To describe the experience with tranexamic acid (TXA) during the care of combat causalities treated in the Spanish military hospital based in Herat (Afghanistan) and to perform an analysis of the literature related to the military setting.
Materials and methodsWith the approval of the appropriate military institutions, an analysis was performed on the use of TXA in combat casualties treated between March and May 2014. Of the 745 patients seen, 10 were due to a firearm/explosive device (combat casualties). A descriptive analysis was performed on the data collected. Absolute and relative frequencies (%) were used for the categorical variables. For central tendency measurements, the arithmetic mean and standard deviation or the median and interquartile range was calculated. The data were obtained from the military records of patients treated in the Herat military hospital.
ResultsAll the patients in this series received TXA within the first 3h after the attack. The most frequent dose used was 1g IV, with bleeding was controlled in 100% of cases. All the patients survived and none of them had secondary effects. These data agree with that recommended in the combat casualties treatment guide followed by military health in other countries in this setting.
ConclusionAll combat casualties were treated with TXA within the first 3h. The most frequent dose used was 1g IV and bleeding was controlled in all cases. All the patients survived with no adverse effects being observed.
Describir la experiencia obtenida con el ácido tranexámico (ATX) durante la atención a bajas de combate en el hospital militar español desplegado en Herat (Afganistán) y analizar la bibliografía relacionada en el ámbito militar.
Material y métodosCon la aprobación de las instituciones militares pertinentes, se analizó la administración de ATX en bajas de combate entre marzo y mayo de 2014. De los 745 pacientes atendidos, 10 fueron por arma de fuego/artefacto explosivo (bajas de combate). El método estadístico empleado fue el descriptivo. Para variables categóricas se emplearon frecuencias absolutas y relativas en tanto por ciento (%). Como índices de la tendencia central, la media aritmética y la desviación estándar o la mediana y el rango intercuartílico. Los datos se obtuvieron del registro militar de pacientes atendidos en el hospital militar español de Herat.
ResultadosEn nuestra serie de datos, todos los pacientes recibieron ATX antes de las 3 primeras horas tras el ataque. La dosis empleada más prevalente fue un gramo iv (intravenoso). La hemorragia fue controlada en el 100% de los casos. Todos los pacientes sobrevivieron y en ninguno se objetivaron efectos secundarios. Estos datos coinciden con lo recomendado en las guías de atención a la baja de combate seguidas por sanidades militares de otros países de nuestro entorno.
ConclusiónTodas las bajas en combate fueron tratadas con ATX durante las 3 primeras horas. La dosis más prevalente fue de un gramo iv. La hemorragia fue controlada en la totalidad de los casos. Todos los pacientes sobrevivieron sin efectos secundarios.
Tranexamic acid (TXA), which is sold in Spain under the name Amchafibrin® (Rottapharm, Italy), is a medicine that slows the physiological fibrinolysis system, preventing the degradation of fibrin. TXA acts by binding to the plasminogen lysine receptor site, preventing the fibrin from joining the complex composed of the plasminogen-plasmin tissue activator and causing its degradation. Another possible effect is that of protecting platelets thanks to inhibition of platelet activation factor.1
TXA is used in the field of traumatology. The results of works published in hip arthroplasty surgery,2 knee surgery3 or patient blood management4 programmes indicate that the use of TXA significantly reduces blood loss and the number of patients who receive transfusions.1–4
Military medicine has recently included TXA in the clinical guides for treating combat casualties.5–7 Controlling haemorrhage is of maximum importance in wounds of this type and, together with the use of tourniquets, topical haemostatic material, the early administration of haemoderivatives and the monitoring of coagulopathy, TXA is a new therapy that has helped increase the survival rate of combat casualties.8,9
The aim of this study is to describe the administration of TXA in combat casualties treated by Spanish military doctors deployed in Herat (Afghanistan).
Materials and methodsThis is a descriptive retrospective study undertaken in the Spanish military hospital at Herat (Afghanistan) from March to May 2014. The inclusion criterion covered all casualties due to firearms or explosive devices that arrived at the emergency department of this hospital. Those patients who had not suffered injury caused by firearms or explosive devices were excluded from the study. The quantitative variables were: age, dose of TXA, haemoglobin level, the New Injury Severity Score (NISS), units of red blood cell concentrates administered, units of fresh frozen plasma administered and units of frozen platelets administered. The selected qualitative dichotomising variables were sex (male or female), TXA administration (yes or no), prescription of TXA by a military doctor (yes or no), when TXA was administered (prehospital, intrahospital), moment of haemoglobin analysis (prehospital, intrahospital), vasopressor use (yes or no), haemorrhage control, by fulfilling the following 4 requisites: recovery of skin and mucous membrane colour+GCS≥14+heart rate<100bpm+SBP>120mmHg, the presence of side effects (yes or no) — pulmonary thromboembolism, deep vein thrombosis — and survival 15 days after hospital discharge (yes or no). Other nominal qualitative variables were: time passed from injury to the administration of TXA (<90min, 90min–3h, >3h), haemorrhage control, cause of injury (firearm, explosive device) and anatomical region affected (head/neck, thorax, abdomen, limbs, and spinal column).
Descriptive statistical method was used. Absolute and relative percentage (%) frequencies were used for categorical variables. The arithmetic mean and standard or median deviation and the interquartile range were used as indexes of the core tendency and dispersion of the quantitative variables.
Pie charts were used as graphic representations.
Data were obtained from the military registry of patients treated in the military hospital at Herat (Afghanistan). A data sheet was prepared using the Excel (Windows® 2007) program, while the statistical application used was the SPSS® version 15 package, respecting patient information in accordance with the data protection law. This study was approved by the relevant military institutions.
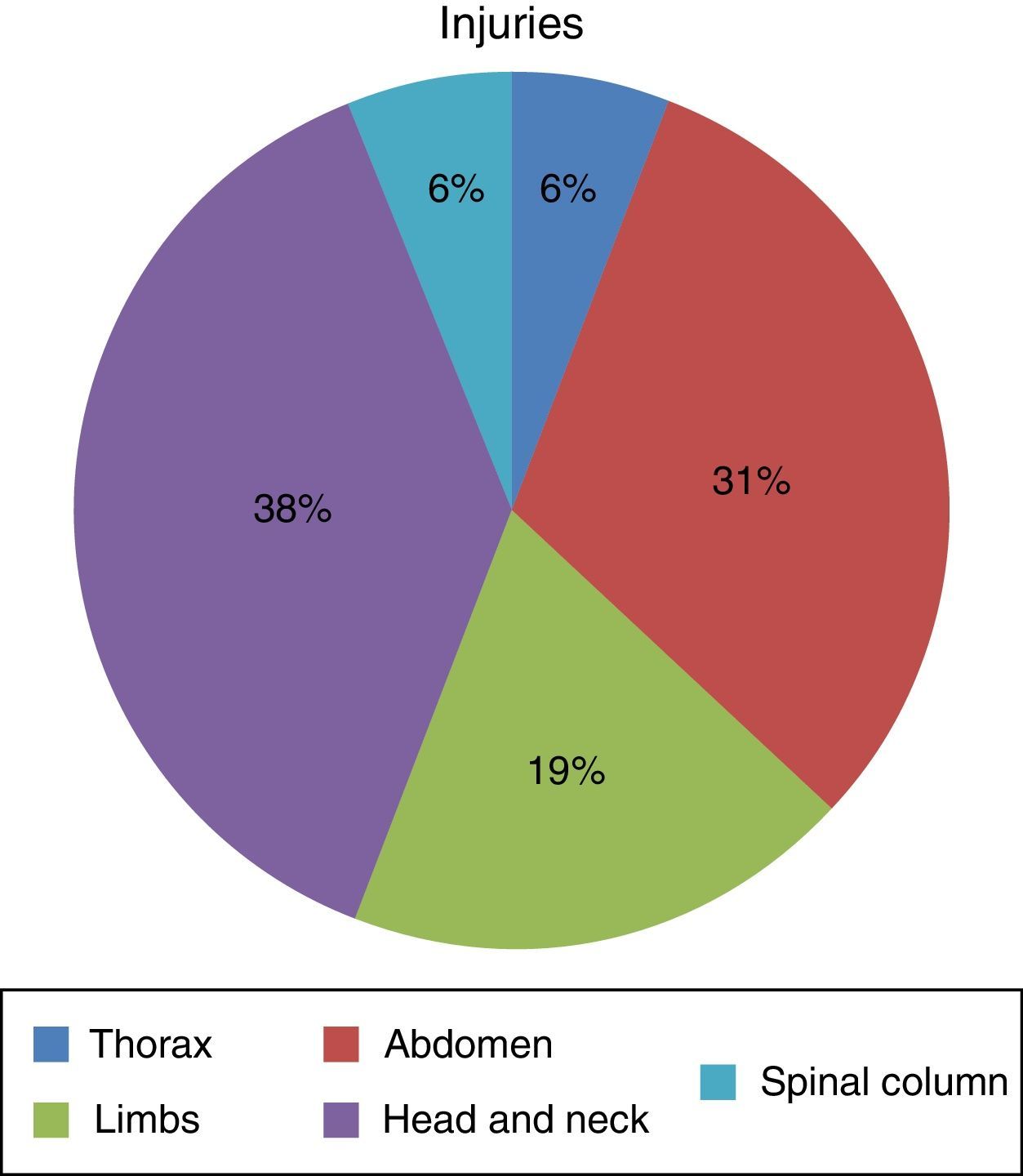
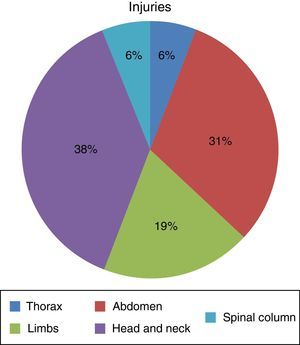
ResultsSeven hundred and forty-five patients were treated in Herat (Afghanistan) military hospital during the period analysed. Of these, 10 were injured by firearm or explosive device (combat casualties) (Table 1). These 10 patients were male and aged from 25 to 30 years old (with an average age of 27.4 years old, SD=1.776) with a NISS Md (IQR)=13 (16.5). Seven suffered injuries caused by an explosive device and the other 3 were injured by firearms. Of a total of 16 anatomical regions affected in these 10 combat casualties: 38% (n=6) corresponded to the head/neck, 31% (n=5) to the abdomen, 19% (n=3) to the limbs, 6% (n=1) to the thorax and 6% (n=1) to the spinal column (Fig. 1) (7 casualties had one region injured, one had 2 injured regions, one had three injured regions and another had 4 injured regions).
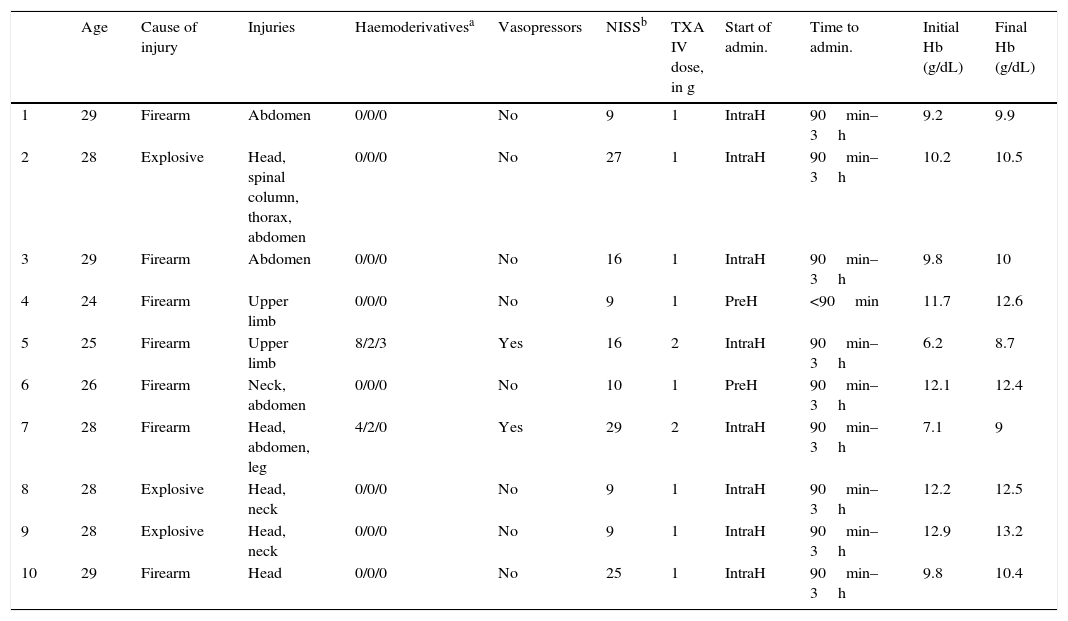
Description of the combat casualties, the cause of injury and treatment with tranexamic acid.
| Age | Cause of injury | Injuries | Haemoderivativesa | Vasopressors | NISSb | TXA IV dose, in g | Start of admin. | Time to admin. | Initial Hb (g/dL) | Final Hb (g/dL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29 | Firearm | Abdomen | 0/0/0 | No | 9 | 1 | IntraH | 90min–3h | 9.2 | 9.9 |
| 2 | 28 | Explosive | Head, spinal column, thorax, abdomen | 0/0/0 | No | 27 | 1 | IntraH | 90min–3h | 10.2 | 10.5 |
| 3 | 29 | Firearm | Abdomen | 0/0/0 | No | 16 | 1 | IntraH | 90min–3h | 9.8 | 10 |
| 4 | 24 | Firearm | Upper limb | 0/0/0 | No | 9 | 1 | PreH | <90min | 11.7 | 12.6 |
| 5 | 25 | Firearm | Upper limb | 8/2/3 | Yes | 16 | 2 | IntraH | 90min–3h | 6.2 | 8.7 |
| 6 | 26 | Firearm | Neck, abdomen | 0/0/0 | No | 10 | 1 | PreH | 90min–3h | 12.1 | 12.4 |
| 7 | 28 | Firearm | Head, abdomen, leg | 4/2/0 | Yes | 29 | 2 | IntraH | 90min–3h | 7.1 | 9 |
| 8 | 28 | Explosive | Head, neck | 0/0/0 | No | 9 | 1 | IntraH | 90min–3h | 12.2 | 12.5 |
| 9 | 28 | Explosive | Head, neck | 0/0/0 | No | 9 | 1 | IntraH | 90min–3h | 12.9 | 13.2 |
| 10 | 29 | Firearm | Head | 0/0/0 | No | 25 | 1 | IntraH | 90min–3h | 9.8 | 10.4 |
Admin.: administration; TXA: tranexamic acid; IntraH: intrahospital; IV: intravenous; PreH: prehospital.
All of these combat casualties were treated using TXA, prescribed in all cases by a medical official. The moment of administration was: prehospital in 20% and intrahospital in 80%. A 1g IV dose was given in 80% of the combat casualties and 20% received 2g IV The latter 20% of cases were patients who received haemoderivatives and vasopressors to stabilise them. The haemorrhage was brought under control in all of the casualties. In 100% of the patients the level of haemoglobin at discharge was higher than it was in the first available analysis, and all measurements were made in the hospital. None of the casualties treated died in the hospital or during the 15 days after discharge from the hospital. There were no side effects (pulmonary thromboembolism or deep vein thrombosis).
DiscussionThe armed conflicts in Iraq and Afghanistan have given rise to notable advances in the diagnosis and treatment of combat casualties, as well as in the implementation of healthcare logistic capacities. In particular, the Spanish armed forces have been deployed in Herat (Afghanistan) since the year 2005. There is a Spanish military hospital in this city which treats NATO troops, the Afghan armed forces and police as well as civilian personnel.10 Military doctors have been deployed crewing armoured ambulances,11 air ambulance helicopters,12 at advanced medical facilities and in Herat military hospital.13 More than 30,000 patients have been treated over 10 years,14 together with approximately 900 combat casualties, so that the logistic-medical footprint (facilities, material, medicines, …) has been deep. Afghanistan is the zone of operations that has evacuated the largest number of casualties to Gómez Ulla Central Military University Hospital, Madrid.15
Control of haemorrhagic shock is one of the basic pillars of military medicine. Haemorrhage is the first preventable cause of death in combat: proper control of bleeding is fundamental for the survival of combatants and is also a challenge within military medicine logistics.16 The addition of TXA to massive haemorrhage and damage control protocols, so that its use is planned in preparation prior to deployment in the combat zone for the treatment of combat casualties, is an advance in military medicine that can be extrapolated to civilian healthcare.
The studies ‘Military Application of Tranexamic Acid in Trauma Emergency Resuscitation Study (MATTERS) I and II’ were carried out by an American and British military research team. MATTERS I5 is a retrospective observational study that compares the administration or non-administration of TXA in patients with haemorrhage who had been treated with at least on unit of red blood cell concentrate. Eight hundred and ninety-six combat casualties in Afghanistan were studied. Of these, 293 received TXA while 603 were not treated with this medication. The mortality in the first group was 17.4% while in the second group it was 23.9% (P=0.03). The study concludes that the use of TXA and haemoderivatives leads to an increase in survival and reduces coagulopathy in combat casualties with haemorrhage. The MATTERS II6 study was published in 2013 with the aim of quantifying the impact of the fibrinogen contained in the cryoprecipitate and TXA on the survival of combat casualties. This retrospective study was undertaken in a military hospital in Afghanistan from 2006 to 2011. One thousand three hundred and thirty-two casualties were selected and included in one of 4 groups: TXA, cryoprecipitate, TXA+cryoprecipitate, no TXA+no cryoprecipitate. This study concluded that mortality is the lowest in the TXA+cryoprecipitate group (11.6%) and in the TXA group (18.2%), compared with the cryoprecipitate group (21.4%) and the no TXA+no cryoprecipitate group (23.6%). TXA and cryoprecipitate were considered to be 2 independent factors that reduce mortality (odds ratio 0.61; 95%; P=0.01 and odds ratio 0.61; 95%; P=0.02, respectively).
TXA is therefore often prescribed in the massive haemorrhage protocols proposed by different military medical corps. The United States guides for military clinical practice7 recommend the administration of 1g of TXA in 100ml IV of saline solution in the first 3h after suffering the injury, and a second dose of 1g in 100ml saline solution during 8h. The procedure selected by British military doctors17 also uses an initial dose of 1g IV TXA in a bolus before 3h have passed since the injury, followed by the perfusion of 1g during 8h. The Spanish protocol for massive haemorrhage in an operations zone18 was proposed at the end of 2011. Like the previous protocols, this one also recommends the use of TXA and subsequent coagulation control by means of thromboelastography.
Israeli medical officials have published 2 papers on their experience of using TXA in combat. In the first of these19 they analyse the use of this medication in the treatment of casualties during the prehospitalisation phase. From 2011 to 2013, 40 casualties were treated using TXA, and the main injury mechanism was penetration (55%). No delays during evacuation caused by the administration of the medication or any adverse effect were detected. The authors propose using TXA from the start of treatment of the trauma.
The previous study is used by Nadler et al.20 to describe civil and military experience with TXA in Israel. Of the 103 casualties analysed, 65 were treated by the Israeli military medical corps. The median Injury Severity Score (ISS) was 16 (9–25). 52% of the casualties had penetrating injuries. In 84% of cases TXA was administered in the first hour following the attack and in 14% from the first to the second hour. Only one casualty was given TXA after the third hour. 75% of the casualties treated by military medical personnel were given the medication under protocol (1g IV TXA must be given to all casualties with at least 2 signs of shock: SBP<90mmHg, HR>11bpm, pallor/sweating, delayed capillary filling or deterioration of the level of awareness, with a penetrating injury in the torso or joints). In this series 2 patients had complications due to pulmonary thromboembolism. Following analysis of this possible adverse effect, a United States series21 of complications found in 296 combat injuries states that 45 had deep vein thrombosis, and that 21 of these had been treated with TXA. Nevertheless, this association is not considered statistically significant.
The French armed forces are involved in many missions in Asia and Africa. They describe their experience with TXA in both continents in one study, and recommend that it be used to treat haemorrhage in combat casualties.22
Additionally, it is a priority to use TXA in the control of damage due to the fibrinolysis that often appears in cases of multiple injuries. A civilian–military study of damage control23 analyses the physiopathology of injuries in adults, and it underlined the importance of the role of TXA as a plasmin and plasmogen inhibitor. Rappold and Pusateri24 of the U.S.A. Defence Department revises the role of the medication in this procedure and concludes that TXA may improve the survival of casualties.
As a result of the long time which western armed forces have been deployed in Iraq and Afghanistan, it is possible to evaluate how the treatment of casualties has evolved and more specifically how TXA is used in such cases. Jansen et al.25 describes how TXA was not used by British troops until 2009, although given the scientific evidence it is now prescribed for the majority of casualties. Pidcoke et al.26 analyses changes in the control of haemorrhage in a study of 3632 casualties, underlining the role of TXA in improving survival.
One of the main advances in military medicine in these 2 major conflicts in the 21st century is the possibility of transfusing haemoderivatives or other haemostatic medicines during transport to hospital. More specifically, O’Reilly et al.27 describes the treatment of 310 casualties in military helicopters, of which 119 (38.4%) received TXA (1g IV), and he considers this medication to be habitual in the prehospital phase.28
On the other hand, the military STAAMP29 study aims to prospectively evaluate the use of TXA compared with a placebo during air transport to hospital as well as treatment in hospital of patients with haemorrhage caused by injury. This study is foreseen to have implications for the care of injury patients and will make it possible to gain evidence on the efficacy, safety profile and optimum dose of TXA.
As the Canadian military doctor Culligan sought earlier administration of TXA, in 2011 he described the possibility of intramuscular (IM) injection by autoinjector at the place of the attack. This author considers that soldiers have experience in the IM administration of drugs such as morphine. He states that this measure would accelerate treatment in casualties with haemorrhage that could not be compressed in the trunk and may increase their survival. After this work was published the Canadian Armed Forces Medical Corps started developing a tranexamic acid autoinjector.30 Wright,31 a British medical lieutenant colonel, also analysed the use of TXA in combat. He considers it to be safe and effective in casualties with haemorrhage, and supports its use without prescription by any member of the armed forces to ensure early universal treatment.
The data obtained in our series follow the same tendency as major international military studies. All casualties were given a suitable dose of TXA in the recommended window period (<3h). In case of massive haemorrhage the dose was doubled and in no case were adverse effects detected.
The authors consider the data studied to be of interest, given that they refer to the first series of combat casualties treated with TXA by Spanish military doctors in an operations zone. The data agree with those of other allied countries.
This study has the following limitations: it is a retrospective study with a limited sample and no control group, in which haemoglobin levels may be affected by multiple variables. Additionally, it was not possible to discover long-term morbimortality as all the patients were taken to civilian Afghan hospital once they had received emergency treatment.
To summarise, in our series of data all of the patients received TXA in the first 3h after suffering an attack. The dose was usually 1g IV, and haemorrhage was controlled in 100% of the casualties. All of the patients survived and no side effects were detected in any of them. These data coincide with the recommendations of different treatment guides for combat casualties followed by the military medical corps of other similar countries.
ConclusionAll of the combat casualties during the period studied were treated with TXA during the first 3h after being attacked. The dose was usually 1g IV, and haemorrhage was controlled in all cases. All of the patients survived and in the selected sample the medication was not found to have any side effects.
The result obtained in this series of cases is similar to those of other studies published by military medical personnel of several allied countries.
The early use of TXA in patients with multiple injuries may be recommended for civilian as well as military use.
Level of evidenceLevel IV.
Ethical responsibilitiesProtection of persons and animalsThe authors declare that no experiments were conducted on human beings or animals for this research.
Confidentiality of dataThe authors declare that no patient data appear in this paper.
Right to privacy and informed consentThe authors declare that no patient data appear in this paper.
Conflict of interestsThe authors have no conflict of interests to declare.
We would like to thank the medical commander Luis Sáenz Casco, of the Intensive Care Department of Gómez Ulla Central Defence Ministry University Hospital, Madrid.
Please cite this article as: Aedo-Martín D, García-Cañas R, Navarro-Suay R, Martínez-Roldán M, Baños-Turza R, Tamburri-Bariain R. Empleo de ácido tranexámico en el herido de combate, experiencia de la sanidad militar española. Serie de casos y revisión de la literatura. Rev Esp Cir Ortop Traumatol. 2016;60:200–205.