Reverse shoulder arthroplasty is becoming a useful tool for many diseases of the shoulder. Any severe glenoid bone defect may affect the fixing of the glenoid component. The aim of this paper is to evaluate the medium-term outcomes of reverse shoulder arthroplasty associated with a glenoplasty.
Materials and methodsA retrospective study was conducted on 5 patients from our hospital, selected due to glenoid defects of different aetiology. All of them where treated with reverse shoulder arthroplasty associated with glenoplasty with bone graft.
ResultsThe minimum follow-up was one year (mean 30.4 months). All grafts were radiologically integrated, with no signs of resorption or necrosis being observed. At 12 months, the Constant score was 66.75 and the mean EVA score was 1.
DiscussionGlenoplasty surgery is technically demanding for restoring original bone size in patients with glenoid structural defects, enabling a reverse shoulder arthroplasty to be implanted. Thus improving both the function and clinical outcomes in selected patients with glenohumeral pathology and providing them with a solution.
La artroplastia invertida se está convirtiendo en una herramienta útil para afecciones muy variadas en el hombro. Un defecto óseo importante de la glena puede afectar a la fijación del componente glenoideo. El propósito de nuestro estudio es evaluar a medio plazo los resultados de la artroplastia invertida de hombro asociados a una glenoplastia.
Material y métodosSe realizó un estudio retrospectivo de 5 pacientes de nuestro hospital con defectos glenoideos de distinta etiología que fueron tratados mediante artroplastia invertida de hombro asociada a glenoplastia.
ResultadosEl seguimiento mínimo de estos pacientes fue de un año (con una media de 30,4 meses). Todos los injertos estaban radiológicamente integrados, sin observarse signos de resorción o necrosis. A los 12 meses el test de Constant era de 66,75 de media y el EVA medio era de 1.
DiscusiónLa glenoplastia es una intervención de alta demanda técnica que consigue restaurar el remanente óseo en pacientes con defectos estructurales, permitiendo así implantar una artroplastia invertida. De esa forma podemos mejorar la función y la clínica en pacientes con diversas afecciones glenohumerales, proporcionándoles una solución.
The indications for anatomical as well as reverse shoulder arthroplasty have increased in number for different pathological processes1,2 (posttraumatic arthritis, rheumatoid arthritis, arthropathy of the rotator cuff, fracture in 4 fragments in the elderly …). Arthroplasty improves pain and function in these patients.3 Apart from the technical difficulty inherent in the implantation of the prosthesis, bone defects in the glenoid may also prevents the proper connection of the implant, increasing the possibility of failure of the surgical operation.4
The aim of this paper is to present an evaluation of the medium-term clinical, radiological and functional results in 5 patients operated using reverse shoulder arthroplasty with associated glenoplasty and also to evaluate the integration of the bone graft used.
Material and methodsWe performed a transversal retrospective study of 5 patients (2 men and 3 women), with an average age of 72.6 years (range 64–85) operated from December 2009 to February 2013. They all presented important glenoid bone defects with different causes: 2 changes of prosthesis (previous partial prosthesis with wear of the glenoid bone and periprosthetic fracture, and change due to septic mobilisation of the previous reverse prosthesis), anterior dislocation fracture in 4 fragments (a Bankart lesion that could not be synthesised due to excessive comminution) and 2 deep luxations (one anterior and one posterior). Glenoplasty was performed in all of them (3 with a humeral head autograft and 2 with an allograft from the tissue bank) together with a reverse shoulder arthroplasty of the Delta XTEND™ Reverse Shoulder System type (DePuy Orthopaedics Inc, a Johnson & Johnson company, Warsaw, U.S.A.). The minimum follow-up time was 12 months (with an average of 30.4 months).
The passive and active mobility of the operated shoulder and homolateral elbow was evaluated using the weighted Constant–Murley test5,6 for functional evaluation. This took place after a 12 month follow-up. Test scores were grouped in bands and several categories were established, from “excellent” with a score of at least 80 points, to “good”, “mediocre” or “poor” when the score was 50 points or less. Constant's test as approved by the Spanish Shoulder and Elbow Society was used to evaluate strength. In this strength (up to a maximum of 25 points) is calculated, if there is no isometric dynamometer, by repeating abduction 3 times with a weight (of up to 12.5kg) and multiplying this weight by 2.
A CT scan was performed on all of the patients to evaluate the osteointegration of the graft. A radiologist specialising in musculoskeletal imaging and an orthopaedic surgeon who were both independent of the study confirmed the absence of resorption, radiotransparent lines or graft descent. Subjective evaluation used the visual analogue scale (VAS score) and a personal satisfaction test.
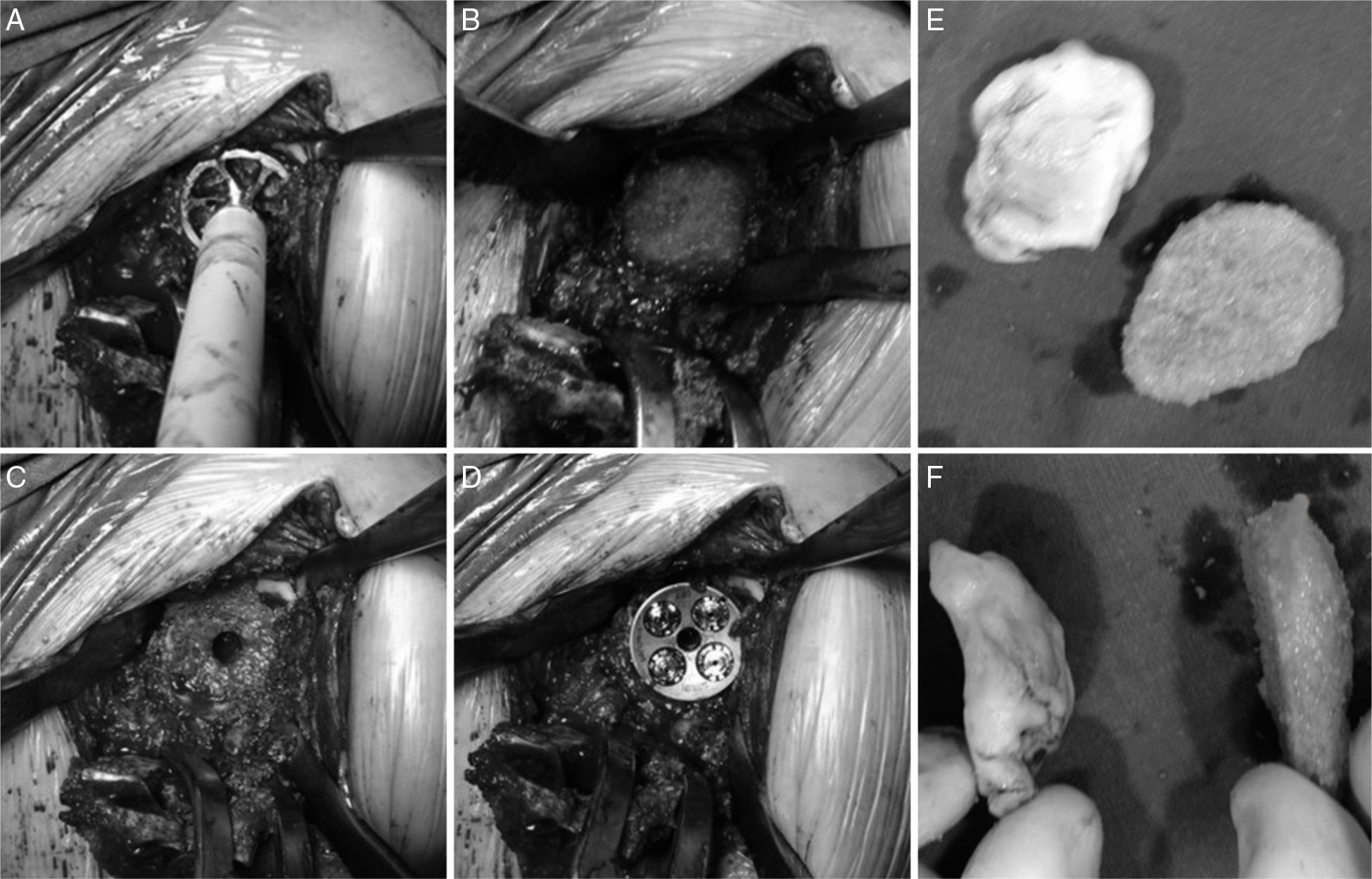
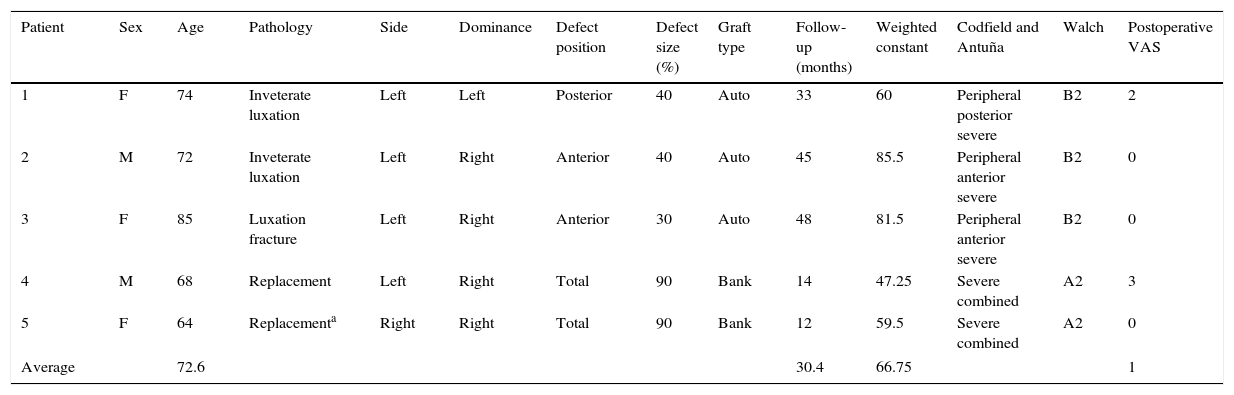
All of the patients were operated under general anaesthesia in the beach chair position with a deltopectoral approach to the shoulder.7 The first step was to prepare the humerus, cutting off the humeral head in the cases in which it was conserved and removing the previous prosthesis in the others. Once this was done there was a complete view of the cavity and glenoid defect. The remaining glenoid bone was abraded together with the defect to be covered until a suitable bed had been created (Fig. 1A and B).
After this the graft was prepared following the technique of Iannotti et al.8 PMMA cement in a malleable state was applied onto the defect to obtain a mould; when it started to set and before it hardened completely it was removed. A ruler was used to mark the reference points that would serve as guides for sculpting the graft into the right shape. Depending on availability, dried humeral head was used in 3 cases while an allograft from the tissue bank was used in 2 cases (Fig. 1E and F).
A small jigsaw was used for this together with a cylindrical rasp. The graft was then placed on the area of the defect to be covered and affixed using 2.4mm cortical screws under compression (2 or 3, depending on each case). These screws are unnecessary in restricted (Walch A2) central defects as they are easily fixed when anchoring the metaglene. Nevertheless, in peripheral (Walch B2) defects we consider that they should be used to improve the fixing of the graft and prevent it from moving during rasping and the positioning of the metaglene.
When implanting these screws the positions that the central lug and the metaglene fixing screws will occupy must be taken into account, while locating the graft-fixing screws in the space between them.
Once the glenoplasty has been completed the neoglene is rasped and the hole is drilled for the metaglene lug (in our series a long lug in one case), putting it into place and impacting it immediately afterwards. The screws, which are all mixed compression and locking screws, are inserted, making sure that they pass through the graft and anchor in the shoulder blade (Fig. 1C and D).
The glenosphere is then put into place. Once the glenoid component is ready the humeral component is prepared, rasping the channel and impacting the rod (cemented in all cases and long in one case) with placement of the corresponding polyethylene.
After surgery the arm was protected by a sling during 4 weeks. As a general rule the elbow and wrist were allowed to move freely immediately after the operation. The patient was told to exercise passively after 48h for the first 2 weeks. Active movements were permitted from the 4th to the 6th weeks, while exercises against resistance were allowed after 12 weeks. The hospital Rehabilitation Department supervised the need for additional physiotherapy.
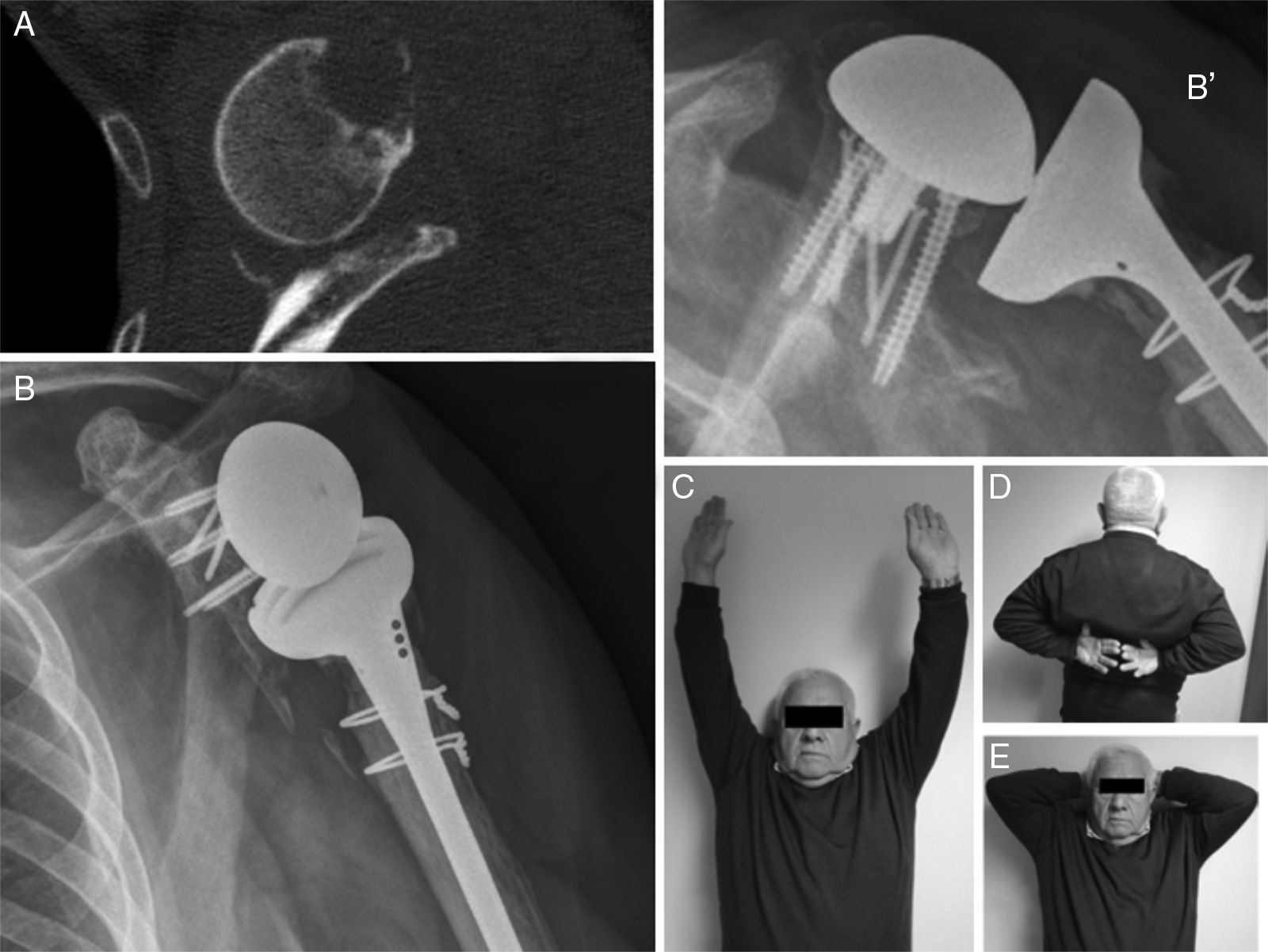
ResultsThe main results are shown in Table 1. We underline that the average weighted Constant test score of the operated shoulders was 66.75 points, which is in the category of “good results”. Two patients had an excellent result (81.5 and 85.5). The results for two patients were less than 65 points, in the group of mediocre results. Only one patient had a result that scored less than 50 points (a poor result) in the case of septic mobilisation of the reverse prosthesis.
| Patient | Sex | Age | Pathology | Side | Dominance | Defect position | Defect size (%) | Graft type | Follow-up (months) | Weighted constant | Codfield and Antuña | Walch | Postoperative VAS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 74 | Inveterate luxation | Left | Left | Posterior | 40 | Auto | 33 | 60 | Peripheral posterior severe | B2 | 2 |
| 2 | M | 72 | Inveterate luxation | Left | Right | Anterior | 40 | Auto | 45 | 85.5 | Peripheral anterior severe | B2 | 0 |
| 3 | F | 85 | Luxation fracture | Left | Right | Anterior | 30 | Auto | 48 | 81.5 | Peripheral anterior severe | B2 | 0 |
| 4 | M | 68 | Replacement | Left | Right | Total | 90 | Bank | 14 | 47.25 | Severe combined | A2 | 3 |
| 5 | F | 64 | Replacementa | Right | Right | Total | 90 | Bank | 12 | 59.5 | Severe combined | A2 | 0 |
| Average | 72.6 | 30.4 | 66.75 | 1 | |||||||||
F: female; M: male.
The replacement corresponds to the periprosthetic fracture in which glenoplasty was used due to the large amount of cavity erosion. The size of the defect was calculated using the preoperative CT scan in those patients when this was available, while for the others this was determined “in situ” during surgery.
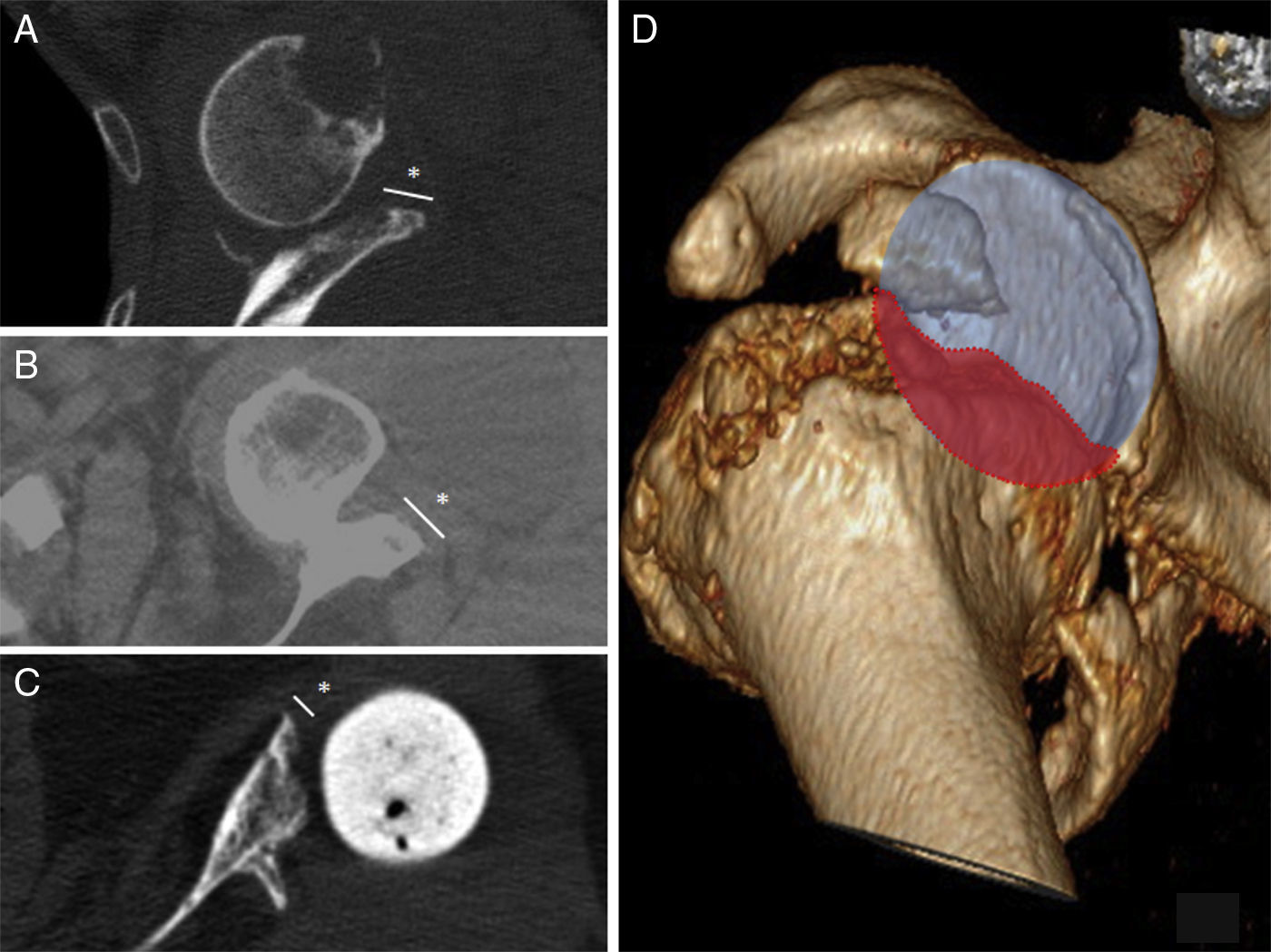
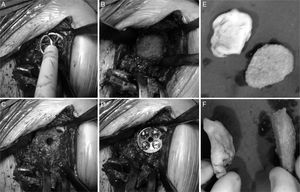
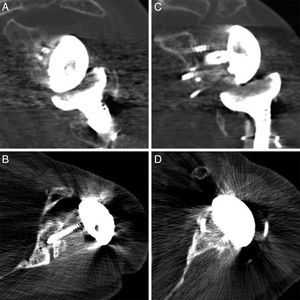
Radiological reports on the CT scans performed state that all of the grafts, auto – as well as alografts, are integrated. No resorption or collapse in them was detected, and no complications arose during the postoperative period (Fig. 2).
The average VAS score was 1 at 12 months (zero in 3 patients), and all of them except one stated that they were highly satisfied with the surgery. The patient operated due to septic mobilisation expressed moderate satisfaction.
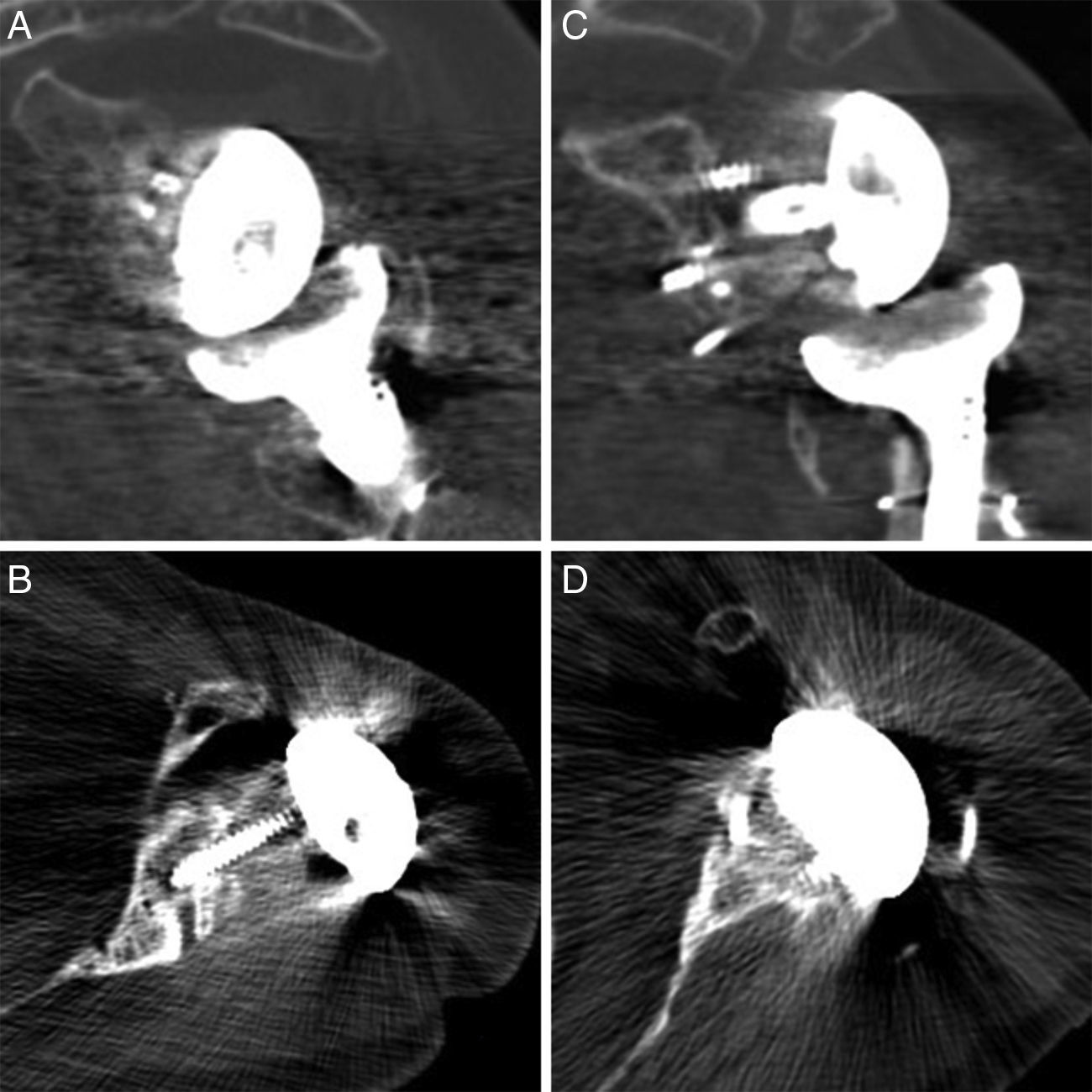
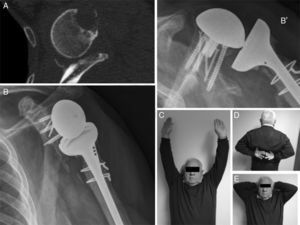
DiscussionGlenoid bone defects reduce the area for fixing screws and therefore make defective implantation more probable.9 Different classifications have been suggested to help locate and manage these glenoid defects, such as the classification systems of Codfield and Antuña,10 Williams et al.,11 Frankle et al.12 or Walch et al.13 The latter may be the simplest, most reproducible and useful for treatment. Walch divides defects into 3 types depending on whether they are central, marginal or total. Although a defect may occur in any zone, the majority are located in the posterior or superior part of the cavity.14 Our sample has one posterior defect, 2 anterior defects and 2 total defects (corresponding to replacements). The size of the defect could be evaluated preoperatively in 3 patients by CT (Fig. 3). Of the other defects without CT, one corresponds to an intraoperative finding while the other is due to replacement because of periprosthetic fracture with erosion of the cavity.
Glenoid defects are sometimes frequently found in patients with glenohumeral problems (osteoarthritis, rheumatoid arthritis, posttraumatic arthritis …) as well as in those with prosthetic replacements.12
We have several options when addressing these defects that may be used alone or in combination.15 The first and the most commonly used is rasping the cavity. This has the drawback of reducing the amount of residual bone and medialising the centre of rotation.16 Another option is to use larger components: this is currently being studied and conclusive results have yet to be obtained.17 The last option is to use bone graft to cover glenoid defects. Glenoplasty may be considered the most suitable technique for severe defects, as it restores the original centre of rotation, permitting conservation of a suitable bone remnant and avoiding excessive abrasion.18 In spite of this grafts are hardly indicated (more so in the case of replacements), at approximately 1% to 3% of cases according to some authors.19
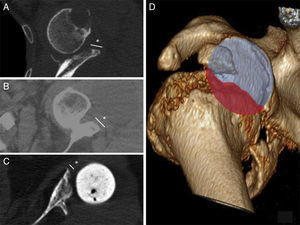
In primary prostheses the most indicated procedure according to the bibliography is to use humeral head autograft18 (which was done in 3 of our patients). Nevertheless, in replacements some authors defend the use of iliac crest graft20 while others prefer tissue bank graft.21,22 The advantages of the iliac crest are that it is more available and is autogenous bone. However, it is a narrow graft which in the majority of cases does not cover the whole defect. It is also usually of low quality in patients of this type, and moreover it adds to the morbidity of the donor zone. Tissue bank graft has the advantages of being more resistant and being able to cover broader defects, although it has a high risk of not integrating correctly or even of resorption. In our series of cases all of the grafts showed satisfactory integration in CT scan images (Fig. 4).
In cases of major glenoid defects the aim of all arthroplasty is to create a stable base that makes it possible to implant the components securely, thereby improving their function and shoulder pain. Although using a bone graft to cover large defects is a hardly used technique it is very useful as it even permits the implantation of a reverse prosthesis, increasing the area of the cavity and, in cases where the defect is total, lateralising the centre of rotation of the joint.23
Many authors consider that lateralising the centre of rotation offers major advantages such as improved internal and external rotation (as more anterior and posterior deltoid fibres are recruited), retensing the remains of the rotator cuff that increases stability as well as increasing humeral projection or offset, making it possible to reduce the rate of infrascapular notching.24
Some authors state that this lateralisation of the centre of rotation may also have drawbacks, given that it increases the stress forces on the bone-prosthesis interface, increasing the risk of the implant loosening and failing.25
Our reverse arthroplasty technique and glenoplasty with a graft makes this lateralisation possible in total defects secondary to revision of the prosthesis, thereby recovering the original surface area and position of the cavity. Nevertheless, once the graft has integrated with the native bone these forces fall considerably, contrary to the lateralisation which occurs in anatomical prostheses.18
Until enlarged implants develop and prove themselves to be effective glenoplasty with a graft will continue to be the recommended technique recommended by many authors26–28 to treat major bone defects. It is currently indicated for: irregular wear or a defect that cannot be corrected by small changes in the version of the components or when the bone remnant is insufficient to support the metaglene.
Although the most suitable technique for implanting the graft has yet to be established, some authors state that while good anchoring is ensured (and thereby bone integration too) there are no contraindications for reverse arthroplasty in these cases.29
Our glenoplasty technique and results are similar to those which appear in the most recent publications.30 Although our series is limited in the number of cases, the good bone integration of the graft in all cases indicates that this, in spite of its demanding technical nature, may be an appropriate option to obtain improved shoulder function and quality of life for these patients.
Ethical responsibilitiesProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors must have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence must be in possession of this document.
The authors would like to thank Dr. Rafael Montero Pérez-Barquero, specialist musculoskeletal radiologist, for his selfless collaboration in this work. We would also like to thank Dr. María Teresa Urbano Luque for her statistical recommendations and support during the whole scientific and editing process.
Please cite this article as: Díaz Miñarro JC, Izquierdo Fernández A, Muñoz Reyes F, Carpintero Lluch R, Uceda Carrascosa P, Muñoz Luna F, et al. La artroplastia invertida de hombro ante defectos óseos glenoideos. Rev Esp Cir Ortop Traumatol. 2016;60:206–213.