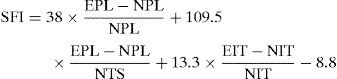This project aims to study the regeneration of non-repairable lesions of peripheral nerve by muscle grafts enhanced with growth factors.
Material and methodsThe experiment was carried out in two phases. The first one compared direct suture of a critical defect in the sciatic nerve of ten rats, with the interposition of autologous muscle graft, denatured by heat, in another ten. The second phase compared ten rats with nerve repair using an acellular muscle graft, with injection of 2 cc of IGF-1 (10mg/ml mecasermin, injectable solution) into the acellular graft of another ten.
A clinical and functional follow-up was carried out including, ambulation, footprint measurement, and “Grasping Test”. The animals were sacrificed at 90–100 days, and samples obtained for macro- and microscopic studies with toluidine blue, hematoxylin-eosin and Masson's trichrome staining.
ResultsThe first experiment showed the characteristic findings of nerve tissue in muscle graft level sections. The second was an enhancement of the results: post-surgical clinical improvement, early ambulation, decrease in the rate of pressure ulcers in toes, recovery of the footprint, and increasing the percentage of nerve endings in distal sciatic regeneration (from 47 to 62%).
ConclusionsIn this study the experimental and clinical possibilities of nerve defect repair by denatured muscle are demonstrated, confirming the suitability of the technique. Furthermore, it confirms our hypothesis with clinical and cellular determinations enriched by the addition of growth factors that promote nerve regeneration.
El objetivo del proyecto es estudiar la regeneración de las lesiones no reparables del nervio periférico, mediante un injerto muscular enriquecido con factores de crecimiento.
Material y métodoLa experimentación se desarrolla en 2 fases: primero, comparamos la sutura directa del defecto crítico en el nervio ciático de 10 ratas, con la interposición de un injerto de músculo autólogo desnaturalizado por calor en otras 10. En la segunda, se comparan 10 ratas con reparación mediante injerto muscular acelular, con la inyección de 2cc de IGF-1 (10mg/ml de mecasermina, en solución inyectable) dentro del injerto acelular de otras 10.
Realizamos el seguimiento clínico y el control funcional de la marcha, medición de la huella plantar y «Grasping Test». Fueron sacrificadas a los 90–100 días, obteniendo muestras para macro y microscopía, con tinciones de azul de toluidina, hematoxilina-eosina y tricrómico de Masson.
ResultadosLa primera experimentación demostró el hallazgo de tejido de características nerviosas en las secciones del injerto muscular. La segunda supuso una potenciación de los resultados: mejoría clínica posquirúrgica, precoz deambulación, descenso en la tasa de úlceras por presión en partes acras, recuperación de la huella plantar, e incremento del porcentaje de terminaciones nerviosas en regeneración del cabo distal (47–62%).
ConclusionesExponemos en este trabajo las posibilidades experimentales y clínicas de la reparación del defecto nervioso mediante músculo desnaturalizado, confirmando la adecuación de la técnica. Además, confirmamos nuestra hipótesis con clínica y determinaciones celulares enriquecidas por la adicción de factores de crecimiento que impulsan la regeneración nerviosa.
The purpose of a nerve graft is to replace a defect in order to allow the maximum number of regenerated nerve fibers to reach their target organ. Millesi et al.1 obtained satisfactory results using saphenous nerve autografts: a technique that became the gold standard. However, the limited availability of nerve tissue for autografts, as well as the incomplete functional recovery obtained in most cases and problems at the donor site have led to the search for other alternatives.
In 1984, Keynes et al.2 found that the basal lamina of muscle cells was useful as a pathway for the passage of regenerating axons, due to the action of laminin from the basal lamina. Subsequently, in 1986, Glasby et al.3 achieved a considerable passage of useful axons through endomysium channels, using denatured skeletal muscle to repair nerve defects, as endomysium channels are similar to endoneurium channels.
Other authors have continued these studies. In 1990, Pereira et al.4 began their studies with denatured muscle grafts as a substitute for mycobacterial granulomatous lesions in peripheral nerves of patients with leprosy. In 1993, Brunelli et al.5 developed an experimental study in rats with vein grafts (which provides guidance for nerve regeneration) filled with fresh skeletal muscle (to prevent the collapse of the vein and provide support for the regeneration of axons), with similar results to traditional nerve grafts. In 1994, DeFranzo et al.6 examined the use of frozen–thawed muscle as a nerve graft material in rats, histologically analyzing the density of axons and their correct myelination.
In 1995, Whitworth et al.7 presented a simple technique for incorporating a deposit of Schwann cells and other essential components in a nerve conduit, with a marked effect on axonal regeneration of defects measuring about 5cm in rabbit sciatic nerves, repairing them with 2–3mm, fresh, autologous nerve grafts interspersed between 1cm muscle grafts. One year later, these same authors, Whitworth et al.,8 published another article which developed different methods to denature muscle grafts, thus enhancing the results, using either dry heat in a microwave oven or by heating in distilled, sterile water. Continuing this line of research, in 1995, Martín Ferrero et al.9 used freeze-dried, denatured skeletal muscle, obtaining good results in terms of functional and histological regeneration, with passage of axons to the distal segment in 62% of the oligofascicular nerves and in 48% of the polyfascicular nerves.
In 2000, Battiston et al.10 described nerve repair with vein grafts filled by muscle in 3cm defects, a critical length in the experimental animals, and superior to the 2cm repair achieved in previous experiments. As observed 6 months after surgery, the quantitative morphometric analysis of myelinated nerve fibers showed a significant increase (P<.05) of the total number (29.7% higher) and density (18.2% higher) compared to the fibers of the non-operated nerves in the control group. They concluded that muscle fibers, in addition to preventing the collapse of the vein, offered a good support for advancing nerves through adhesion molecules present in their basal lamina, and also increased neurite extension. Meanwhile, in 2004, Oliveira et al.11 used a rat sciatic nerve model to demonstrate that there were no significant differences in the morphology and function of regeneration with devitalized skeletal muscle grafts and conventional nerves for the repair of a 5mm segmental lesion.
The description of the clinical use of tissue versus synthetic grafts was initially developed 1 year later by Battiston et al.12 Clinical data suggested that simple, monotissue, biological conduits, either from vein or skeletal muscle, offered good chances of success when used in defects not exceeding 2cm. The same was true of silicone tubes. For gaps greater than 30mm it was necessary to use combined, vein–muscle or nerve–vein, biological bridges. Moreover, bioabsorbable polyglycolic acid conduits represented a reasonable option in certain clinical situations, based on encouraging results obtained up to that date.
Given the above, we considered comparing different methods to treat skeletal (striated) muscle in order to enhance its characteristics as a graft for neural defects. In 1997, Hall13 examined the cellular and acellular elements which facilitate axonal regeneration, as well as the use of acellular muscle grafts in the repair of lesions in the peripheral nervous system. In 2001, Liu et al.14 used immunohistochemical staining on neurofilaments and protein S-100 to demonstrate that axons and Schwann cells grew faster in chemically acellularized muscle grafts than in those denaturated by freezing–thawing.
We also investigated other substances which could promote the regeneration of nerve tissue, such as neurogenic growth factors (NGF), epidermal growth factors (EGF) and platelet-derived growth factors (PDGF), with varying results. In 1997, Welch et al.15 concluded that the combined administration of PDGF and insulin-like growth factor 1 (IGF-1) did not improve the regeneration of peripheral nerves 6 weeks after surgery. In 2009, Yu et al.16 studied the histological effect of NGF and found better axonal diameter, number of axons and myelin thickness with NGF in nerves repaired using acellular grafts, compared to acellular grafts alone and acellular grafts with fibrin glue.
We then began a project that was awarded a support grant for research projects from SECOT Foundation 2010–2012: «Regeneración de las lesiones críticas del nervio periférico con factores de crecimiento. Estudio experimental» (“Regeneration of critical injuries of the peripheral nerve with growth factors”). In this project, we aimed to determine the best method to denature muscle grafts, as well as to investigate whether the added use of growth factors (IGF-1) improved axonal regeneration or not, compared with previous studies by our group in which growth factors were not used.
Material and methodsThis was a controlled clinical trial, with level I scientific evidence. For this study we used male Wistar rats with a mean weight of 278g. We created a critical defect of 15mm in the sciatic nerve and attempted to bridge it with a muscle graft. Thereafter, we conducted a clinical and biological study in 2 phases.
First phaseAs control group we used 10 rats in which a critical defect was created in the sciatic nerve and which was repaired using direct termino-terminal suture.
As case group we used 10 animals in which a critical defect was created in the same sciatic nerve (5 right and 5 left hind legs) which was then bridged using an autologous muscle graft. The rats were anesthetized with ketamine hydrochloride (Imalgene®) at doses of 0.13ml per 100g body weight and xylazine (Rompun®) at doses of 0.02ml per 100g body weight, both injected intraperitoneally. Next, we performed a posterior approach to the sciatic nerve.
The grafts were obtained from a block of the gluteus maximus muscle of each rat and denatured by heat in a microwave oven at 250W for 60s, so that the muscle reached a temperature between 60 and 70°C, according to the protocol described by Whitworth et al.7
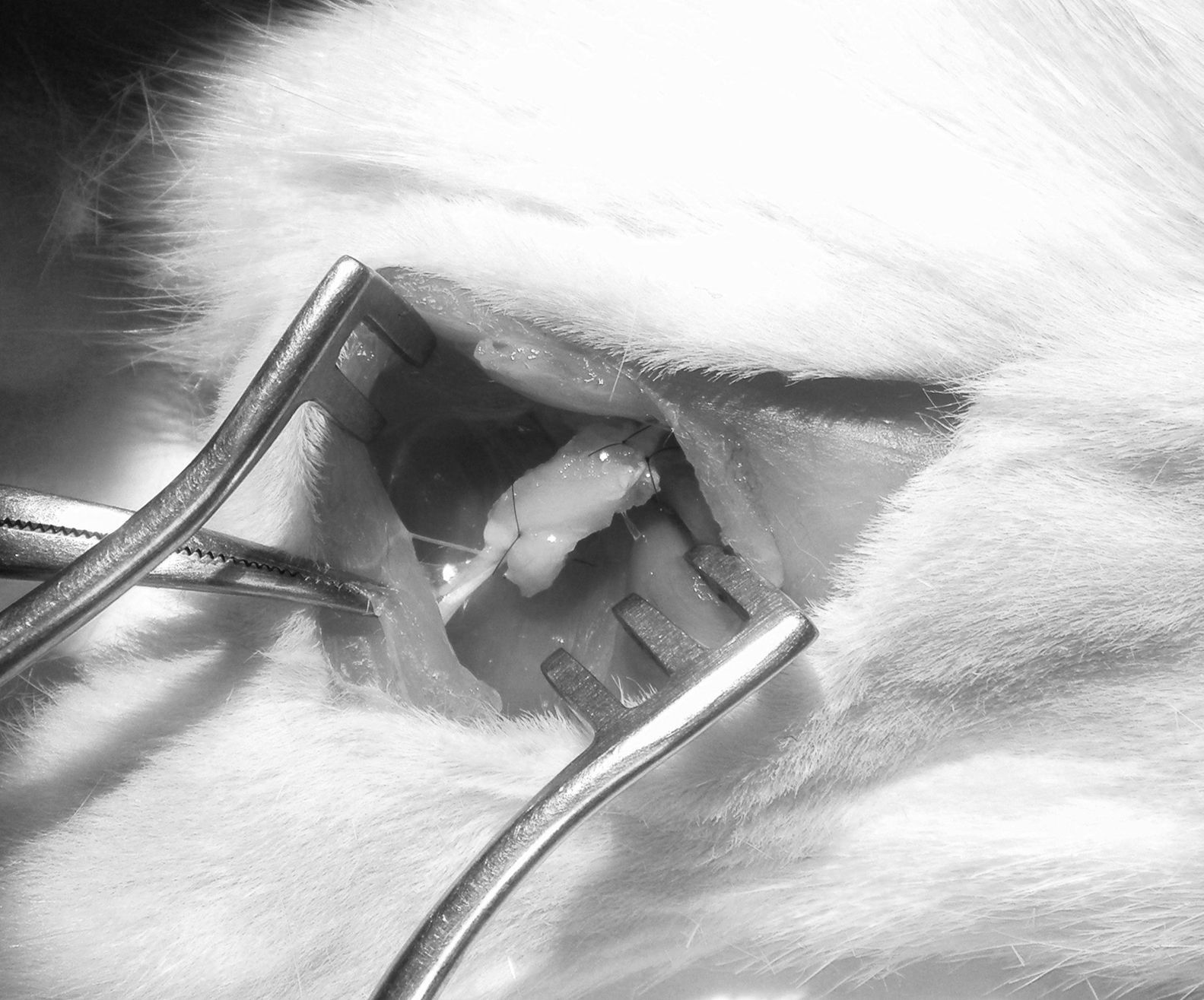
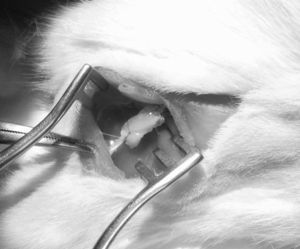
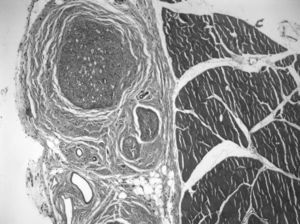
Next, we created a defect of 15mm in the sciatic nerve and used the autograft to bridge the critical nerve lesion (Fig. 1). This was fixed with termino-terminal 7/0 monofilament sutures and closed by layers.
Second phaseWe selected 20 male Wistar rats with weights between 250 and 300g, in which difficulty for walking and leg dissymmetry was ruled out. The first 10 became the controls, which underwent the same surgical procedure, but without including growth factors. The next 10 completed the entire protocol set forth below.
We obtained grafts for all the animals studied from 2 donor rats. The 2 specimens were sacrificed according to the guidelines of the Regularization Act on the use of experimental animals and 2 separate blocks were extracted from the gluteus medius muscle of both legs. Two grafts were reserved in order to analyze the cell extraction process selected. This consisted of 7h in distilled water, which was changed 3 times every 2–3h, 1 night in Triton detergent and 24h in deoxycholate. Next, they were washed in buffer and the entire process was repeated. Finally, they were passed to another buffer and stored at 4°C once the pieces had been obtained.17
We started the surgical protocol by preoperative intraperitoneal anesthesia with ketamine (75mg/kg) plus xylazine (10mg/kg), (Imalgene® 1000 and Rompun®). We then exposed the sciatic nerve through a posterolateral approach. Once this was located, it was sectioned to generate a 15mm defect, placing the acellular muscular block as a bridge, with 2, 7/0 polypropylene sutures at each end at an epineuro-epimysial level. Within the graft, we injected 2cc of Increlex® (10mg/ml of mecasermin in an injectable solution), thus contributing IGF-1 regeneration-promoting factors. Next, we sealed the sutures with fibrin (Tissucol®, Baxter, Valencia, Spain). The wound was closed in layers with skin resorbable agents.
Postoperative careThe rats were awakened from anesthesia in their cage, 20min after the end of surgery, together with another rat in the same circumstances, due to their gregarious nature. An Elizabethan collar was placed on each rat, so as to prevent autophagia of the limb. The antibiotics administered were Calimicina® LA (oxytetracycline) at doses of 60mg/kg, repeated at 72h, in an interscapular subcutaneous injection according to veterinary guidelines. Analgesia administered was ibuprofen 20mg/ml (Dalsy®, Abbott, Madrid) at doses of 10ml/l of drinking water (200mg ibuprofen/l of drinking water). Cures of the surgical wound were monitored until their closure.
Functional study methodThe rats were weighed and filmed every 2 weeks to record their progress in ambulation. Recovery of motor function was analyzed by the pattern of motion. The Sciatic Functional Index (SFI) is a useful parameter to assess progress during ambulation. We placed a wooden tunnel for the rats to traverse and thus leave marks on a paper after passing through an ink filter. The values obtained from the prints were applied to the formula proposed by Bain et al.18 and the results obtained expressed functional loss as a percentage. A value of 0 represented normal function or absence of dysfunction, whilst a value of −100 represented a total loss of function (Fig. 2).
De Medinaceli et al.19 described the following distances of the experimental (E) and normal (N) legs: tread distance (PL) between the tip of the third digit and the calcaneus, tread width (TS) between the first and fifth digits, and the intermediate tread width (ITS) between the second and the fourth digits, to describe the SFI using the formula:
The “Grasping Test” consisted in raising the rat by its tail, allowing it to grip the cage with its front paws. They were able to do so if they controlled the motility of their hind limbs. Gripping with 4 digits and an extended elbow was accepted as valid. In addition, it enabled us to observe if any digits were missing from the operated limb and if the animal was able to stretch them.
For the functional study we also counted the myelinated axons, evaluating the mean in the preparations according to the location of the section and the therapeutic option chosen for each animal. This was done after the sacrifice of the animals and sizing of the parts, during the observation of the microscopic sections. Subsequently, the results were analyzed by a computer program with incorporated morphometry (JAVA, Jandel Scientific®).
Statistical comparisons were performed with 2-way ANOVA using the statistical software StatView 5.0. Differences of P<.05 between the values were considered as statistically significant.
Animal sacrificeThe rats were sacrificed at 90–100 days after surgery, with mean weights around 450–500g. This was done through an intracardiac injection of sodium pentobarbital (200mg/kg). The surgical approach was repeated, dissecting the segment of the sciatic nerve which included the muscle graft. From this we obtained samples and sections for macro- and microscopic preparations with toluidine blue, hematoxylin–eosin and Masson trichrome staining. To complete this study, the explanted pieces were carved and cut into 7 pieces, all different from each other in cross section and size, but equal in all pieces. Even sections were embedded in resin for toluidine blue staining and odd sections were embedded in paraffin for hematoxylin–eosin and Masson trichrome staining.
ResultsFirst phaseThe functional recovery study showed how the animals regained partial mobility of the limb. During the “Grasping test”, 6 cases could extend the leg and digits, but could not separate them. The rest could not extend or separate the digits. Four animals presented trophic disorders in the intervened distal limb: atrophy of 1 or more digits and ulcers from maintaining a supine position. The remaining 6 rats had all their digits and no ulcers, but, in general, presented amyotrophy of the operated limb.
Preoperatively, the values of the Sciatic Functional Index reflected a normal function (SFI: −7±3.37), whereas at 7 days (−96±6.29), 1 month (−82.24±2.40) and 2 months (−47±11.90) postoperatively, these values decreased, thus indicating an expressive functional loss, reaching values close to normality at the end of the third month (−22.23±2.71).
Macroscopically, the site of the muscle graft appeared whitish and thin. Furthermore, in 4 animals the distal ends became degenerated (lipid degeneration) and presented formation of neuromas. There was a correlation between the appearance of the graft and operated limb function, that is, those grafts whose appearance was that of a nerve and with a less degenerated distal end corresponded to limbs with increased mobility and lower trophic alterations.
Microscopically, in the proximal end we could observe axons of a similar size. The characteristic structure of a healthy nerve section was preserved: with typical myelinated fibers of different diameters distributed into individual fascicles (the mean number of myelinated fibers in each preparation was 5865±911). Over 60% of the samples presented degeneration figures, that is, axons which were more stained by toluidine blue, due to myelin and axonal destructuring (Wallerian degeneration: with advanced axonal loss, myelin debris, vacuoles and macrophages phagocytizing degenerated glycine and mastocytes). In 2 animals we identified sections with mast cells, with a nucleus and granules in the cytoplasm, due to the immune response developed.
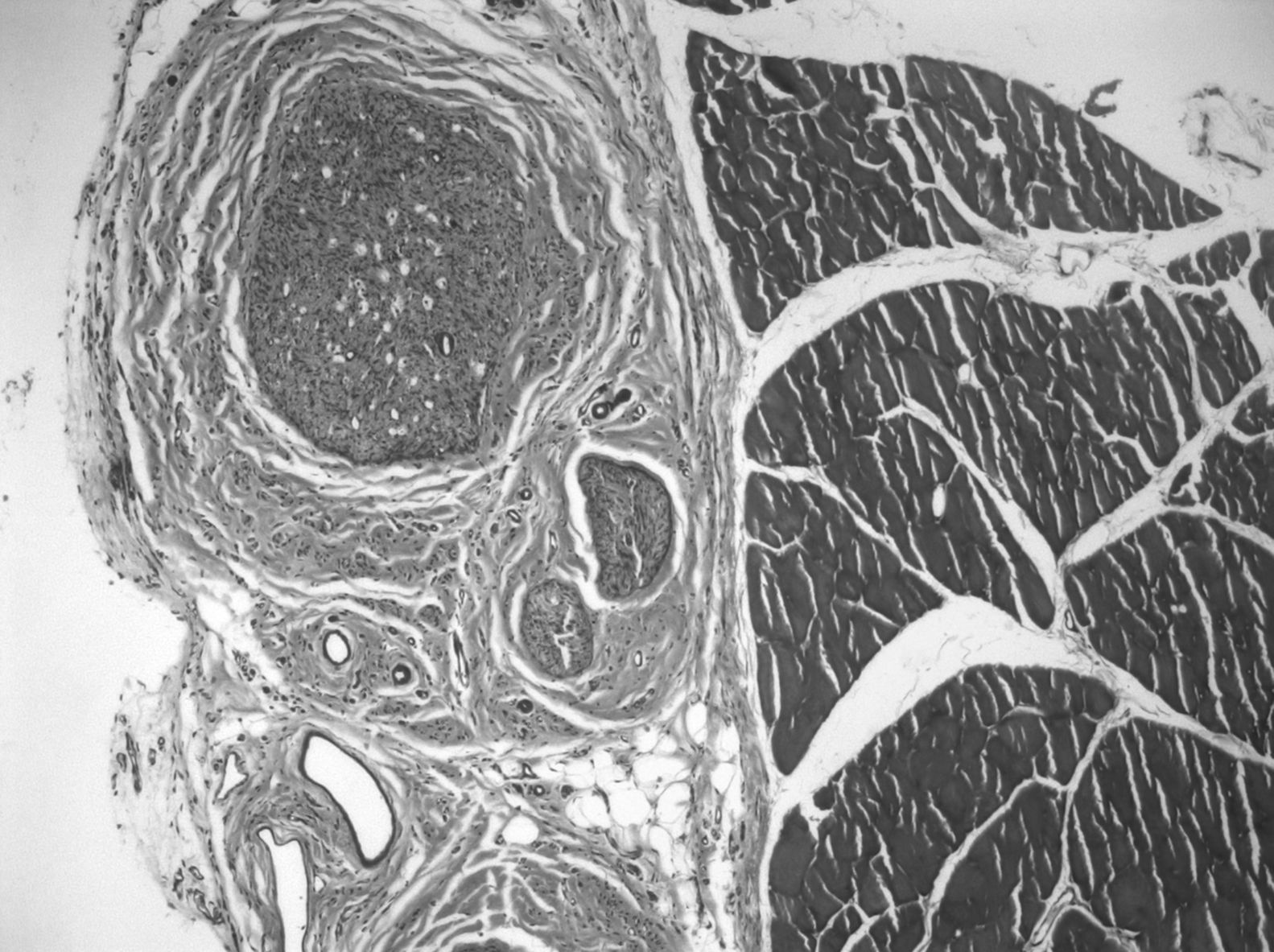
In the graft, the histological findings were more varied. In 68% of the preparations we could observe areas of muscle cells and nerve tissue in place, showing axons with normal morphological characteristics. In 3 cases, we observed nerve-muscle interface sections, with interdigitation of degenerated muscle fibers and axons. Since this was the junction area of the proximal end and the graft, it could be that the 3D structure made the graft larger than the original nerve. The most common finding was axons with very different sizes and histological characteristics (polymorphs), typical of nerve regeneration, being observed in the thickness of the graft by light microscopy. The mean number of myelinated fibers in these preparations was 3519±1560. We observed signs of regenerating nerve advancing in its growth and supported by the bed of muscle cells in the connective tissue located between them: the axons did not grow inside the endomysium, but within its thickness, following its basal lamina on the outside (Fig. 3).
In the distal end we observed adipose and muscular tissue and nerve regeneration areas, as well as more regular myelinated fibers with more space between them (the mean number of myelinated fibers in each preparation was 2756±1156, that is, 47% of those found in the proximal end). Firstly, these fibers degenerated and then their space was invaded by regenerated fibers with a predominance of connective tissue with some undifferentiated cell nuclei and small groups of regenerating axons. The discovery of numerous degenerated axons could be due to the fact that they did not find their target and, therefore, became degenerated. Furthermore, we observed Schwann cell nuclei loaded with axonal debris and degenerated myelin forming stellate shapes and vacuoles, with a typical central nucleus surrounded peripherally by axons forming Bungner regeneration bands.
Second phaseThe functional recovery study showed how the experimental group did not present pressure ulcers in the limbs nor digit losses, although 80% of the controls presented digital flexion contracture in the affected leg, as did 45% of cases which, without extending the digits, achieved a more functional neutral position during the “Grasping Test”.
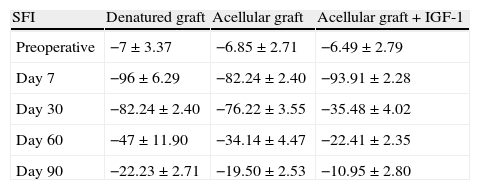
SFI assessment showed the same functional pattern between the different groups studied over time (preoperatively and at 7, 30, 60 and 90 days postoperatively). Values between 0 and −20 obtained in the preoperative period reflected a normal function. Values close to −100, between days 7 and 30 after surgery showed a complete loss of function, thus indicating an absence of innervation during this period. However, it is true that the values were more satisfactory at 30 days than they were without IGF-1 treatment. These values improved from day 60 to 90 after surgery, showing a gradual return of function which coincided with the period when muscle reinnervation began. On day 90 after surgery, these values reached levels close to normality, characteristic of functional recovery, possibly related to a poly-innervation peak (Table 1).
Mean values of the Sciatic Functional Index for the 3 main study groups, according to the date on which plantar prints were measured.
| SFI | Denatured graft | Acellular graft | Acellular graft+IGF-1 |
| Preoperative | −7±3.37 | −6.85±2.71 | −6.49±2.79 |
| Day 7 | −96±6.29 | −82.24±2.40 | −93.91±2.28 |
| Day 30 | −82.24±2.40 | −76.22±3.55 | −35.48±4.02 |
| Day 60 | −47±11.90 | −34.14±4.47 | −22.41±2.35 |
| Day 90 | −22.23±2.71 | −19.50±2.53 | −10.95±2.80 |
SFI: Sciatic Functional Index.
Macroscopically, after the animals were sacrificed for the extraction of the specimens, our attention was drawn to the excessive adherence between contiguous planes, which hindered dissection, as well as the observation of a beige band with a tubular and apparently homogeneous structure between the 2 sutures of the graft, which we then sectioned according to our protocol.
Microscopically, when analyzing the sections, at the level of the proximal end we observed a nerve which appeared normal, with correct, regular and orderly fascicular distribution and perfectly defined by its 3 sheaths, with scarce connective tissue.
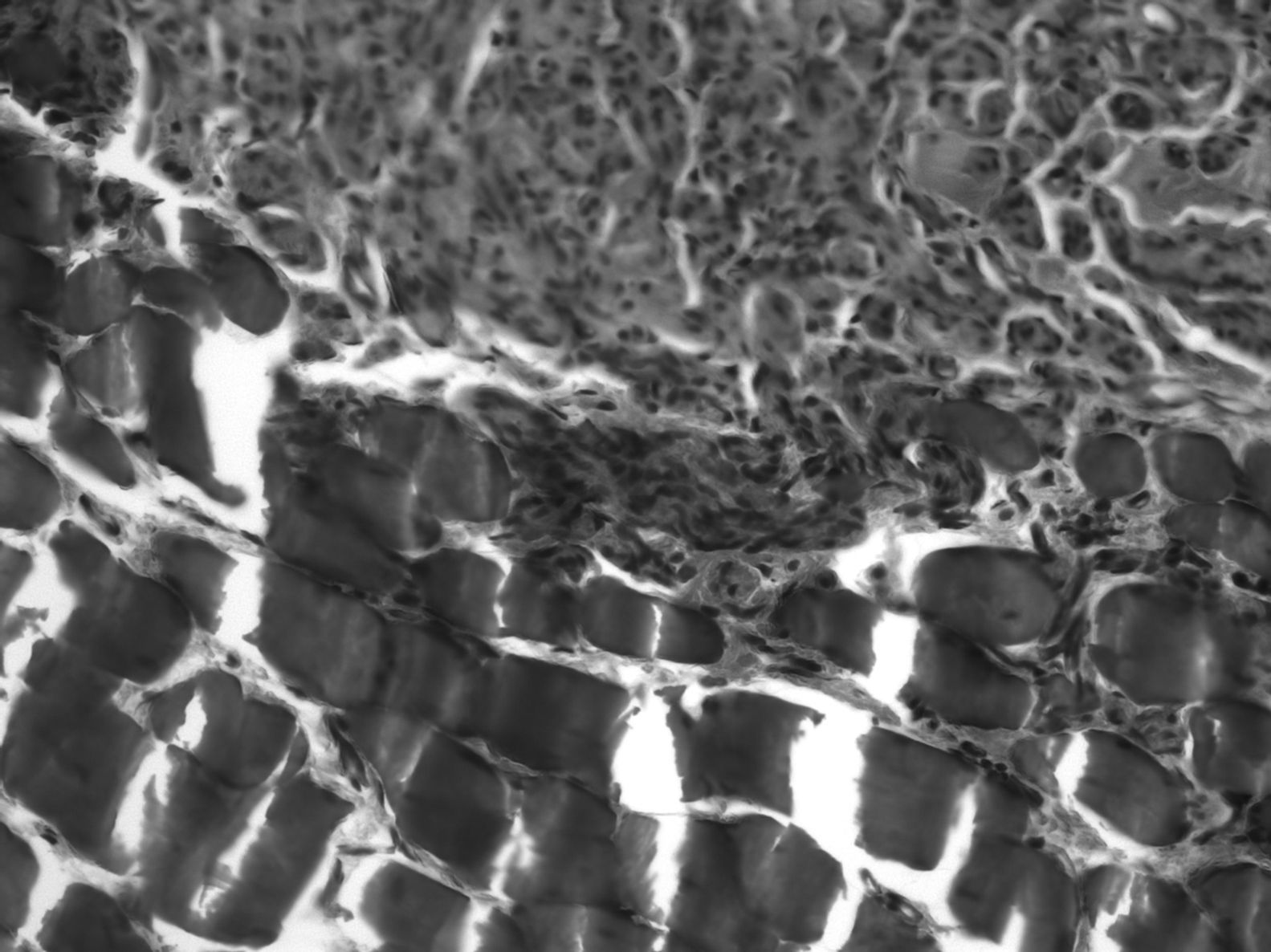
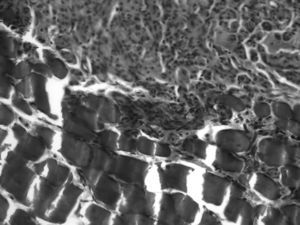
In 80% of cases, at the level of the proximal suture of the muscle graft, we observed how the regenerated nerve used the muscle as a bridge on which to support a distal advance, with numerous small nerve fascicles separated by abundant connective tissue and a multitude of blood vessels (Fig. 4). The mean number of axons in the proximal segment of the animals with IGF-1 treatment was 5740±739, similar to the 10 controls: 5651±931.
In the distal suture of the graft we observed nerves with regenerative characteristics, that is, small, scattered fascicles, large vessels and abundant connective tissue. We also observed some nerve indentations between acellular muscle fibers. The mean number of axons at the level of the graft in animals with IGF-1 was 4385±938 (with 76% of the axons passing to the graft). In the 10 controls this figure was 4143±1030 (73%).
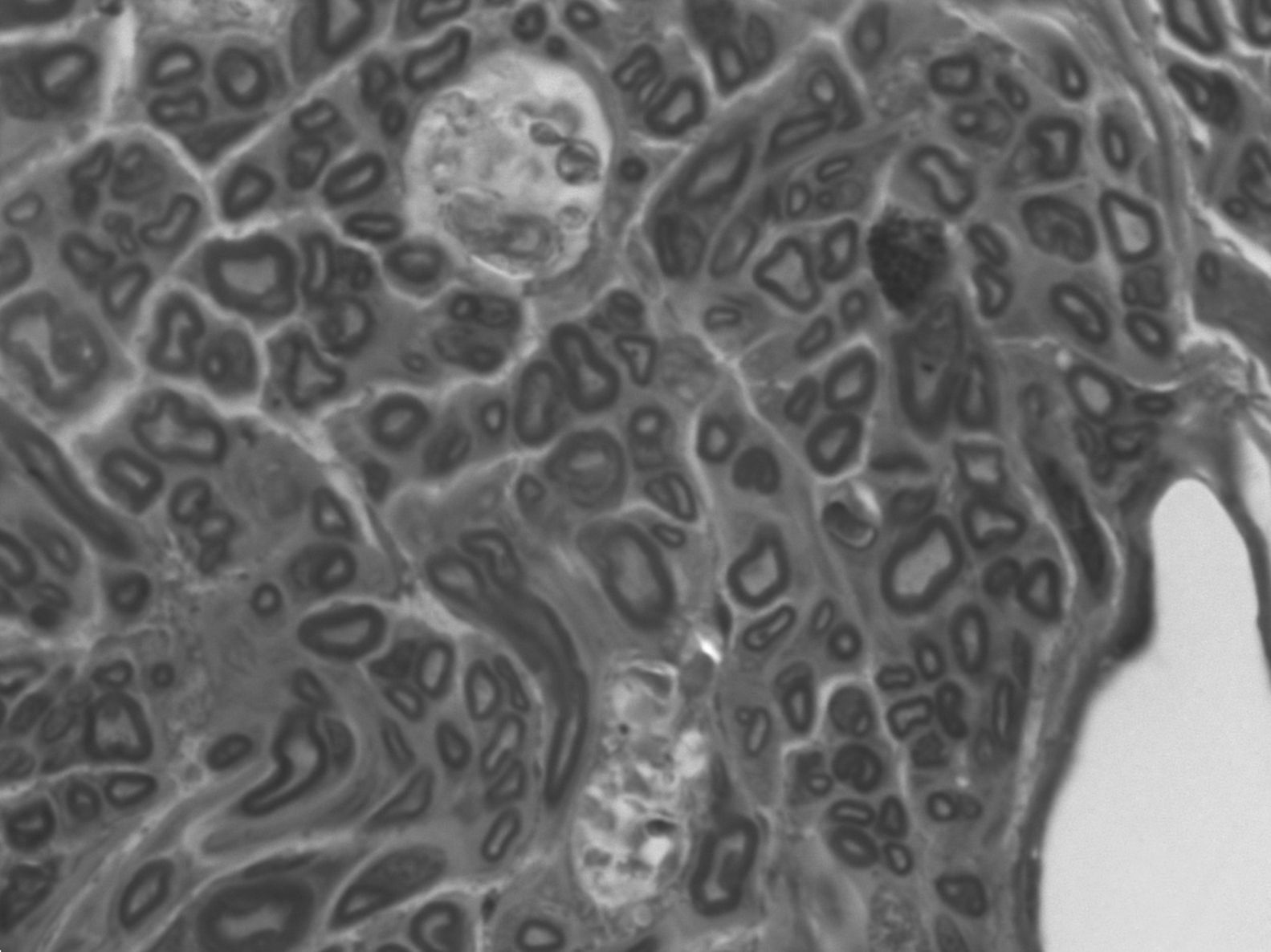
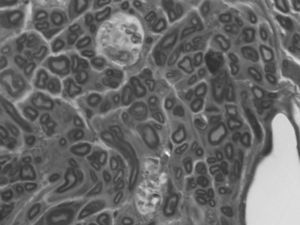
In the distal end there was no clear division into fascicles. There was scarce connective tissue between the fibers but, nevertheless, the epineurium was quite developed. This disorder could be justified: the fibers followed a chaotic trajectory, corresponding to a regenerating nerve whose fibers are still seeking their path. There were a greater number of myelinated fibers in the untreated group, with a smaller diameter and more irregular arrangements (the mean number of axons in the distal end of the animals with IGF-1 was 3552±566, 62% of axons from the proximal end. The mean in control animals was 3183±867, 56%) (Fig. 5). The lower axonal density and the presence of fibers with a smaller diameter showed that the damaged axons were growing and at different rates.
DiscussionPeripheral nerve injuries are a major cause of morbidity and disability in affected patients and generate high economic costs from a global perspective. Noble et al.20 estimated that peripheral nerve injuries affect 2.8% of trauma patients, many of whom become secondarily disabled for life. Repair of the critical defect in these nerves is a challenge for current research, requiring the discovery of economically and biologically feasible techniques, due to the conflict generated by classical bridging with autologous nerve.
This research project has attempted to advance the investigations regarding different methods to present a muscle graft as a bridge in critically injured nerves, and the available options to enrich it biologically. The current project was supported on experimentation with growth factors.15,16
In the first phase of our research we proved that there was passage of axons through a striated muscle graft denatured in a microwave, and that nerve fibers rested on the basal lamina of the muscle fiber (endomysium).7 These results confirmed that the technique selected was adequate, with discovery of tissue showing nerve characteristics in the biological samples obtained from the sciatic nerve at the level of the contributed muscle graft.
These fairly satisfactory results, with partial and disorganized passage of axons, axonal pathways outside the basal lamina of muscle fibers instead of their interior, along with poor functional results, led us to consider a new method of presenting and enriching the graft.
During the second phase of the experiment, we achieved more satisfactory clinical outcomes, with recovery of ambulation in both groups of animals, with growth factor and without it, in which we created a critical defect in the sciatic nerve. During the first 2 postoperative days we observed the appearance of pressure ulcers, as well as several digital losses, in the controls but not in the case group. Furthermore, muscle atrophy of the operated leg, which appeared in 100% of animals, was more pronounced in the control group.
According to Varejão et al.,21 the SFI is the most reliable method to analyze functional recovery, allowing the integration of sensory and motor systems. The results obtained in the functional test were positive in both experiments. Although differences were detected at 30 days of surgery in the treatment with IGF-1, these were not significant, as the values converged toward the end of the follow-up period.
Regarding analysis of the sections under light microscopy, the results were in favor of IGF-1 as a promoter of axonal regeneration, with a larger number of myelinated axons per field, higher axonal density and a larger diameter of the nerve at the distal level being observed. Although it is true that there were several animals in which we did not locate the nerve in the center sections, we did observe a distally regenerated nerve, which makes us think that we could have lost some tissue during the shaping of the sections.
From the analysis of the results obtained we can say that the acellular muscle employed as a graft was a useful bridge for advancing, regenerating axons, perhaps due to the structural proximity of laminin to the nerve sheaths. Thus, we demonstrated that cell determinations in the samples obtained from the case group, with growth factors promoting nerve regeneration at that level, exceeded those obtained from the control group. In 2 cases we verified the passage of axons between the muscle fibers of the graft, but in the remaining animals it was used as a vehicle for passage.
We do not know the half-life of IGF-1 deposited locally in the muscle graft, in our attempt to seal it inside with fibrin glue (Tissucol®, Baxter, Valencia, Spain) placed at the level of the sutures. It may be that it enhances regeneration in the early stages (SFI improvement at 30 days), but is insufficient to maintain it at that level, thus the results achieved by controls converged, without IGF-1 in its action plateau (functional results became equalized at 3 months). We know that several authors, including Kanje et al.,22 Sjöberg and Kanje23 and Peter et al.,24 have described pulsed or continuous releases of local IGF-1 through pumps connected by a catheter from the abdomen of the rat, with an enhancement of the functional and histological nerve results following animal sacrifice.
There are multiple new questions that we shall have to continue investigating through future experiments. Some of the most notable are: the axon uses the endomysium to pass through the muscle, but could it also pass through the interstitial endomysium? Could fibrin used as a seal for our sutures generate more fibrosis and hinder dissection of the sections, even with loss of material? Since muscle seems to be a good vehicle for IGF-1, how could we extend the half-life?
This opens up a new line of research in which we shall try to find a physical and/or pharmacological stimulation to extend survival of the IGF-1 factor to enhance nerve regeneration in critical defects of the PNS.
ConclusionsThe results obtained during the first phase of the investigation confirm that the technique selected was adequate, with the finding of tissue with nerve characteristics in the cuts and sections of the sciatic nerve at the level of the muscle graft. During the second experiment we confirmed our hypothesis by showing that cell determinations in the samples obtained from the case group, with growth factors enhancing nerve regeneration at that level, exceeded those obtained in the control group.
Ethical responsibilitiesProtection of people and animalsThe authors declare that this investigation adhered to the ethical guidelines of the Committee on Responsible Human Experimentation, as well as the World Medical Association and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that this work does not reflect any patient data.
Right to privacy and informed consentThe authors declare that this work does not reflect any patient data.
Conflict of interestsThe authors have no conflict of interests to declare.
The authors wish to thank the SECOT Foundation.
Please, cite this article as: García Medrano B, et al. Regeneración de las lesiones críticas del nervio periférico con factores de crecimiento. Estudio experimental. Rev Esp Cir Ortop Traumatol. 2013;57:162–9.