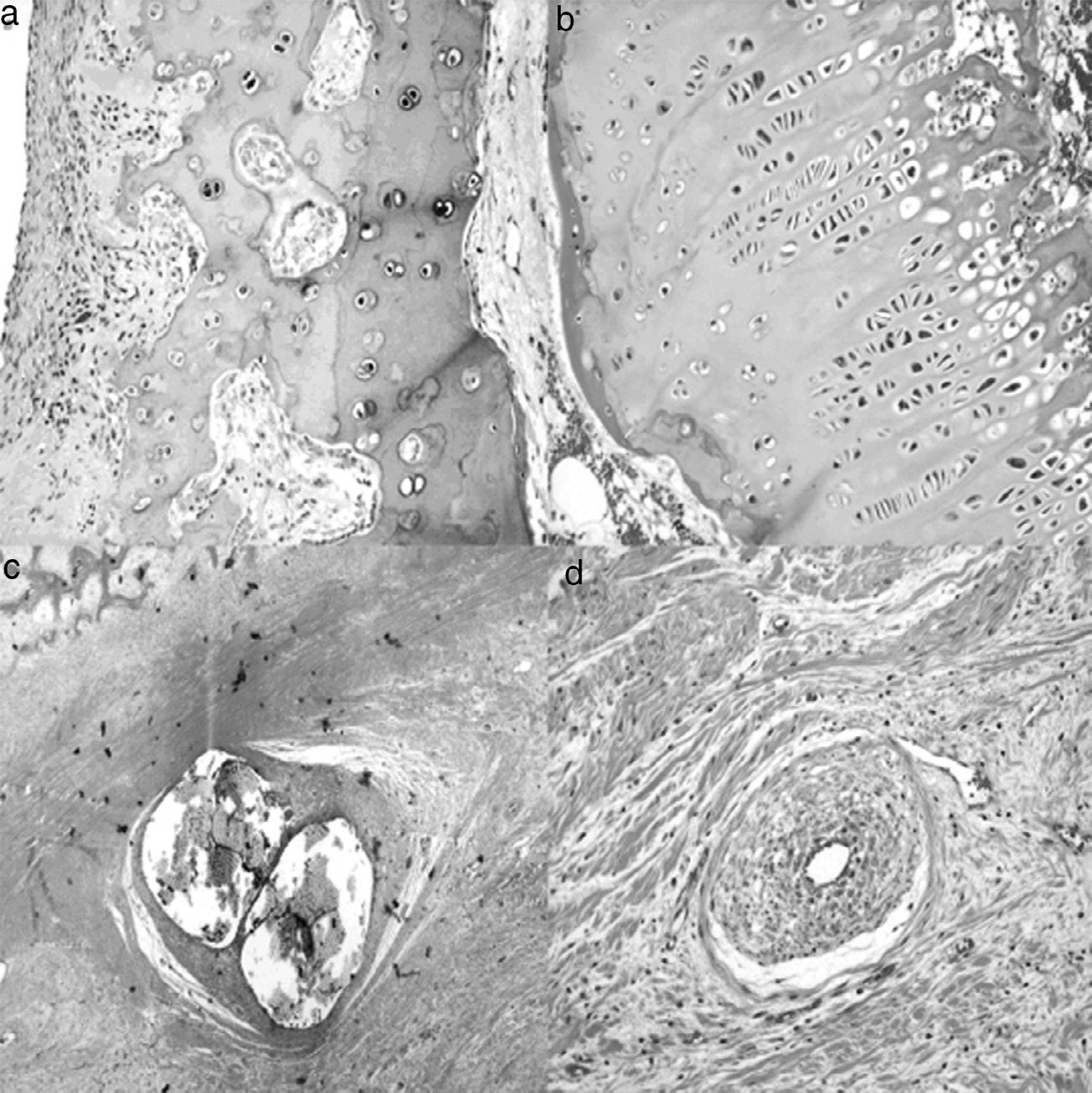
At 8 weeks evolution, the animals presented subchondral fibrosis, with an irregular, devitalized and thin joint cartilage, without any observations of chondroblastic activity. Furthermore, areas of angiogenesis with more fatty tissue appeared next to the subchondral bone. The physis continued to present loss of color and an irregular appearance. Closer to the diaphysis, the medulla presented an increased activity and cellularity, whilst the trochanter presented less development than the control side with closure of the growth cartilage. The part in contact with the ligation presented areas of fibrosis with bone remodeling and areas of neovascularization (Fig. 4).
Histology at 8 weeks after surgery. (a) There is a marked subchondral fibrosis in the articular cartilage causing an irregular articular cartilage with loss of thickness and without chondroblastic activity (H&E, ×10). (b) The physis shows premature closure, is irregular and without chondroblastic activity (H&E, ×10). (c) Marked periosteal reaction in the area corresponding to the double ligation, with fibrosis, bone remodeling and large areas of angiogenesis (H&E, ×2). (d) Areas of neovascularization associated with fibrosis; medium-caliber vessels with marked plexiform hyperplasia of the wall and recanalization thereof (H&E, ×10).
In the samples taken at 12 weeks evolution we appreciated an articular cartilage similar to that of the previous group, closure of the growth cartilage, increase in the areas of cartilage in the bone trabeculae and an increased number of fat cells in the diaphyseal bone marrow. The trochanteric physeal cartilage was completely closed. Moreover, the periosteum in contact with the ligation presented an increase in fibrous tissue with blood vessels in its deep layer (Fig. 5).
Histology at 12 weeks after the intervention. (a) No significant differences were observed in the histological structure of articular cartilage between operated and control animals (H&E, ×4). (b) The area of physeal proliferation and differentiation was reduced in control animals, due to premature ossification thereof (H&E, ×10). (c) The periosteum presents a fibrotic reaction around the suture which does not collapse the blood vessels of the deep layer (H&E, ×2). (d) No vascular changes in the areas of periosteal reaction (H&E, ×10).
Since the size and the mechanical properties of their skeleton are comparable to those of humans, sheep can be useful animals for experimental work related to the pathophysiology of the musculoskeletal system. Sheep present the haversian bone remodeling process, are genetically close to man, are docile, inexpensive to maintain, available in good numbers in all areas of the world and, lastly, present fewer ethical constraints that most domestic pets.12,28,29 Mazoochian et al.30 used the finite element method to compare human and sheep hips, concluding that sheep represent a suitable experimental model to conduct preclinical studies of the hip.
One of the main problems in experimental studies of LCPD is matching the age of the animals with humans in development and, obviously, quadruped load. Experimental works regarding LCPD are not very common in recent years and differ notably according to the chosen animal and necrosis induction technique. Bone necrosis of the femoral head in animal models is not related to that of humans; evolution in pigs is different to that in humans.11 In adult rabbit models with femoral ischemic necrosis, vascular invasion of the medullary spaces of the “dead” portion of the femoral head takes place by proliferating mesenchymal cells and the capillaries of the adjacent living metaphysis; these MSCs can differentiate into the osteoblast lineage and form new bone by apposition, replacing the necrotic bone.31
Various authors have employed aggressive methods to achieve bone necrosis. Crawford et al.16 induced necrosis in goats caused by ablation of the physis with electrocautery. Jin et al.32 dislocated the femoral head and applied liquid nitrogen, as did Velez et al.24,25 in mature sheep, using liquid nitrogen and subsequently ligating the cephalic vessels. In rabbits, Aimaiti et al.33 injected intramuscular methylprednisolone and used electrocoagulation of the femoral head vessels, obtaining necrosis at 8 weeks.
Simank et al.34 and Manggold et al.35 injected ethanol in an ovine model of bone necrosis of the proximal end of the femur and found the same bone alterations observed clinically, including an intact articular cartilage, preserved proximal trabecular structure of the femur and maintained microcirculation; they observed greater damage in the femoral head and noted that bone induction began around 12 weeks after injection. Furthermore, they compared the size of the bone and demonstrated that it was similar to developing human femurs. TajraFeitosa et al.23 used the same methodology by infiltration of pure ethanol into the femoral head. Six weeks after the induction of necrosis they carried out drillings in the control group and bone marrow infiltration in the experimental group, observing that bone regeneration was faster than in humans, and that this was better than in the experimental group after injection of mesenchymal cells.
We have employed an avascular experimental model causing a progressive ischemia without directly damaging the bone or cartilage in order to create a necrosis similar to that observed in patients with LCPD. The most commonly cited works in the literature employing this method used skeletally immature pigs. Rowe et al.36,37 reported the experimental pig model for studying the pathogenesis of LCPD. They noted that the most evident changes took place in the early stages of vascular deprivation. These results are similar to those obtained in our work, with the greatest changes being observed at 4 weeks and nearly full morphological restoration at 12 weeks. Kim et al.38–40 described a method to generate ischemic necrosis of the femoral head in developing pigs, aged between 5 and 6 weeks and with a weight of 5–6kg, through ligation around the femoral neck and section of the round ligament. Then, using a needle, they pierced from the articular cartilage to the subchondral bone, confirming the lack of bleeding for ischemia. Our ischemic model was carried out through a double ligation around the femoral neck and complete section of the round ligament. The absence of severe alterations with fragmentation of the femoral head with acetabular inconsistency could be caused by the fact that lambs are highly resistant animals and are able to move very easily after surgery. Section of the round ligament led to the dislocation of the hip in many cases, reducing load transmission through the proximal end of the femur and thus preventing the aggravation of injuries. The dislocation caused the animals to become prostrate, facilitating wound infections with abscesses that occasionally caused suppurative arthritis. In addition, due to the proposed model, we observed some cases of necrosis in the disinserted muscles. Moreover, section of the round ligament was not always achieved in full as the femoral head could not be dislocated. This could explain why an intense necrosis was not achieved in some cases, although we must not forget that, at this age, the blood supply in lambs through this structure only represents 10% of the total blood supply reaching the proximal end of the femur.41
In children, the proximal femoral physis acts as a barrier for the metaphyseal vessels, so that the vascularization of the proximal femoral epiphysis depends exclusively on the extraosseous retinacular vessels.42 The retinacular vessels come from the vascular ring around the base of the neck, ascend throughout the surface of the neck and enter peripherally through the base of the proximal femoral epiphysis, leaving the head vulnerable to ischemia.
Revascularization also depends on the peripheral retinacular vessels, and requires a long time to reach the central portion of the femoral head following an ischemic phenomenon. Therefore, perforations are considered to be a good choice to accelerate the revascularization process, establishing channels for the passage of vessels through the physeal barrier.43 However, these perforations may also damage the growth cartilage, so this procedure is only recommended in elderly patients with poor regenerative capacity and, therefore, with little risk of developmental alterations,44 although it has been reported that perforations under 7% of the physis do not affect subsequent growth.31,44
An osteoclastic resorption process has been observed to predominate during the repair process, albeit without achieving bone formation.38–40,45,46 Moreover, the keys of the necrotic process include early bone loss, absence of bone formation and persistence of vessel-rich fibrous tissue in the area of bone resorption. These changes cause a progressive collapse of the femoral head. Meanwhile, Gong et al.44 described bone formation by endochondral ossification in the growth cartilage surrounding the secondary ossification nucleus, without new bone apposition being observed in the central medullar cavity of the proximal femoral epiphysis.
The findings obtained in our study show significant changes in the proximal end of the femur of the lambs. Ischemia caused by the double ligation in immature skeletons affected both bone and cartilage, whilst also producing histologically visible morphological alterations. Our objective was the development of an experimental model of ischemic necrosis in lambs, as we considered these to be accessible animals, easy to handle and maintain.
Our model has allowed us to observe morphological changes, with hypertrophy of the femoral head and thickening of the neck, when compared with the control group. From the 4th week after surgery we also observed the onset of changes in the ossification center, becoming more evident at 8 weeks and acquiring a whitish appearance in relation to the control group. After this point, the changes were not so evident.
The histological study showed signs of ischemia from the 4th week after surgery, being more evident on the 8th week, with an increase of fibrosis and larger areas of endochondral ossification, presenting an irregular joint cartilage, thinner and with no chondroblastic activity. This process extended to the ossification nucleus, presenting a whitish macroscopic appearance and a greater degree of ossification. There was an increase of fat cells in the marrow, both epiphyseal and metaphyseal, and an early closure of the physis. No signs of necrosis were observed at any point in the areas where the ligations were applied. We observed an increase of fibrosis in the periosteum with multiple areas of angiogenesis, without reaching a collapse of the vessels, which presented proliferation of the middle and adventitial layers with external plexiform hyperplasia.
The changes observed in the articular cartilage were not obvious. The articular cartilage lacks vessels and the mechanism by which nutrients reach the cartilage has been discussed for a long time.47 Some authors believe that nutrients reach the cartilage solely through the synovial fluid,48–50 whilst other studies indicate that there is also a vascular supply from the proximal metaphysis of the femur which nourishes the articular cartilage. This fact has been demonstrated in rabbits51–53 and lambs.54
Kalhor et al.41 worked with 35 hips from 28 fresh adult human cadavers and observed that the internal circumflex artery (ICA) offered the main blood supply to the proximal end of the femur in 29 cases, whilst the inferior gluteal artery did so in 6 cases. In these specimens, the artery of the round ligament had no contribution, whilst the ICA was observed to consistently lead to a branch with a smaller caliber, the inferior-medial genicular artery. The number and caliber of the superior retinacular vessels revealed their predominance in the vascularization of the femoral head, although the inferior retinacular arteries were always present. To reduce the risk of ischemic necrosis, branches of the ICA and the inferior gluteal artery pass through the space between the quadratus femoris and piriformis muscles and must be protected during surgery. Since all intracapsular vessels penetrate near the distal insertions, distal capsulotomy involves a much greater risk of causing necrosis than proximal capsulotomy. This arterial distribution leads us to believe that it is very difficult to achieve complete ischemia, and that we possibly had a transitional process of hypertension of the area caused by compression of the suture. The radiological changes observed were not similar to those obtained in humans with phases of necrosis, fragmentation, reossification and remodeling, so we consider that lambs are not a good experimental model for the study of Leg-Calvé-Perthes disease.
Level of evidenceLevel of evidence I.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation adhered to the ethical guidelines of the Committee on Responsible Human Experimentation, as well as the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols of their workplace on the publication of patient data and that all patients included in the study received sufficient information and gave their written informed consent to participate in the study.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Antonio and Mauricio Riosalido Award 2013 for works related to Orthopedic and Traumatology surgery.
Please cite this article as: Martínez-Álvarez S, Epeldegui-Torre T, Manso-Díaz G, Rodríguez-Bertos A, Forriol F. Inducción experimental de la enfermedad de Perthes en corderos. Rev Esp Cir Ortop Traumatol. 2014;58:68–77.