The aim of this study was to study the viability and multipotential capacity of adipose derived mesenchymal stem cells, after being labelled with superparamagnetic iron oxide particles. The final aim is to monitor stem cells after being implanted by cellular therapy and tissue engineering techniques in the field of Orthopaedics and Traumatology.
MethodTen biopsies of adipose tissue were processed in order to isolate adipose derived mesenchymal stem cells, which were cultured under standard in vitro culture conditions until pre-confluent state. Cells in 1P and 2P were labelled with superparamagnetic iron oxide particles (SPIO), using protamine sulphate as a transfection agent. After confirming labelling efficiency with Prussian blue staining, we studied the labelling effect on the growth and multipotency of stem cells by analysing their in vitro growth curves and their capacity to differentiate into adipogenic, osteogenic and chondrogenic cell lines. We also tried to view the labelled stem cells using 3T magnetic resonance imaging.
Results and conclusionAdipose derived stem cells labelled with SPIO showed a proliferation and differentiation capacity similar to control stem cells. These cells were visible on MR images after having implanted in a bone piece. Therefore, in vitro cell labelling of adipose derived mesenchymal stem cells with SPIO could be useful for in vivo cell tracking by MRI in bone tissue engineering.
El objetivo principal de este estudio es comprobar la inocuidad del marcaje de células madre mesenquimales derivadas de tejido adiposo con partículas de hierro superparamagnético (SPIO) en términos de proliferación y multipotencialidad celular. El objetivo final es posibilitar el seguimiento celular in vivo mediante resonancia magnética con vistas a una posible aplicación en técnicas de terapia celular e ingeniería tisular en el área de la Cirugía Ortopédica y Traumatología.
MétodoMediante protocolos estandarizados se aislaron y cultivaron células mesenquimales procedentes de 10 muestras de tejido graso. En pases 1P y 2P, dichas células fueron marcadas con SPIO, empleando sulfato de protamina como agente transfectante. Tras comprobar la eficiencia del marcaje mediante tinción con azul de Prusia, se analizó el efecto del mismo sobre el crecimiento y la multipotencialidad de las poblaciones celulares mediante el estudio de su crecimiento in vitro y su capacidad de diferenciación hacia diferentes estirpes celulares. Finalmente, se estudió la posibilidad de localizar las poblaciones celulares marcadas mediante resonancia magnética de 3 Teslas.
Resultados y conclusiónLas poblaciones celulares aisladas a partir de las muestras de grasa subcutánea marcadas con SPIO, muestran patrones de crecimiento y multipotencialidad similares a las poblaciones control. Dichas células son visibles mediante imagen por resonancia magnética una vez implantadas en una pieza ósea de control. Los resultados obtenidos en este estudio indican que esta metodología podría aplicarse a la monitorización de las células implantadas en defectos óseos.
In recent years, cell therapy and tissue engineering based on mesenchymal stem cells have acquired great importance in the development of new therapeutic strategies aimed at the treatment of several pathologies of the skeletal system.1 However, there are still many unanswered questions accompanying this kind of technique. One of the issues most disturbing the researchers and clinicians is that referring to the biodistribution of these cells after implantation.2–4
The study of the location and distribution of implanted mesenchymal stem cells is essential to specify the safety and efficacy of these techniques. In addition, ensuring the feasibility and permanence of the cells at the implant site is fundamental to guarantee their possible therapeutic effect.2–4 With these aims, several methods have been designed for cell marking location, including labelling with fluorochromes such as fluorescent green protein or transfection with plasmids coding for fluorescent proteins.2,5 Other techniques use radioisotopes5 or implant stem cells from a donor of a different gender or species from the recipient, using the Y chromosome as a “natural” marker.6 These and other methods, although they have been useful in the location of implanted cell populations, present certain limitations, such as the need to take tissue biopsies for subsequent analysis in view of the impossibility of tracking them in vivo.
Imaging technology based on magnetic resonance (MRI), on the other hand, allows highly sophisticated in vivo images to be obtained in real time. In addition, it is a non-invasive sensitive technique with sufficient specificity that can be carried out in a relatively short space of time.4,5 For all these reasons, it is a good option when designing in vivo tracking techniques that can be applied in humans.
For MRI tracking, the cells to be implanted must first be marked with biocompatible magnetic particles, which has previously been done using particles of superparamagnetic iron oxide (SPIO) experimentally in the areas of cardiac surgery,5 neurosurgery7 and in intervertebral pathology.8
Considering that the paramagnetic marking technique for cell tracking has not been applied to the skeletal system, we have studied its innocuousness in populations of adult adipose-derived mesenchymal stem cells (ADMSCs), as these are easy to obtain and are widely available, at the same time as we have analyzed the possibility of locating, using MRI, the marked cell population after they have been implanted in bone tissue.
Materials and MethodsObtaining Adipose Tissue SamplesThe samples of adipose tissue were obtained from adult sheep of the Asaaf breed that had been sacrificed after use in experimental protocols that did not interfere with the present study. In the operating room and under strict conditions of asepsis, between 5 and 10g of adipose tissue were collected from the tails of 10 animals. The samples were maintained for 6–8h at room temperature in a sterilizing solution made up of Dulbecco's modified eagle's medium (DMEM-PAA Laboratories, GMBH) supplemented by antibiotics and anti-fungal agents (vancomycin (Normon), tobramycin (Braun), sulphametoxazol (Almirall) and amphotericin B (Sigma)).
Obtaining Blood Samples and Preparation of MatricesFor the subsequent chondrogenic differentiation of the cells in a fibrin matrix, during the surgery to extract the adipose tissue, ovine blood samples were also collected in blood extraction tubes containing sodium citrate as an anti-clotting agent. These tubes were subjected to a 700×g centrifuge cycle. The plasma fraction was then collected and preserved at −20°C until used.
For the formation of the matrix, the plasma fraction was thawed and added to culture medium, calcium chloride and tranexamic acid together with the cell suspension, then kept at 37°C until polymerized.
Isolation and Culture of ADMSCsAfter the corresponding sterilization period and once in the cell culture laboratory, the samples of adipose tissue were processed to obtain ADMSCs. Briefly, the procedure used consisted in several washes with a phosphate buffered saline solution (PBS), followed by mechanical digestion and, finally, enzymatic digestion with type I collagenase (Sigma) 2mg/mL in DMEM at 37°C until the tissue is completely digested (20–24h). The samples thus obtained were centrifuged at 720×g for 10min and the stromal vascular fraction (SVF) was re-suspended in standard ADMSC culture medium, consisting in DMEM supplemented with 10% foetal bovine serum (FBS, Linus), 1% penicillin/streptomycin (PAA Lab.) and 1% glutamine (PAA Lab.), supplemented with 2.5ng/mL of basic fibroblastic growth factor (FGFb, Sigma). The cell population was quantified using the trypan blue method in a Neuvawer chamber. Finally, the cell suspensions were seeded on 2-well culture plates (2cm2) (Becton Dickinson) at a density of 30,000cells/cm2 to constitute the primary culture (0P) and were incubated at 37°C and 5% CO2 in an atmosphere with 95% humidity. After 48h had elapsed, the cultures were subjected to rinsing with PBS to eliminate the erythrocyte component, leaving in the wells only those cells capable of adhering to them (ADMSCs).
The cultured cells were observed every day and the culture medium replaced every 2–3 days. When the cells were found to be in a preconfluent state, they were trypsinized and subcultured on 24-well plates. Briefly, the medium was eliminated; the well was rinsed with DMEM and trypsin/EDTA (0.05/0.02%) (PAA Lab.) was added and left to act at 37°C for 2min. The cells were recovered, neutralized and centrifuged at 720×g.
For subsequent studies, the secondary cultures (1P and 2P) were seeded at a density of 5000cells/cm2 on surfaces of 2cm2.
Labelling of Cell Populations with SPIOOnce the primary cultures reached a preconfluent state and prior to their subculture, the cell populations were labelled using SPIO (Endorem®, Guerbet Laboratories). For this purpose, they were incubated with a labelling solution consisting in 50μL/mL of Endorem® in DMEM and 6mg/mL of protamine sulphate, used as a transfection agent to facilitate the internalization of the iron particles by the cultured cells.9 Following a 12-h incubation period at 37°C, the wells were rinsed with PBS and 10μ/mL of heparin to eliminate the labelling remnants that had not penetrated into the cells. In order to verify the effectiveness of the labelling, one well in each experimental case was subjected to Prussian blue staining and, after corresponding fixation with 4% paraformaldehyde at 4 C for 30min, a solution of 10% potassium ferrocyanide in 20% hydrochloric acid was added for 30min. After contrasting the cell nuclei with the Nuclear Fast Red agent, the presence of blue particles was observed under phase contrast microscopy inside the cells subjected to the SPIO labelling procedure.
Innocuousness of SPIO LabellingIn order to confirm the innocuousness of the ferrous labelling in in vitro ADMSCs, the growth patterns and differentiation of the labelled cell populations were studied and compared with those obtained in the control cultures (not labelled). These studies were performed in the 1P and 2P stages of each of the samples analyzed.
In order to study the proliferation pattern, growth curves were established in wells with a surface area of 2cm2 at an initial density of 5000cells/cm2 and were stored under standard culture conditions. For 10 days, the wells were trypsinized in duplicate every 48h and the number of cells was evaluated using the Trypan blue method. For the statistical data processing of cell proliferation in the cultures labelled with SPIO and the control cultures, SPSS v. 17.0 software was used.
To study the multipotency of the cell populations under study, the procedure continued with the adipogenic, osteogenic and chondrogenic induction of SPIO-labelled ADMSCs and the control populations. For this purpose, cells were cultivated on standard culture medium supplemented with specific additives and subsequently characterized using basic stains.
For their differentiation into adipocytic phenotype cells, the ADMSCs were cultivated in a base medium supplemented with 1μM of dexamethasone (Sigma), 0.5mM of IBMX (Sigma), 10μM of insulin (Sigma) and 200μM of indometacin (Sigma). After 7 days of induction, it was stained with Oil Red O (Sigma).
For osteogenic induction, the cells were cultivated in base medium with 5% of foetal bovine serum and 10μM of dexamethasone, 150mM of l-ascorbic acid (Biomedia) and 10 of ß-glycerolphosphate (Sigma), with induction being completed with the addition of 10ng/mL of morphogenetic protein 2 (BMP2) (Sigma) to the medium during the first 48h. After 21 days of induction, staining was performed with Alizarin Red (Sigma).
Finally, for chondrogenic induction, 1×106 ADMSCs were seeded in 100μL of fibrin matrix prepared from ovine plasma. These matrices were cultivated in chondrogenic culture medium, consisting in standard medium supplemented with 50μg/mL of ascorbic acid, 6.25μg/mL of insulin and 10ng/mL of TGF ß1 (Sigma) for 21 days. The characterization of chondrogenic differentiation was effected by microscopic observation of the cell morphology, and by studying histological sections of the matrices, subjected to Alcian blue staining.
Visualization of Labelled Cell Populations Using MRIThe labelled cells from 1P were trypsinized and transported in culture medium to the facilities at the University of León to verify their visibility with MRI.
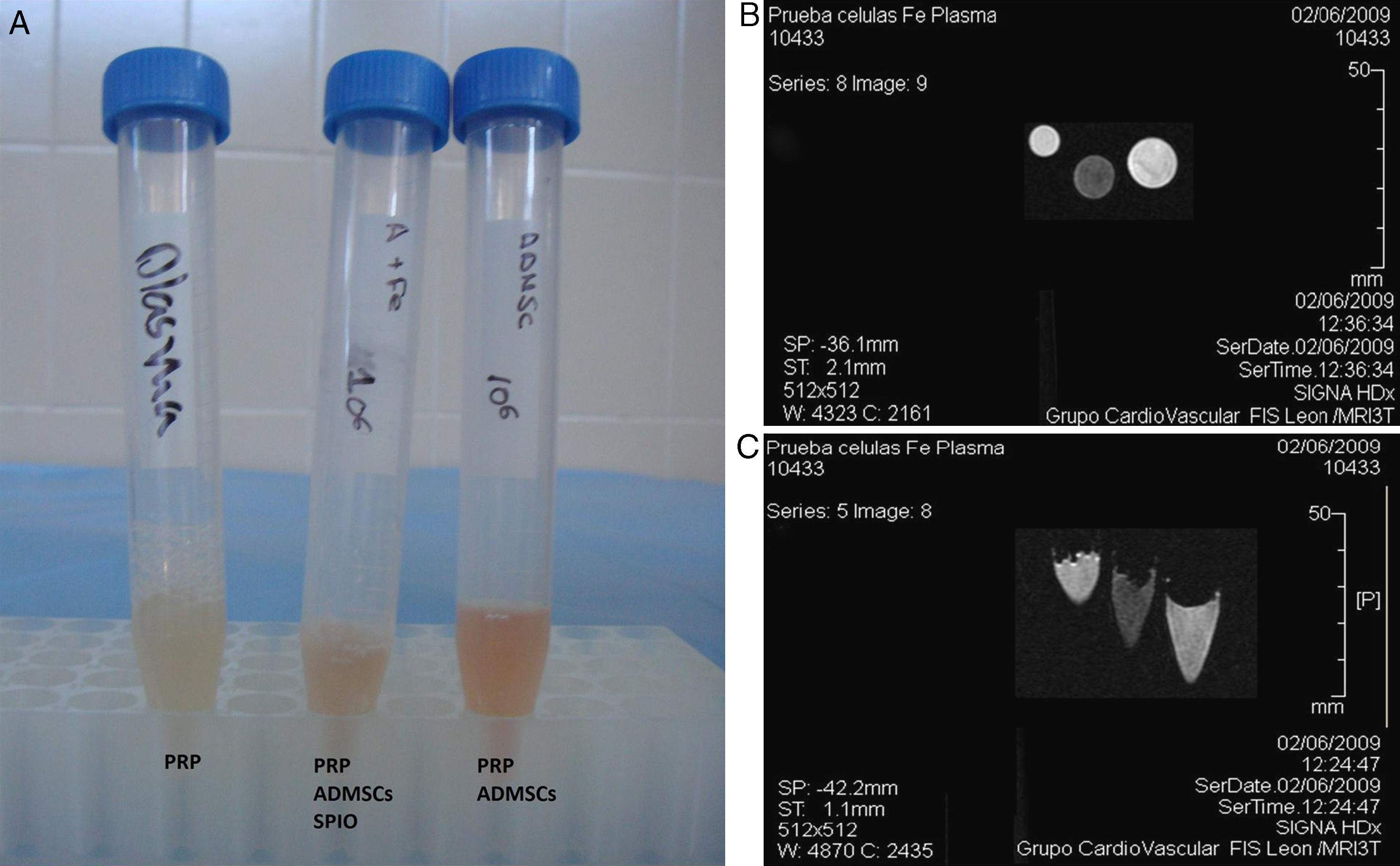
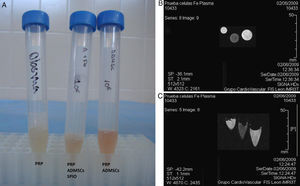
An initial trial studied the differences between the images obtained from labelled cells embedded in platelet-rich plasma (PRP), unlabelled cells embedded in PRP and PRP on its own. For this purpose, the cells were mixed with ovine plasma and clotting was provoked with calcium chloride in test tubes. The samples were inserted, in triplicate, into a 3T MRI machine.
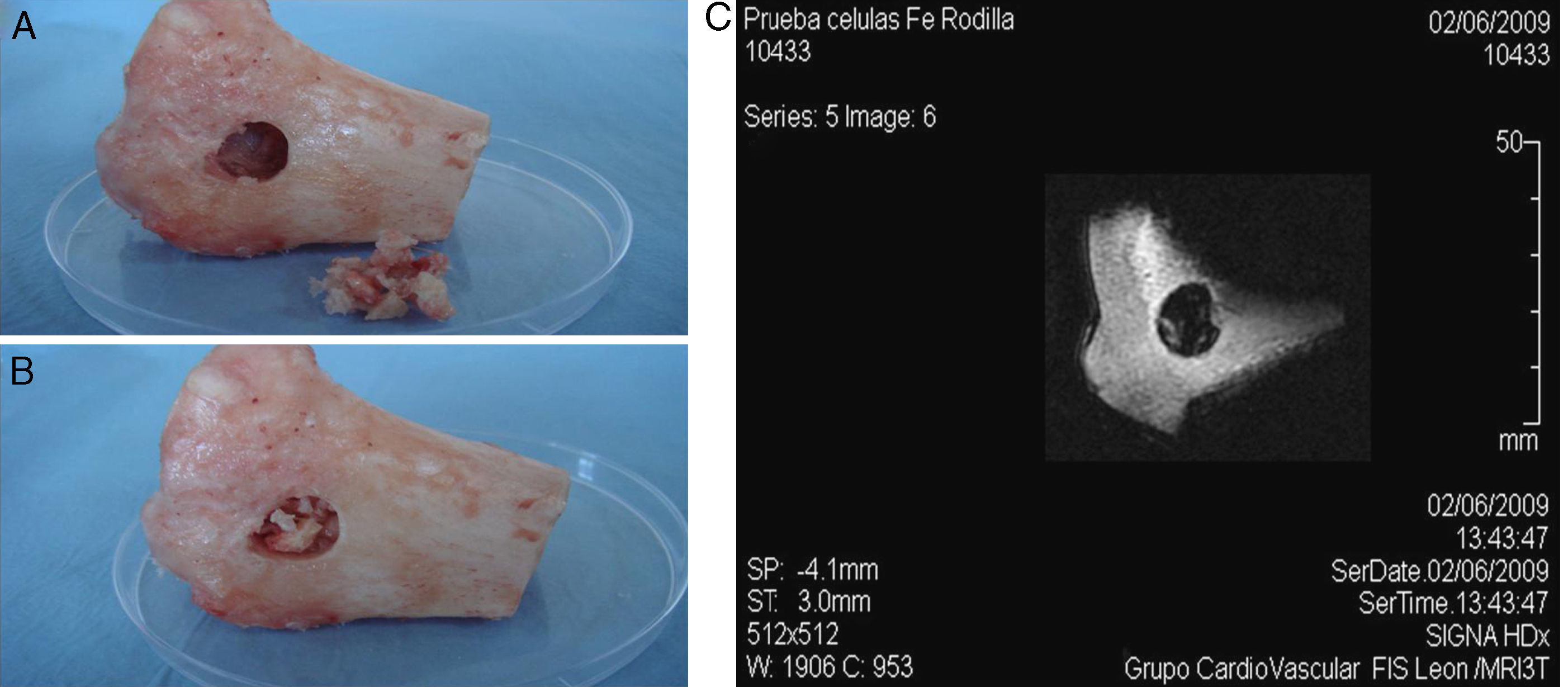
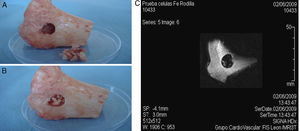
Secondly, three cavitatory defects of 13mm×11mm were made on the internal side of separate tibial plateaux from adult sheep that had been sacrificed following other experimental tests.10 Inside these bone defects, 1.5×106 labelled ADMSCs embedded in PRP and freeze-dried bone xenograft were implanted, in an attempt to reproduce the ideal treatment conditions for this kind of pathology using tissue engineering techniques.11 Immediately afterwards, an attempt was made to locate the labelled cell population inside the bone defect using MRI.
ResultsIsolation of ADMSCs and Primary CultureOf the 10 samples of ovine adipose tissue obtained, three were discarded due to bacterial contamination. From the remaining 7 samples the mean cell yield obtained was 172±132cells per mg of digested tissue, giving rise to the same number of viable primary cultures.
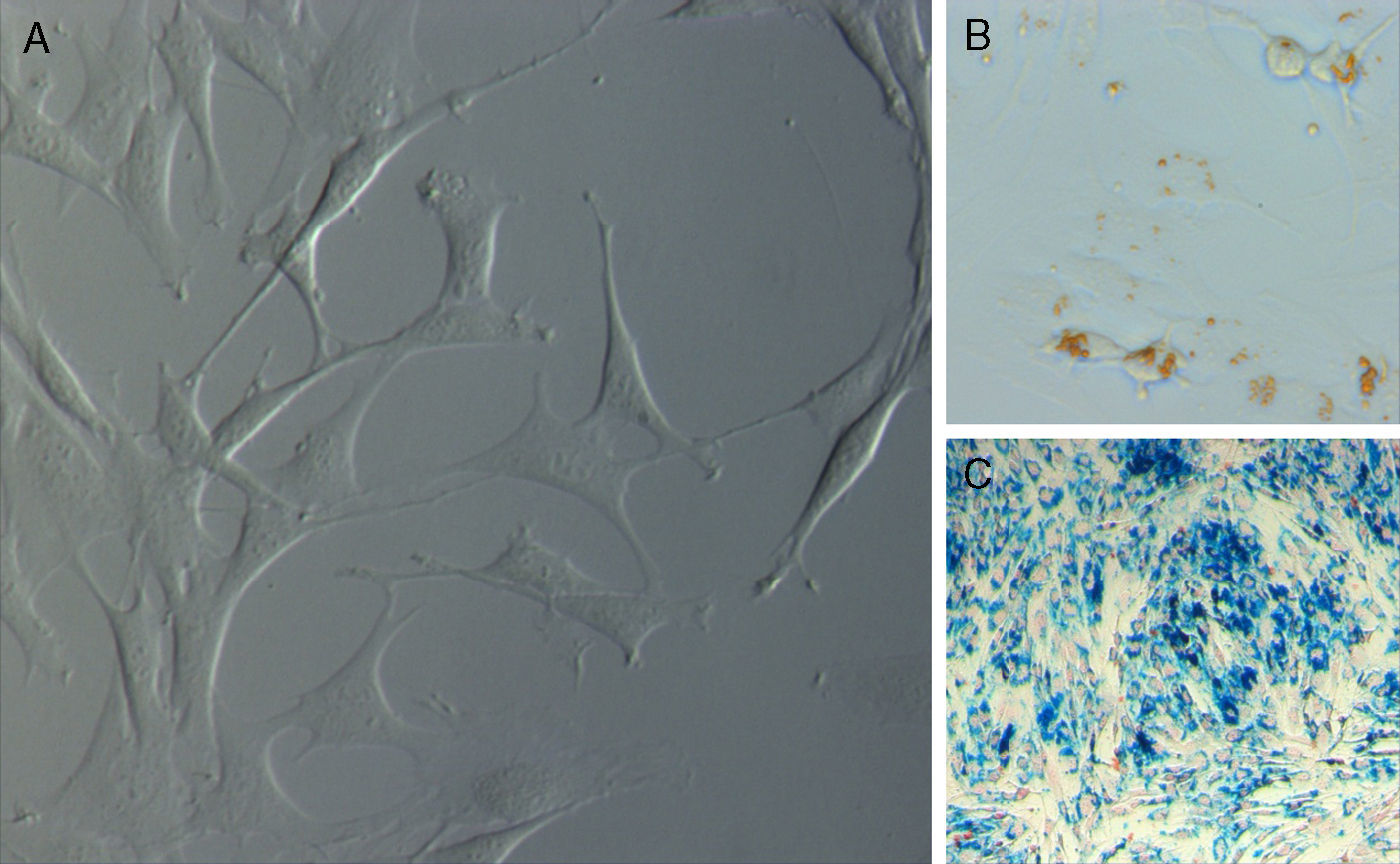
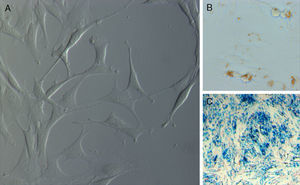
When the wells were rinsed 48h after the establishment of the primary culture, the adhering cells evolved from a rounded morphology towards a bipolar or tripolar spindle-like shape, with long cytoplasmatic prolongations, and proliferated uniformly without forming colonies until the creation of a single layer (Fig. 1A).
The primary cultures took an average of 9 days to reach the preconfluent state with the mean expansion rate achieved being 4 times the number of original cells.
Labelling of ADMSCs with SPIOThe labelling method used turned out to be effective. This labelling was visible through phase contrast microscopy and was observed as dark brownish particles inside the culture cells.
Prussian blue staining confirmed the presence of iron particles inside more than 90% of culture cells, as they were seen as blue coloured particles (Fig. 1B and C).
Innocuousness of Labelling with SPIOThe growth patterns and multipotency of the ADMSC populations were not altered by intracellular labelling with SPIO.
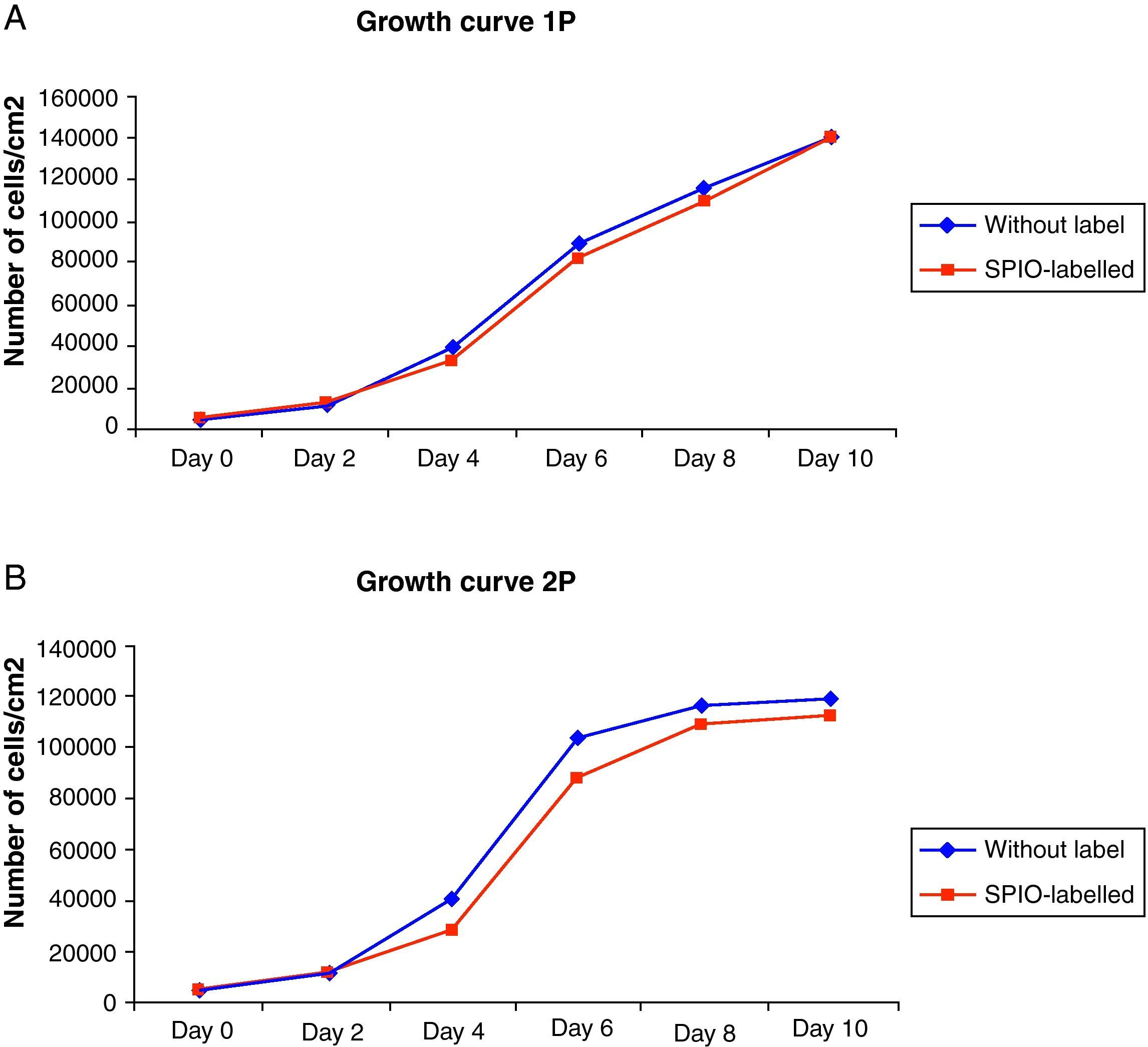
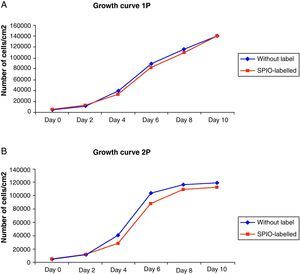
The growth curves for the SPIO-labelled ADMSC cultures showed a standard pattern in which a short latency phase could be seen, followed by an exponential growth phase until the stationary phase was reached after 8–10 days. No statistically significant differences were observed with respect to the control cell populations. Nonetheless, following the procedure for labelling with SPIO, a slight decline in the number of cells was observed with respect to the control cells due the fact that the repeated rinsing to eliminate iron not internalized removed some of the culture cells (Fig. 2).
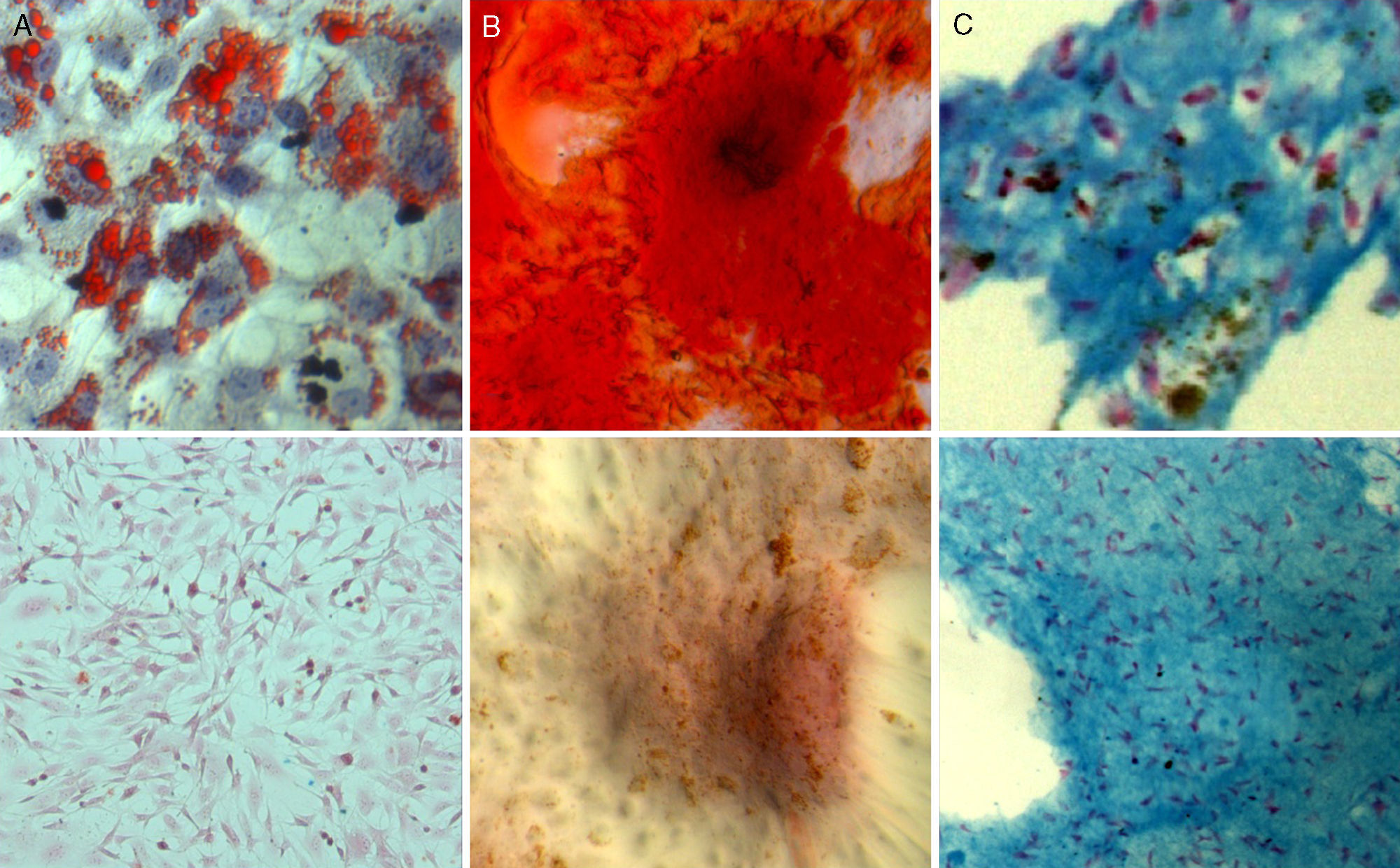
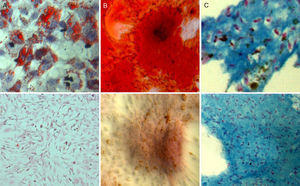
With respect to multipotency, the labelled cell populations retained their ability to differentiate into different cell strains in a manner similar to the control populations (Fig. 3).
- 1.
Adipogenic differentiation: after three days of adipogenic induction, a change was observed in the morphology of the culture cells, which changed from showing a fibroblastic morphology with fine prolongations to a more star-shaped morphology. After 7 days of culture in an adipogenic medium, Oil Red O staining revealed the formation of lipid vacuoles inside labelled cells.
- 2.
Osteogenic differentiation: the cultivation of SPIO-labelled ADMSCs in osteogenic medium triggered a change in the growth patterns and distribution of the culture cells which was visible in the formation of cell nodes that showed signs of mineralization after staining with Alizarin Red. This mineralization began to be evident after 7 days of induction and was more intense after 14 and 21 days of culture in osteogenic medium.
- 3.
Chondrogenic differentiation: three-dimensional cultivation in fibrin matrix of the cell populations labelled in chondrogenic medium for 21 days brought about a change in the cell morphology which changed from a spindle-like shape to another rounded shape. The cells were arranged in lagoons inside the matrix and Alcian blue staining showed the presence of proteoglycans in the areas of the matrix close to the differentiated cells.
Fig. 4 shows the MR image obtained of the labelled cell populations and the control cell populations embedded in ovine plasma. It can be clearly seen that the SPIO-labelled cell populations show a hypotense signal with respect to the muscle in T2/T2*, whereas the tubes containing unlabelled cell populations show no changes in signal intensity.
Similarly, when implanting the SPIO-labelled cells together with sheep plasma and freeze-dried bone xenograft in a cavitatory type defect made on the bone, it was possible to locate the labelled cell populations using MRI as it showed a hypotense signal in T2/T2* (Fig. 5).
DiscussionIn the last few years, medical and scientific societies have shown enormous interest in adult stem cells and their therapeutic potential. In the field of orthopaedic surgery, they have been applied in the treatment of avascular necroses of the head of the femur12 and femoral condyles,13 in the reconstruction of bone defects following tumour surgery14 or in the consolidation of fractures.15 Nonetheless, the application of tissue engineering techniques with adult stem cells for the treatment of lesions in the bone system is still in its early days, as we still do not know the mechanisms through which cell therapy influences disease treatment.
The study of the biodistribution and permanence of adult stem cells after they have been implanted in a living body is essential to ensure the safety and efficacy of any therapy based on the use of stem cells.2–4 Different techniques have been used for this purpose, such as X-rays, tomography (computed tomography, positron emission tomography or single photon emission computed tomography),3,5 or optical methods based on fluorescent or bioluminiscent molecules.2,5
Magnetic resonance is a particularly interesting way of obtaining images that are useful for tracking cells in the living body, as it allows multiplanar images to be obtained non-invasively in real time with a high-contrast resolution.4,5 In addition, it allows long-term monitoring to take place for the possible proliferation and migration of the implanted cells.
In order to track implanted cell populations, these must first be marked with an agent that is visible with MR imaging.
Contrast agents can be classified depending on whether they produce contrast in T1 or in T2/T2*. T1 contrast agents use gadolinium and enable tracking of implanted cell populations for up to 6 weeks but this requires high concentrations to be visible.5 T2/T2* contrast agents are the most widely used for MRI cell tracking as the use of SPIO allows the detection of a small number of cells for 12 weeks.5 Furthermore, its use in humans has been approved by the FDA, as it is biocompatible, safe and non-toxic.4,16
The present study analyzes the effectiveness of labelling adult stem cells derived from ovine adipose tissue with SPIO (Endorem®), as well as its effect on cell viability and multipotency.
With regard to the labelling methodology used in the study, a low concentration of iron was used (50μL/mL) with protamine sulphate as the trasfection reagent, giving a high rate of internalization of SPIO particles after 12h of incubation, similar to that obtained with other reagents such as lipofectamine17 or polylysine.18
As for the innocuousness of labelling, the results of the study show that the SPIO particles do not affect the viability or the multipotency of ovine ADMSCs, similarly to what has already been described by other groups with other cell types such as myofibroblasts and mononuclear bone marrow cells8,19 or even ADMSCs of human origin.20 Labelled cell populations show similar proliferation patterns to the control populations, and are capable of differentiating into mature cells of different cell strains (adipocytic, chondrocytic and osteoblastic) in a way analogous to what was described by Arbab et al. for other cell types.21
MRI observation of labelled cell populations embedded in gelified plasma shows that they can be perfectly distinguished from control cell populations. As in prior studies,3,4,7,8,16 labelling produces a drop in the intensity of the signal in T2/T2*, making it possible to use the technique for in vivo tracking of cell populations implanted in bone tissue similar to what has already been described in cardiac,5 brain7 and intervertebral8 tissues.
ConclusionIn the light of the results of the present study, we can conclude that the labelling of ovine ADMSCs populations with SPIO and protamine sulphate is innocuous and enables cell populations to proliferate and differentiate in a manner similar to the control populations. This labelling is visible using MRI and allows the tracking of cells implanted in bone defects.
In this way, the results of the present study provide sufficient data to continue investigating through studies with laboratory animals to enable the tracking of cells implanted in bone defects using magnetic resonance imaging techniques.
Level of EvidenceExperimental study II.
Protection of Human and Animal SubjectsThe authors will declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of DataThe authors declare that no patient data appear in this article.
Right to Privacy and Informed ConsentThe authors declare that no patient data appear in this article.
FundingThe Provincial Council of León and the Social Work Department of the Caja España savings bank.
Conflict of InterestThe authors have no conflict of interests to declare.
The Provincial Council of León and the Social Work Department of the Caja España savings bank.
Please cite this article as: López-Laguna M, et al. Marcaje de células madre mesenquimales derivadas de tejido adiposo para su localización y seguimiento mediante RM en terapias de regeneración ósea. Rev esp cir ortop traumatol. 2011;55(5):369–377.
Article presented at the SECOT Congress.