The proximal humerus is a common site for primary bone sarcomas, of which chondrosarcoma represents 15%. There are few reports about this select group of tumours. We set out to analyse a group of patients with proximal humerus chondrosarcoma treated with surgery and to assess their long term surgical and oncological outcomes.
Material and methodsA retrospective review was performed and all patients with a proximal humerus chondrosarcoma treated with surgery were included in the study. Overall survival and local recurrence rates were analysed. Post-operative complications were recorded and limb salvage surgery failures classified according to the Henderson classification.
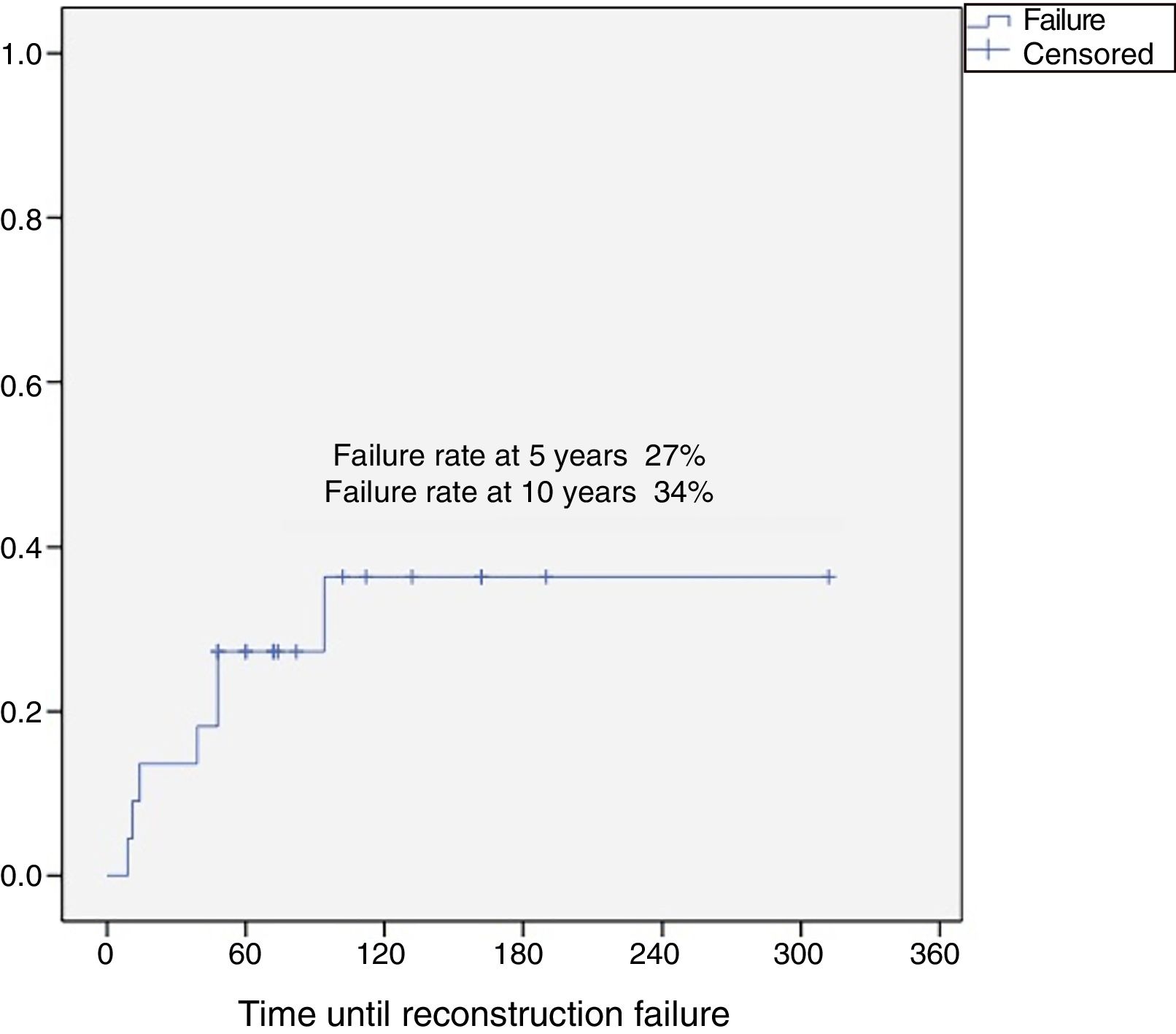
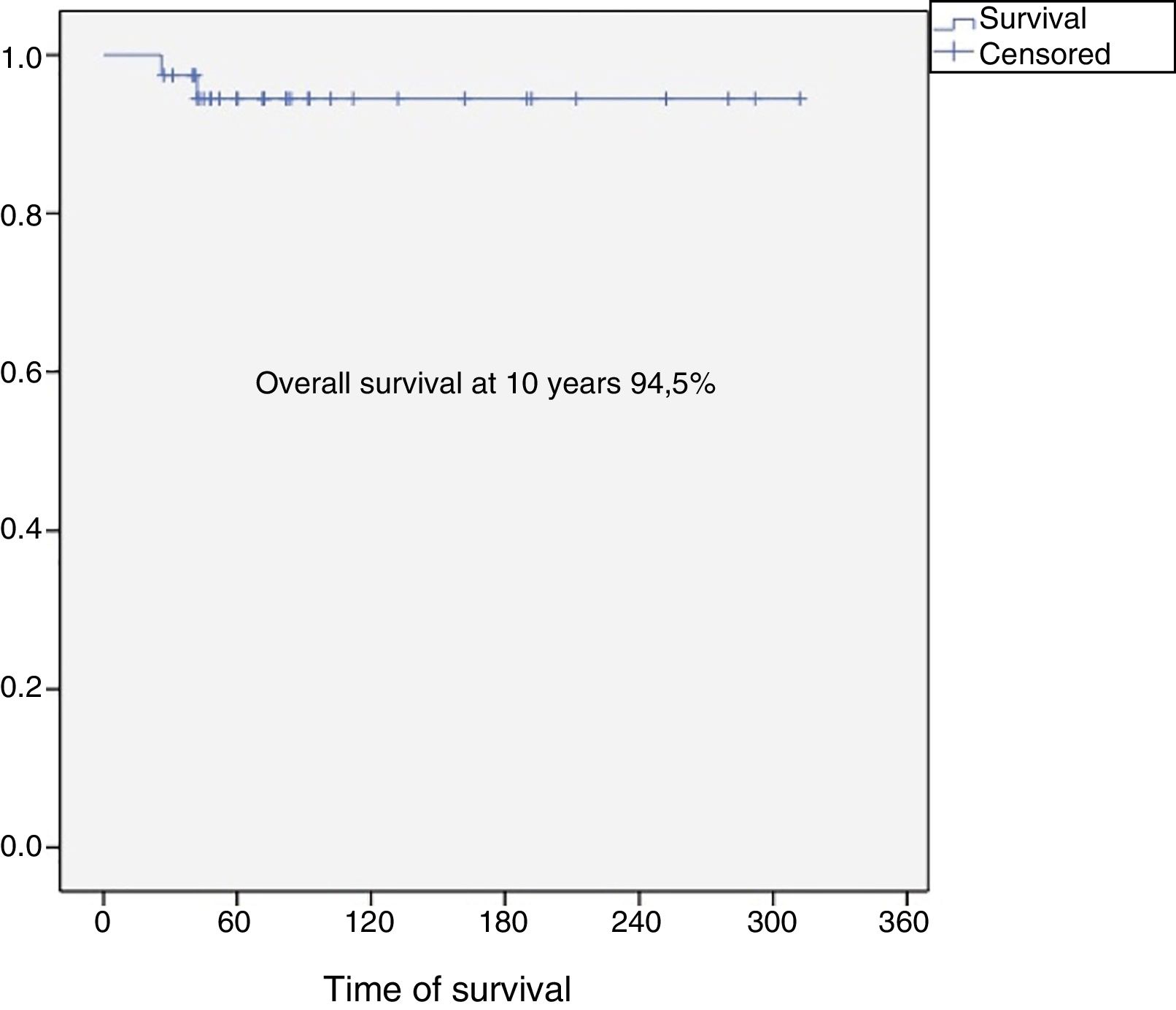
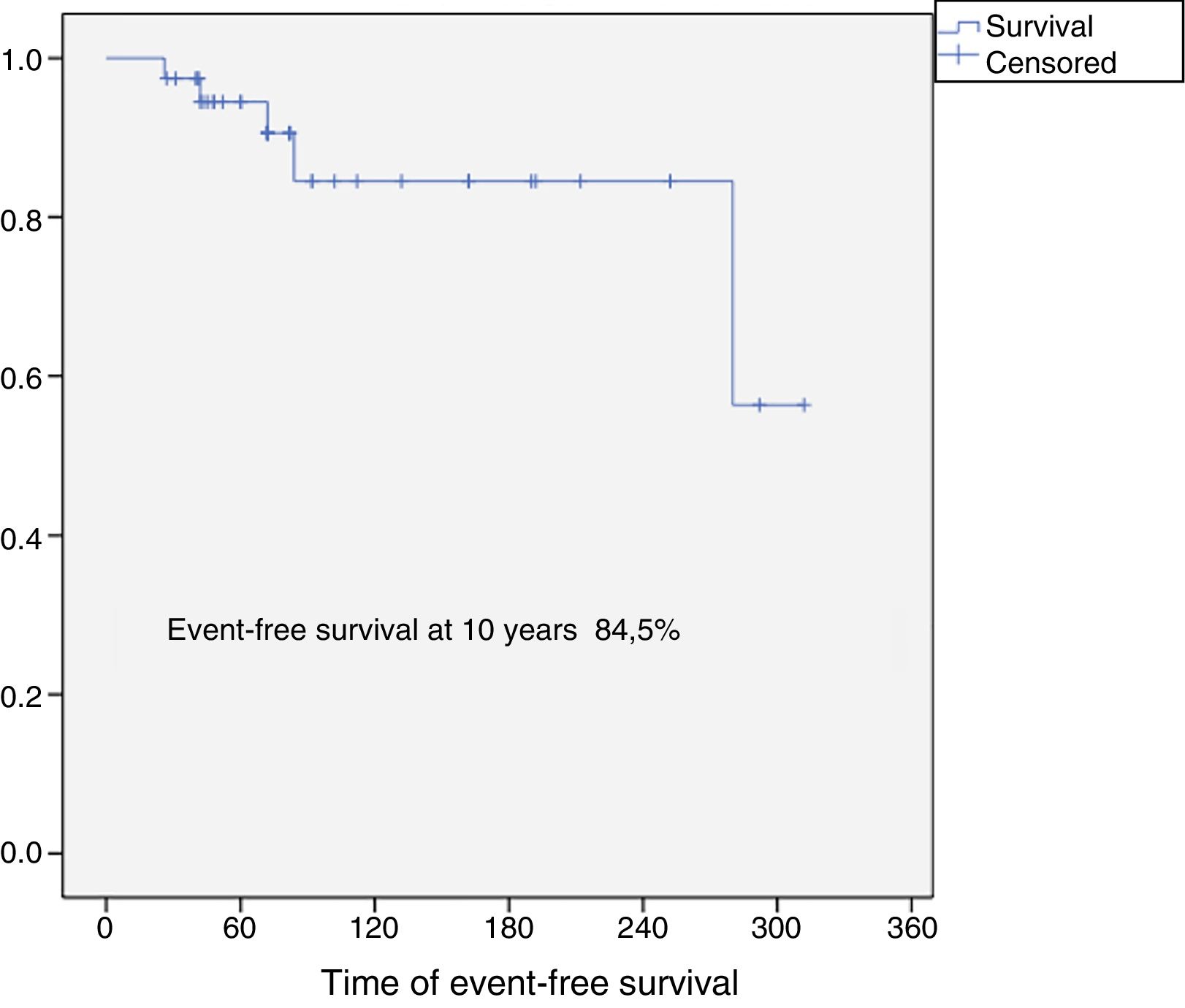
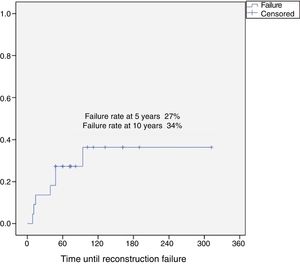
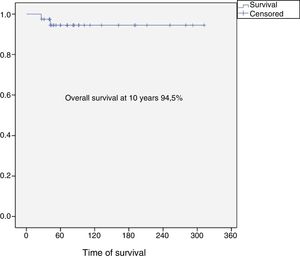
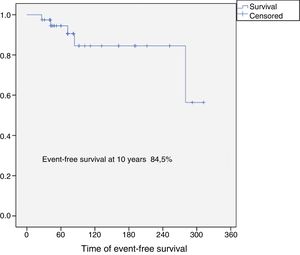
Results37 patients were included in the study. The median age was 46 years (SD: 15.6, range: 17–24), 24 (65%) were female and the mean follow-up was 8.5 years (SD: 6.4, range: 2–26). Eighteen patients were classified as grade 1 (49%), 15 as grade 2 (40%), 2 as grade 3 (5%) and 2 dedifferentiated chondrosarcomas (5%). The 10-year overall survival was 94.5% and the 10-year event-free survival was 84.5%. Five patients developed local recurrences (13%) and none of them was grade 1. The reconstruction failure rate was 27% at 5 years and 34% at 10 years. There were no complications or local recurrence in patients treated with curettage.
ConclusionProximal humerus chondrosarcoma presented high survival rates. Curettage and bone grafting is a safe procedure, with low risk of complications and local recurrence for grade 1 chondrosarcomas and should be the first indication for the proximal humerus. Reconstruction of the proximal humerus after a wide resection has a 5-year failure rate of 27% and 10-year failure rate of 34%.
El húmero proximal es una localización frecuente de los sarcomas óseos y el condrosarcoma representa el 15%. Existen pocos trabajos sobre este grupo seleccionado de tumores. El objetivo del estudio fue analizar a un grupo de pacientes con condrosarcoma del húmero proximal tratados con cirugía y evaluar los resultados quirúrgicos y oncológicos a largo plazo.
Material y métodosSe realizó una búsqueda retrospectiva y se incluyeron en el estudio todos los pacientes con condrosarcoma de húmero proximal tratados quirúrgicamente en una única institución. Se analizaron la supervivencia global y la tasa de recurrencia local. Las complicaciones postoperatorias fueron registradas y los fracasos de la cirugía de conservación de miembros se clasificaron de acuerdo a la clasificación de Henderson.
ResultadosTreinta y siete pacientes fueron incluidos en el estudio. La mediana de edad fue de 46 años (DE: 15,6; rango: 17-24), 24 (65%) eran mujeres y la media de seguimiento fue de 8,5 años (DE: 6,4; rango: 2-26). Diez y ocho fueron clasificados como grado 1 (49%), 15 como grado 2 (40%), 2 como grado 3 (5%) y 2 condrosarcomas desdiferenciados (5%). La supervivencia global a 10 años fue del 94,5% y la supervivencia libre de eventos a 10 años del 84,5%. Cinco pacientes desarrollaron recurrencias locales (13%) y ninguno de ellos fue grado 1. Las tasas de fracaso en la reconstrucción fue del 27% a los 5 años y del 34% a los 10 años. No se registraron complicaciones ni recurrencia local en los pacientes tratados con raspado.
ConclusiónEl condrosarcoma de húmero proximal presento altas tasas de supervivencia. El raspado y relleno con injerto óseo es un procedimiento seguro, con bajo riesgo de complicaciones y recurrencia local para los condrosarcomas de grado 1 y debe ser la primera indicación para el húmero proximal. La reconstrucción del húmero proximal después de una resección en bloque tiene una tasa de fracaso del 27% a 5 años y del 34% a 10 años.
Proximal humerus chondrosarcoma is the second commonest primary bone sarcoma, and principally affects the pelvis and the metaphysis of the long bones.1,2 Chondrosarcomas are resistant to chemotherapy and radiotherapy, and therefore treatment is based on surgery.3,4 The histological grade and anatomical site have been described as major prognostic factors in relation to overall survival and local recurrence.5 Low histological grade chondrosarcomas (Grade 1) are characterised by local aggressiveness and they rarely spread systemically, therefore high rates of survival have been reported for this particular group.6,7 Furthermore, high grade dedifferentiated chondrosarcomas or those located in the axial skeleton (pelvis/vertebral spine) have a poorer prognosis.8 Although the proximal humerus is a common site for primary bone sarcomas, chondrosarcoma in particular accounts for less than 15%, and there are few papers on this specific tumour group.9 The aim of the study was to analyse a group of patients with proximal humerus chondrosarcoma treated with surgery, and to assess the surgical and oncological outcomes in terms of: surgery failure rate, postoperative complications, overall survival, and rate of local recurrence.
Material and methodsWe undertook a retrospective review from our oncological database, and analysed all the patients diagnosed with proximal humerus chondrosarcoma treated between 1 January 1990 and 31 December 2013 (23-year study period). We only included patients with a minimum of 24 months’ follow-up, who were treated surgically. Following approval by our institution's research committee, we obtained the demographic characteristics of the patients from the hospital's clinical data including age, sex, histological grade of the tumour, type of surgery and reconstruction. We used the World Health Organisation's criteria for histological classification that defines grade 1 chondrosarcoma as moderately cellular neoplasms with hyperchromatic spheroidal nuclei, uniform in size; grade 2 as more cellular and containing a greater degree of nuclear atypia, hypercromasia and nuclear size; grade 3 lesions as more cellular and pleomorphic than grade 2, with easily detected mitoses.10,11 Dedifferentiated chondrosarcoma is defined by the presence of a biphasic tumour composed of a conventional chondrosarcoma and a high-grade non-cartilaginous sarcoma.11 We should highlight that the latest classification also includes changes to low-grade chondrosarcomas, which are now classified as “atypical enchondromas”.11
In the cases where the histological specimen showed a mixed grade, the highest grade was taken as the final classification. For grade 1 chondrosarcomas, the diagnosis was always supported by the clinical and radiological findings as discussed in multidisciplinary meetings. All lesions classified as typical enchondromas were excluded from the study.12,13
The surgical treatment included: curettage and fill with fragmented banked bone graft or en bloc resection reconstructed with modular prostheses, structural grafts or a combination of both. All the intermediate and high grade chondrosarcomas were treated with en bloc oncological resections. For the low-grade chondrosarcomas, either curettage or en bloc resection was indicated by consensus in a multidisciplinary committee comprising pathologists, radiologists and orthopaedic surgeons. Different reconstruction techniques were used, including osteoarticular and intercalary homograft. In the 3 techniques mentioned, the resection included the humeral head. In contrast, reconstruction with an intercalary transplant required salvage of the proximal humeral epiphysis, and therefore the humero-glenoid joint. The major postoperative complications were recorded, and limb-salvage surgery failures were classified according to Henderson.14 Henderson's classification defines 5 groups: types 1–2–3 classified as mechanical failures, and types 4–5 as non-mechanical failures. Type 1: soft tissue failure (1A: failure of function/1B: failure of coverage. Type 2: aseptic loosening for endoprostheses (2A: early<2 years after implantation/2B: late>2 years after implantation) graft-host non-union for the homograft (2A: hypertrophic/2B: atrophic). Type 3: structural failure (3A: implant or fixation/3B: bone or graft). Type 4: infection (4A: early<2 years for endoprostheses or <6 months for homografts/4B: late>2 years for endoprostheses or >6 months for homografts). Type 5: tumour progression (5A: soft tissue/5B: bone).14
We analysed the disease-free and overall survival of the full series according to the Kaplan–Meier method. Survival was defined as the time from the date of diagnosis to the date of death or the date of the last follow-up. The reconstruction failure rate was analysed. Statistical significance was established as p≤.05.
ResultsPatients and tumour characteristicsThirty seven patients were included in the study. The median age was 46 years (SD: 15.6; range: 17–74), 24 (65%) were female and the mean follow-up was 8.5 years (SD: 6.4; range: 2–26). Eighteen patients were classified as low grade (grade 1, 49%), 15 patients were intermediate grade (grade 2, 40%), 2 were high grade (grade 3, 5%), and 2 were dedifferentiated chondrosarcomas (5%).
Clinical outcomesAll the patients were treated surgically and none of them underwent adjuvant treatment. Thirty-five patients underwent limb-salvage surgery with later reconstruction, and 2 patients were treated with shoulder disarticulation due to involvement of the neurovascular bundle by the primary tumour.
In the group of patients who were treated with limb-salvage surgery, 13 patients diagnosed with a grade 1 chondrosarcoma were treated with curettage and banked bone graft to reconstruct the bone defect. Twenty-two patients (5 grade 1; 15 grade 2; one grade 3; and one dedifferentiated) underwent en bloc resection with free margins, and the patients were reconstructed using different methods: graft-prosthesis composite (n: 11), osteoarticular homograft (n: 5), intercalary homograft (n: 4) and endoprosthetic replacement of the proximal humerus (n: 2). The margins of the 25 patients were classified as disease free.
No postoperative complications were observed in the group treated with curettage. There were 6 non-oncological complications that required a further surgical intervention; all of them were in the group treated with en bloc resection. The complications were: 2 non-unions, one resorption of the homograft, one fracture/osteoarticular collapse, one aseptic loosening of a composite and one proximal dislocation of a humeral prosthesis. For the cases of massive resorption and aseptic loosening, revision surgery was performed to the proximal endoprosthesis of the humerus. The 2 cases of non-union were treated with an autogenous iliac crest bone graft, and both patients achieved full healing at 12 weeks. The patient with the fracture/osteoarticular collapse was converted to a graft-prosthesis composite, maintaining part of the original transplant. For the prosthetic dislocation, an open reduction and stabilisation with prolene mesh were performed.
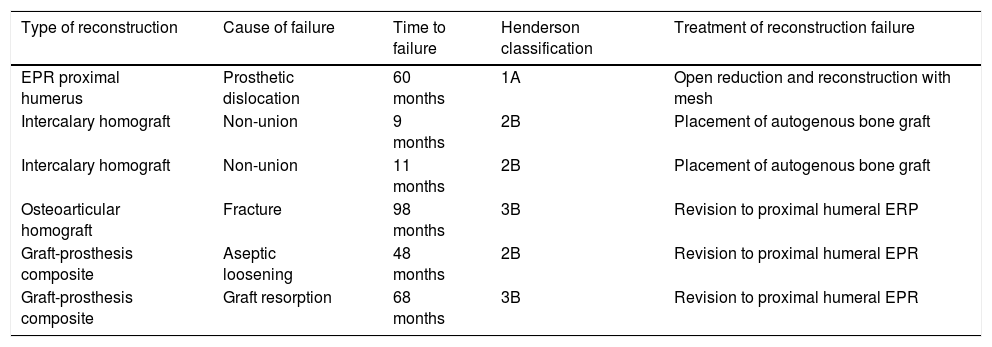
The reconstruction failure rate was 27% at 5 years, and 34% at 10 years (Fig. 1). According to Henderson's classification, the failures of the reconstructions with endoprosthesis were: Henderson type 1A (soft tissue failure). The failures of the reconstruction for homografts were classified as Henderson type 2B (non-union) in 2 cases, Henderson type 3B (structural failure) in one case, and 3 patients were Henderson type 5 (local recurrence). There were 2 failures in two patients reconstructed with a graft-prosthesis composite: one aseptic loosening (Henderson 2B), and one resorption of the homograft (Henderson type 3B) (Table 1).
Description of the cases of reconstruction failure according to the type of reconstruction.
| Type of reconstruction | Cause of failure | Time to failure | Henderson classification | Treatment of reconstruction failure |
|---|---|---|---|---|
| EPR proximal humerus | Prosthetic dislocation | 60 months | 1A | Open reduction and reconstruction with mesh |
| Intercalary homograft | Non-union | 9 months | 2B | Placement of autogenous bone graft |
| Intercalary homograft | Non-union | 11 months | 2B | Placement of autogenous bone graft |
| Osteoarticular homograft | Fracture | 98 months | 3B | Revision to proximal humeral ERP |
| Graft-prosthesis composite | Aseptic loosening | 48 months | 2B | Revision to proximal humeral EPR |
| Graft-prosthesis composite | Graft resorption | 68 months | 3B | Revision to proximal humeral EPR |
EPR: endoprosthetic replacement.
The overall survival of the entire series at 10 years was 94.5% (95% CI: 86–100), and disease-free survival at 10 years was 84.5% (95% CI: 77–99) (Figs. 2 and 3). All the patients with low, intermediate or high grade chondrosarcoma of the proximal humerus were alive at the last follow-up. However, the 2 patients with dedifferentiated chondrosarcoma died at 26 and 42 months from diagnosis due to lung metastases.
None of the patients diagnosed with proximal humerus chondrosarcoma included in the study had metastases on diagnosis, and only 3 developed lung metastases during follow-up. The patients with lung metastases received chemotherapy using the adriamycin+ifosfamide regimen.
The probability of developing metastases from grade 1 and grade 2 proximal humeral chondrosarcoma was 0% (0/33), 50% from grade 3 (1/2), and 100% (2/2) for the patients with dedifferentiated chondrosarcoma.
Five patients (13%) had local recurrence (2 of 15 were grade 2, one of 2 were grade 3, and 2 of 2 dedifferentiated) within a mean of 39 months (range, 6–94). None of the patients with grade 1 chondrosarcoma, irrespective of whether they had undergone curettage or en bloc resection, had local recurrence at the last check-up. All the patients with local recurrence were treated by further oncological surgery to achieve a complete resection with free margins. The limb-salvage procedure was possible for 4/5 patients, while one patient required disarticulation of the upper limb due to vascular compromise and tumour spread. Neither chemotherapy nor radiotherapy was used to treat recurrence in this series. The histological diagnosis of local recurrence showed that one patient had a higher grade compared to that of the original diagnosis (grade 3 dedifferentiated).
DiscussionThe proximal humerus is a common site for sarcomas to develop, 15% of which are chondrosarcomas.9 Because chemotherapy and radiotherapy have proved unsuccessful in managing these types of tumour, surgical resection is the treatment of choice.15,16 We present a group of 37 patients with a histological diagnosis of chondrosarcoma located in the proximal humerus and treated surgically, and we studied the clinical and oncological outcomes.
Scapulohumeral disarticulation was the treatment of choice for these patients for several decades.3,4 However, the development of new surgical techniques, and the introduction of new diagnostic methods has enabled limb-salvage surgery for most patients, as shown in this series (35 of 37 patients).5 Scapulohumeral disarticulation would be indicated at present only for cases where the axillary vascular bundle or the brachial plexus are compromised. The indication for an intralesional resection (curettage) or oncological en bloc resection will depend not only on the tumour's histological grade, but also on the imaging conditions such as cortical scalloping, tumour size, invasion of soft tissue, or articular spread, and the clinical feature of pain.17–19 Donati et al. reported in their series of 31 that the major aggressiveness criteria for grade 1 chondrosarcomas were cortical erosion and bone deformity.20
The rate of overall survival in our series was 94.5% at 10 years, similar to other publications. We know that chondrosarcoma of the proximal humerus has better oncological outcomes compared to the other long bones.9 Mourikis et al., in their series of 31 patients with proximal humerus chondrosarcoma, reported a 96% survival rate, and agreed that proximal humeral chondrosarcoma seems to be less aggressive and malignant that chondrosarcomas in other sites (pelvis, spine and bones of other limbs).9
The histological grade and location have been described as important prognostic factors for chondrosarcoma.17–23 High grade and dedifferentiated chondrosarcomas have a poor prognosis, with lower survival rates. The tumour grade and an axial/pelvic location are reported as the principal negative prognosis factors for overall survival.21
Our reconstruction failure rate for limb-salvage surgeries, after en bloc resections, was 27% at 5 years and 34% at 10 years. Reconstruction of the proximal humerus after resection of an aggressive tumour has been the subject of debate. Different techniques have been described, including biological reconstructions (vascularised fibula, free fibula and osteoarticular homografts), endoprosthetic replacement or a combination of both.9,24–27 The few studies, and the few patients included in all of them, make it difficult to establish a conclusion in terms of outcomes. It is worth highlighting that it was not possible to undertake a detailed analysis of the type of reconstruction in relation to the failure rate in our series due to the few cases; this is a limitation of this study.
Intralesional curettage was only considered an option for patients with grade 1 chondrosarcoma. In our series, intralesional resections for low-grade chondrosarcoma proved a safe procedure for the long bones, with a low risk of recurrence regardless of the different adjuvant therapies used (phenol, cement, cryosurgery).22,23,28–30 However, 5 patients with low-grade chondrosarcoma were treated with en bloc resection, after multidisciplinary discussion due to the radiological findings that raised the suspicion that they were more aggressive (size >5cm, cortical scalloping, oedema on MRI or hyperuptake on bone scintigraphy).20 On the other hand, we all know that free margins after oncological resection minimise the development of local recurrence.3–5 Although tumour-free margins were achieved for the 25 patients who underwent en bloc resection, 5 developed a local recurrence. None of the patients with grade 1 proximal humeral chondrosarcoma had local recurrence or distant metastases. According to our findings, recurrence and distant disease seem to be significantly related to the histological grade of proximal humeral chondrosarcomas. Oncological en bloc resections or radical resections are complex operations, which demand extensive muscular resections, carry a high risk of postoperative complications and poorer functional outcomes, therefore they should be indicated with caution for patients with low-grade proximal humerus chondrosarcomas, based on their low recurrence rate and probability of metastasis.21–30 We should highlight that the possibility of different diagnoses between biopsy and the histological results after the final resection reported previously make this group of lesions even more complicated to manage.12,13
We acknowledge the limitations of our study in that it is retrospective and includes patients treated with different reconstructive techniques over a prolonged period of time (23 years). However, there are few reports in the literature on the oncological and clinical outcomes of proximal humeral chondrosarcoma.
ConclusionProximal humeral chondrosarcoma has high survival rates (overall survival rate of the series at 10 years: 94.5%), and a better oncological prognosis compared to chondrosarcomas located in other long bones. Surgery is always the treatment of choice since these tumours are resistant to chemotherapy and radiotherapy. For grade 1 chondrosarcomas, curettage and fill with banked bone graft is a safe procedure, with a low risk of complications and local recurrence, and should be the first indication for this group of tumours. Reconstruction of the proximal humerus after en bloc resection of a chondrosarcoma has a failure rate of 27% at 5 years, and 34% at 10 years.
Conflict of interestsThe authors have no conflict of interests to declare.
Please cite this article as: Albergo JI, Farfalli Luis GL, Ayerza MA, Muscolo DL, Aponte-Tinao LA. Condrosarcoma de húmero proximal. Resultados clínicos y oncológicos a largo plazo. Rev Esp Cir Ortop Traumatol. 2019;63:181–186.