Age is one of the principal risk factors for development of frailty fractures. Age pyramids show a population that is becoming increasingly more elderly, with an increasing incidence of fractures, and the forecasts for the future are truly alarming. Adequate handling of these patients who are especially at risk, at both the preventive and care levels, with a well-defined orthogeriatric model is necessary to respond to this clinical challenge. The objective of this review is to analyze the efficacy of the different strategies for the handling of geriatric patients with fracture risk.
La edad es uno de los principales factores de riesgo para desarrollar una fractura por fragilidad. Las pirámides de edad muestran una población cada vez más envejecida y la incidencia de fracturas es cada vez mayor, siendo las previsiones para el futuro verdaderamente preocupantes. Un adecuado manejo de estos pacientes de especial riesgo, tanto a nivel preventivo como asistencial con un modelo ortogeriátrico bien definido se hacen necesarias para hacer frente a este reto clínico. En esta revisión queremos realizar un análisis de la eficacia de las diferentes estrategias de manejo del paciente geriátrico con riesgo de fractura.
As the age of the population increases, the incidence of osteoporosis and its direct consequence, fragility fractures, are also increasing.1 The presence of a fragility fracture multiplies the risk of a second fracture by three, which in turn multiplies the risk of a third fracture by five, and of a fourth by eight2. It has been calculated that in the first year following a fracture, between 9% and 14% of patients will suffer another, despite the competing risk of increased mortality associated with them.2,3 The increased mortality related to osteoporotic fractures varies with location, advanced age, and the time elapsed following the fracture.4–6
Hip fractures are associated with the greatest number of complications, functional deterioration, need for more assistance, and mortality of up to 30% one year after the fracture.7–10 This problem also represents a significant expense for healthcare systems, surpassing even the cost of other frequent pathologies such as diabetes.11–13
Numerous drugs have shown an improvement in regard to parameters of bone mineral density, reduction of fractures, and mortality in patients with osteoporosis.4,14–17 Decisions by the administration, guidelines of scientific societies, and prices of the treatments themselves are variables that can influence both the increased detection and treatment of the problem as well as its repercussions in terms of the incidence of fractures. Consequently, the publication of the 2005 NICE Guidelines on the secondary prevention of fractures, along with the commercialization of the generic of alendronate, encouraged an increase in the prescription of the drug, with a reduction in major fractures and hip fractures later observed in the United Kingdom.18 On the other hand, in the US, the reduction in the reimbursement for densitometry in 2007 resulted in a decrease in the diagnosis of osteoporosis in the years that followed and halted the downward evolution of the incidence of hip fractures that had been observed since the year 2000.19
We are therefore dealing with a pathology that is very prevalent in the elderly, incapacitating, that reduces survival to a large degree, that involves a high cost for the care of the direct consequences, which are fractures, and for which treatments exist that can reduce its incidence. These reasons are sufficient to treat this process.
This article reviews the efficacy and cost-effectiveness of treatment of osteoporosis in the elderly, as well as its effect on mortality and aspects to be taken into account before initiating prescription of the treatment.
Efficacy of anti-osteoporotic drugs in the elderly and cost-effectivenessCalcium and vitamin DAt least three meta-analyses have shown that treatment with calcium and vitamin D reduce the incidence of fractures,20–22 by percentages that range from 6% to 23% in the reduction of non-vertebral fractures and 16–30% for hip fractures. Several other studies have found a relationship between low vitamin D levels and increased incidence of osteoporotic fractures.23–25
Nevertheless, a recent meta-analysis did not find decreased risk of fracture in patients of the community treated with calcium and/or vitamin D, although the age criterion for inclusion was older than 50 years of age, so patients were not overwhelmingly those with greater risk of fractures.26 Hiligsman showed that this treatment is cost-effective in women over the age of 60 diagnosed with osteoporosis, but it is much more effective to treat women over the age of 80 with this diagnosis.27
Although some publications have suggested that calcium supplementation could be associated with increased cardiovascular risk,28,29 recently doubts have arising regarding defects in the methodology that condition the validity of the results of these studies30 and therefore the NOF (National Osteoporosis Foundation) as well as the ASPC (American Society for Preventive Cardiology) have published that there is no evidence demonstrating cardiovascular problems at calcium doses between 2000 and 2500mg/day.31
According to the recent Clinical Practice Guidelines (CPG) of the American College of Physicians with a moderate degree of evidence, that the efficacy of calcium and vitamin D in the reduction of fractures is uncertain.17
BiphosphonatesAccording to most of the Clinical Guidelines, biphosphonates are considered to be first-line drugs in the treatment of osteoporosis due to their low cost and long-term safety, making them the most cost-effective, especially the generics alendronate and zoledronate.32
EfficacyThis group of drugs can reduce the incidence of osteoporotic fractures, both vertebral and non-vertebral, and hip fractures.33,34 The cumulative benefit in the reduction of fractures over 3 years varies between an RR of 0.3 and 1. However, not all have shown the same efficacy.14 In a recent meta-analysis of 39,197 patients34 the evidence of the efficacy of biphosphonates in reducing osteoporotic fractures was updated. In this case, zoledronic acid was once again found to be the most effective, with an OR 0.61 (0.49–0.76) in comparison with alendronate OR 0.64 (0.48–0.84) or risedronate OR 0.74 (0.63–0.85).
Most pivotal studies have been done on patients younger than 80 years of age, but in recent years sub-analysis results in the elderly population have become available. Hochberg,35 showed that alendronate reduces the incidence of the most prevalent fractures. They found a reduction in the absolute risk of the set of vertebral, wrist, and hip fracture, and that effect increased with age; in other words, it is more effective in patients between 75 and 85 years of age because they are the population with the highest risk. A post hoc analysis of data from the largest alendronate study (Fracture Intervention Trial, FIT) of an elderly population with non-vertebral fracture, suggested, without achieving statistical significance, that alendronate was less effective in reducing fractures in women with T-scores above −2.5 DE than in women with osteoporosis.36 Boonen37 carried out a post hoc analysis in women over the age of 80, combining 3 large randomized double-blind clinical trials with risedronate (HIP, VERT-NA and VERT-MN). It showed that in the first year, the reduction of vertebral fractures with risedronate was 81%, with similar results in subjects older and younger than 80 years of age, finding no effect on hip fractures. The number of patients needed to treat (NNT) for one year to avoid a vertebral fracture is 12. The same author38 did a post hoc of the Horizon comparing annual zoledronic acid with a placebo in women over the age of 75 with 3-year follow-up and it showed a reduction in vertebral and non-vertebral fractures with a hazard ratio of 0.34 and 0.73, respectively. As with the previous work, it did not manage to demonstrate a reduction in hip fractures, attributing this to the multiple extra-skeletal factors that favour this type of fracture. Also, Nordström,39 in a study on 93,601 patients, showed that the reduction in the risk of fracture after treatment with bisphosphonate is similar in patients over the age of 80 with regard to younger patients (25% lower risk of another hip fracture after a previous fracture). Another detail shows a meta-analysis, meta-regression of 13 studies on the efficacy of antiresorptive drugs in elderly subjects from 70–80 years of age, finding that the reduction in the risk of vertebral fracture increases with age, with body mass index, and duration of the treatment, although once again, this effect was not observed in hip fractures.40 In 6 of the studies, the average age was over 70, although just two studies were aimed at elderly women: TROPOS (74y) and HIP (70–79 and >80y).
EfficiencyThere are several experiences that show that treatment with biphosphonates in the elderly is equally or more cost effective than at other ages because they are the population with the highest risk of fracture. Many of the studies used the Markov model, which was created to assess the cost-effectiveness of the treatment of patients with osteoporosis in different countries. It represents the possible health transitions from the start of treatment, such as: prevention of fractures up to 100 years of age, including fractures of the radius, vertebra, hip, other osteoporotic fractures, fracture after vertebral fracture, fracture after hip fracture, and death. In 6-month cycles, patients could remain healthy, suffer fractures, or death. Nayak41 used a simulation to study the cost of performing screening using DXA and treatment with alendronate if the result was compatible with osteoporosis in women over the age of 65. They compared it with treatment only after a fragility fracture. The simulation assessed the cost of the screening, of the treatment, of the adverse effects and mortality, of the repercussion on admittance to residences, of the quality-adjusted life years (QALY) and of the increase in cost-effectiveness ratios (ICERs). They found, assuming a cost of alendronate between 400 and 800 dollars per year, that screening and pertinent treatment is highly cost effective, but if the cost of alendronate drops to 200$ per year, the screening and treatment decision is much more inexpensive than treating the consequences of osteoporosis. A similar study done in China42 showed that treatment of osteoporosis in women over the age of 75 is more cost effective than in younger women, varying the ratio with the treatment threshold and the cost of the treatment in China. They found that treatment in elderly patients with more than a 3% risk of suffering hip fracture within 10 years is cost effective, while other authors increase that risk to 6.5% to achieve cost-effectiveness in males of the same age.43
Tjeerd-Peter van Staa44 published the first economic model in the field of osteoporosis applying individual fracture and mortality risks. Treatment with biphosphonates was cost-effective in patients with a 5-year risk of osteoporotic fracture of 9.3%. It was effective provided that bone mineral density (BMD) was low, and was less effective if BMD was normal. In 2005, Kanis45 published models in which treatment with biphosphonates was cost-effective. Therefore, a drug that reduces fractures by 35% and at an annual cost of 500$ would be cost-effective if the 10-year probability of hip fracture was 1.2% in women 50 years of age and 7.2% in women 85 years of age. This study found that younger patients with several clinical risk factors had fracture risk and mortality comparable to older patients without risk factors and those patients had similar intervention thresholds when clinical risk factors were taken into account. It was found that treatment with biphosphonates reduced costs in almost all women older than 80 years of age and in patients with low body mass index (BMI). They observed that the avoided costs resulting from the prevented fractures exceeded the cost of the intervention.
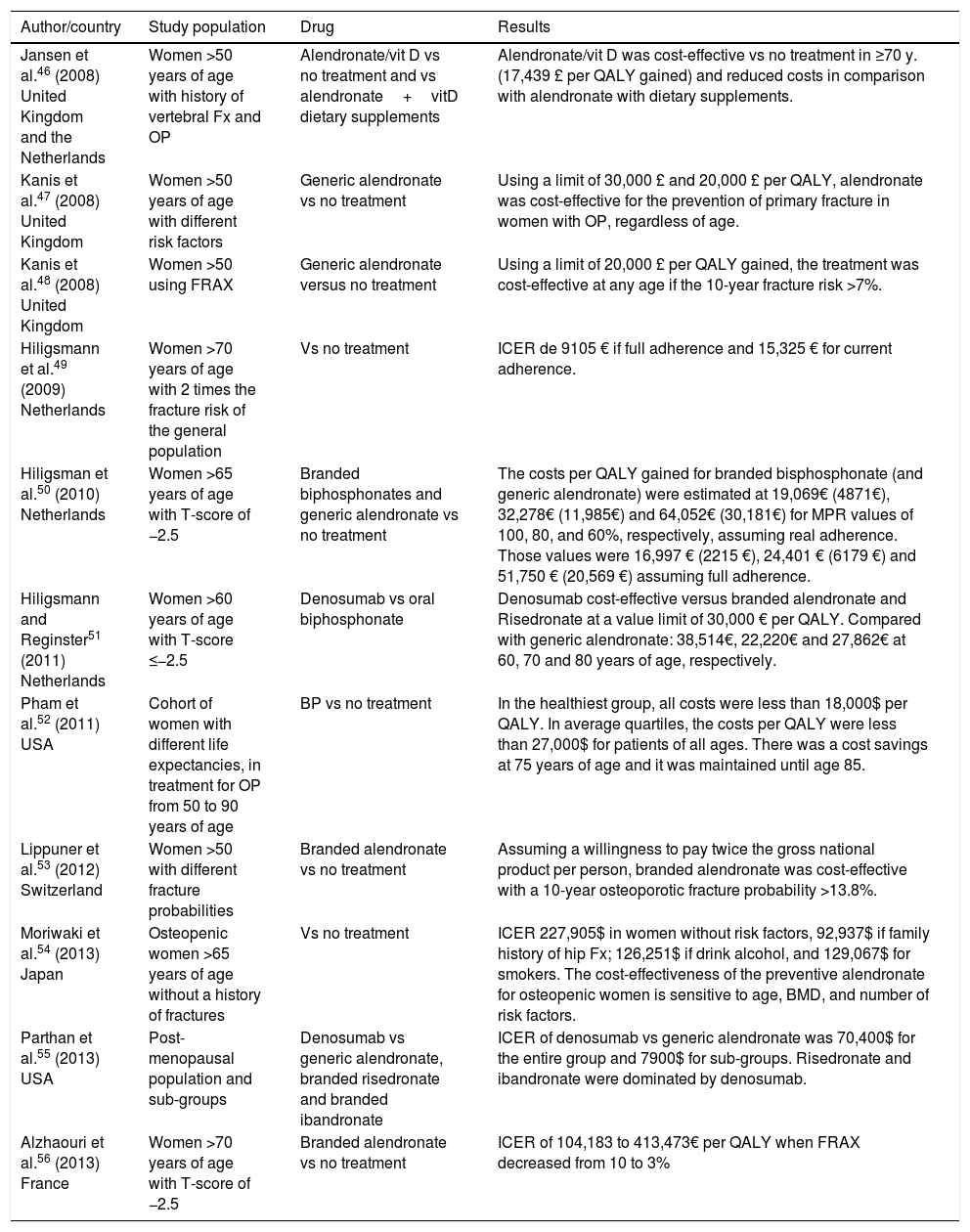
Tables 1–3 show studies on the cost-efficiency of biphosphonates (alendronate, risedronate, and zoledronic acid) that showed efficacy in reducing vertebral fractures (VF), non-vertebral fractures (NVF) and hip fractures (HF).
Cost-effectiveness of alendronate.
| Author/country | Study population | Drug | Results |
|---|---|---|---|
| Jansen et al.46 (2008) United Kingdom and the Netherlands | Women >50 years of age with history of vertebral Fx and OP | Alendronate/vit D vs no treatment and vs alendronate+vitD dietary supplements | Alendronate/vit D was cost-effective vs no treatment in ≥70 y. (17,439 £ per QALY gained) and reduced costs in comparison with alendronate with dietary supplements. |
| Kanis et al.47 (2008) United Kingdom | Women >50 years of age with different risk factors | Generic alendronate vs no treatment | Using a limit of 30,000 £ and 20,000 £ per QALY, alendronate was cost-effective for the prevention of primary fracture in women with OP, regardless of age. |
| Kanis et al.48 (2008) United Kingdom | Women >50 using FRAX | Generic alendronate versus no treatment | Using a limit of 20,000 £ per QALY gained, the treatment was cost-effective at any age if the 10-year fracture risk >7%. |
| Hiligsmann et al.49 (2009) Netherlands | Women >70 years of age with 2 times the fracture risk of the general population | Vs no treatment | ICER de 9105 € if full adherence and 15,325 € for current adherence. |
| Hiligsman et al.50 (2010) Netherlands | Women >65 years of age with T-score of −2.5 | Branded biphosphonates and generic alendronate vs no treatment | The costs per QALY gained for branded bisphosphonate (and generic alendronate) were estimated at 19,069€ (4871€), 32,278€ (11,985€) and 64,052€ (30,181€) for MPR values of 100, 80, and 60%, respectively, assuming real adherence. Those values were 16,997 € (2215 €), 24,401 € (6179 €) and 51,750 € (20,569 €) assuming full adherence. |
| Hiligsmann and Reginster51 (2011) Netherlands | Women >60 years of age with T-score ≤−2.5 | Denosumab vs oral biphosphonate | Denosumab cost-effective versus branded alendronate and Risedronate at a value limit of 30,000 € per QALY. Compared with generic alendronate: 38,514€, 22,220€ and 27,862€ at 60, 70 and 80 years of age, respectively. |
| Pham et al.52 (2011) USA | Cohort of women with different life expectancies, in treatment for OP from 50 to 90 years of age | BP vs no treatment | In the healthiest group, all costs were less than 18,000$ per QALY. In average quartiles, the costs per QALY were less than 27,000$ for patients of all ages. There was a cost savings at 75 years of age and it was maintained until age 85. |
| Lippuner et al.53 (2012) Switzerland | Women >50 with different fracture probabilities | Branded alendronate vs no treatment | Assuming a willingness to pay twice the gross national product per person, branded alendronate was cost-effective with a 10-year osteoporotic fracture probability >13.8%. |
| Moriwaki et al.54 (2013) Japan | Osteopenic women >65 years of age without a history of fractures | Vs no treatment | ICER 227,905$ in women without risk factors, 92,937$ if family history of hip Fx; 126,251$ if drink alcohol, and 129,067$ for smokers. The cost-effectiveness of the preventive alendronate for osteopenic women is sensitive to age, BMD, and number of risk factors. |
| Parthan et al.55 (2013) USA | Post-menopausal population and sub-groups | Denosumab vs generic alendronate, branded risedronate and branded ibandronate | ICER of denosumab vs generic alendronate was 70,400$ for the entire group and 7900$ for sub-groups. Risedronate and ibandronate were dominated by denosumab. |
| Alzhaouri et al.56 (2013) France | Women >70 years of age with T-score of −2.5 | Branded alendronate vs no treatment | ICER of 104,183 to 413,473€ per QALY when FRAX decreased from 10 to 3% |
ICER: incremental cost effectiveness ratio; QALY: quality-adjusted life years; FRAX: tool that makes it possible to calculate fracture risk.
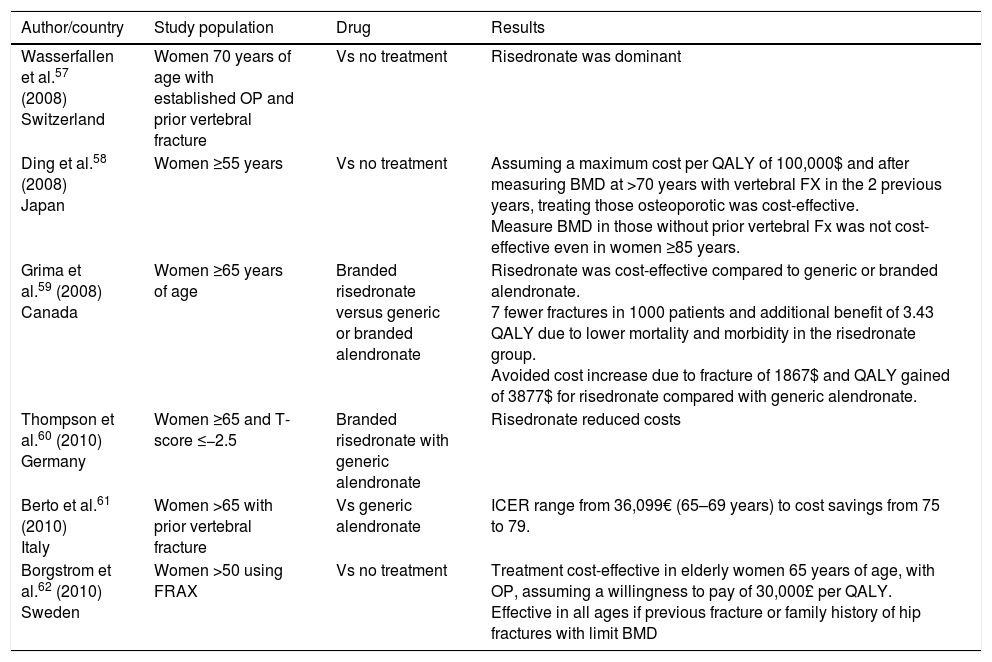
Cost-effectiveness of risedronate.
| Author/country | Study population | Drug | Results |
|---|---|---|---|
| Wasserfallen et al.57 (2008) Switzerland | Women 70 years of age with established OP and prior vertebral fracture | Vs no treatment | Risedronate was dominant |
| Ding et al.58 (2008) Japan | Women ≥55 years | Vs no treatment | Assuming a maximum cost per QALY of 100,000$ and after measuring BMD at >70 years with vertebral FX in the 2 previous years, treating those osteoporotic was cost-effective. Measure BMD in those without prior vertebral Fx was not cost-effective even in women ≥85 years. |
| Grima et al.59 (2008) Canada | Women ≥65 years of age | Branded risedronate versus generic or branded alendronate | Risedronate was cost-effective compared to generic or branded alendronate. 7 fewer fractures in 1000 patients and additional benefit of 3.43 QALY due to lower mortality and morbidity in the risedronate group. Avoided cost increase due to fracture of 1867$ and QALY gained of 3877$ for risedronate compared with generic alendronate. |
| Thompson et al.60 (2010) Germany | Women ≥65 and T-score ≤−2.5 | Branded risedronate with generic alendronate | Risedronate reduced costs |
| Berto et al.61 (2010) Italy | Women >65 with prior vertebral fracture | Vs generic alendronate | ICER range from 36,099€ (65–69 years) to cost savings from 75 to 79. |
| Borgstrom et al.62 (2010) Sweden | Women >50 using FRAX | Vs no treatment | Treatment cost-effective in elderly women 65 years of age, with OP, assuming a willingness to pay of 30,000£ per QALY. Effective in all ages if previous fracture or family history of hip fractures with limit BMD |
Cost-effectiveness of zoledronic acid.
| Author/country | Study population | Drug | Results |
|---|---|---|---|
| Akehurst et al.63 (2011) Finland, Norway, and the Netherlands | Women 50–80 years of age, previous fracture and T-score −2.5 | Vs calcium/vit D, biphosphonates | Reduced costs with regard to Ca/vitD and branded alendronate. Compared with generic alendronate) |
| Fardellone et al.64 (2010) France | Women >65 years of age | Vs other treatments | The cost per vertebral fracture avoided was 1497€ versus 1685€ |
| Moriwaki et al.65 (2017) Japan | Women 70 years of age, with OP and prior vertebral Fracture | Vs weekly alendronate | Zoledronic acid less effective and more costly for those with a risk factor (tobacco, alcohol, prior family history of fractures). Alendronate dominates but with very small increase in QALY. Considering the advantage of the yearly dosage (better adherence), zoledronic acid could be a cost-effective option. |
This is an antiresorptive monoclonal antibody drug that inhibits the formation, activation, and survival of osteoclasts by blocking the RANK/RANKL/OPG system. There are publications that show that Denosumab increases BMD more than biphosphonates66 and this increase was found to be continuous and almost linear, from the 3rd to the 8th year of treatment. As with other antiresorptives decreases cortical porosity and also provides a certain degree of remodelling with an increase in cortical thickness and strength.67 On the other hand, the increased BMD gained during the first two years of treatment with denosumab, has been observed to be lost during the first year after abandonment of the treatment68 and an increase in vertebral fractures was observed after withdrawal, so it is recommended that the treatment be continued with another drug to maintain the BMD.
EfficacyIn regard to data on efficacy in elderly patients, this drug reduced vertebral fractures, non-vertebral fractures, and hip fractures in both patients over and under 75 years of age, with similar BMD increases, and without changes in terms of side effects in both age groups.69 After 3 years of treatment, a reduction in the risk of new radiological vertebral fractures of 68% was achieved, and a reduction of 40% for hip fractures, and 20% for non-vertebral fractures.70 The absolute reduction of fractures was 4.8%, 0.3%, and 1.5% for new vertebral fractures, hip fractures, and non-vertebral fractures, respectively. A post hoc study showed a significant reduction in new vertebral and hip fractures in women over 75, as well as in the sub-groups of women with fracture risk.71,72 In the long-term treatment group of the phase 3 randomized study of the FREEDOM (extension study from year 3 to 10), the annualized incidence of patients with new vertebral fractures, non-vertebral fractures, and hip fractures during the extension phase remained at levels similar to the incidence observed during the FREEDOM study (first three years). The cumulative incidence of new vertebral fractures and non-vertebral fractures in the extension phase was lower than the estimated incidence for the virtual twin placebo group.73
EfficiencyIn regard to cost-effectiveness, which is also evaluated frequently using the Markov model, there are several publications with a series of favourable results in terms of efficacy for denosumab (Table 4). In these studies, the good results for Denosumab in terms of cost-effectiveness are attributed to the persistence and improved efficacy of this drug.74 In a recent review of the subject, denosumab was found to be more cost effective in the elderly, patients with a history of fractures, and with lower BMD.75
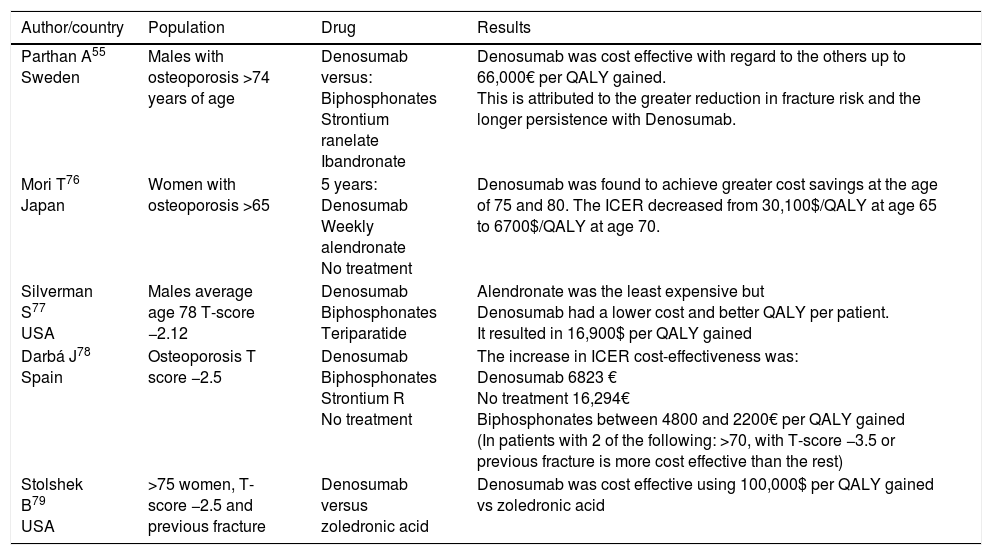
Cost-effectiveness of denosumab.
| Author/country | Population | Drug | Results |
|---|---|---|---|
| Parthan A55 Sweden | Males with osteoporosis >74 years of age | Denosumab versus: Biphosphonates Strontium ranelate Ibandronate | Denosumab was cost effective with regard to the others up to 66,000€ per QALY gained. This is attributed to the greater reduction in fracture risk and the longer persistence with Denosumab. |
| Mori T76 Japan | Women with osteoporosis >65 | 5 years: Denosumab Weekly alendronate No treatment | Denosumab was found to achieve greater cost savings at the age of 75 and 80. The ICER decreased from 30,100$/QALY at age 65 to 6700$/QALY at age 70. |
| Silverman S77 USA | Males average age 78 T-score −2.12 | Denosumab Biphosphonates Teriparatide | Alendronate was the least expensive but Denosumab had a lower cost and better QALY per patient. It resulted in 16,900$ per QALY gained |
| Darbá J78 Spain | Osteoporosis T score −2.5 | Denosumab Biphosphonates Strontium R No treatment | The increase in ICER cost-effectiveness was: Denosumab 6823 € No treatment 16,294€ Biphosphonates between 4800 and 2200€ per QALY gained (In patients with 2 of the following: >70, with T-score −3.5 or previous fracture is more cost effective than the rest) |
| Stolshek B79 USA | >75 women, T-score −2.5 and previous fracture | Denosumab versus zoledronic acid | Denosumab was cost effective using 100,000$ per QALY gained vs zoledronic acid |
Teriparatide or PTH 1–34 (amino-terminal recombinant fragment of the parathyroid hormone) is currently the only one of the available drugs considered to be an anabolic steroid, due to its capacity to stimulate bone formation. Conceptually, and taking into account that age is one of the main risk factors for the development of bone fragility, it could be a good option for increasing bone strength in elderly patients with a marked structural alteration of the bone at both the cortical and trabecular levels.
EfficacyIn the reference or pivotal trial of the drug,80 the Fracture Prevention Trial (FPT), which analyzed the effect of the drug in comparison with a placebo in 1637 post-menopausal women with at least one vertebral fracture and with an average follow-up of 21 months, a significant reduction was found in the rate of new vertebral fractures (65 and 69%, respectively) and non-vertebral fractures (53 and 54%). In addition, the risk of multiple vertebral fractures (77 and 86%) and fractures considered moderate or severe (90 and 78%) was also lower. A favourable effect in the increase in BMD in the lumbar column (9.7% and 13.7%) and in the femoral neck (2.8% and 5.1%) was also noted. But the average age of these patients was 69. It is known that the average age of the patients in studies to determine the efficacy of drugs for osteoporosis is 64, while the average age of the patients who suffer hip fractures is 84.81 In this reference study, an initial screening of 9347 women was done, followed by a sub-analysis with the patients of the FPT, comparing patients younger and older than 75 years of age.82 Remodelling markers, BMD, fracture risk, and adverse effects were evaluated, with similar results, especially in regard to the reduction in new vertebral fractures, with an NNT (number needed to treat) of 11 in both groups, concluding that age does not affect the safety and efficacy of the drug, making it a valid alternative in this type of patient. Similar results were obtained in other sub-analyses of the same cohort of patients.83
Other works analyze the effect of teriparatide in patients older and younger than 80 years of age.84 They study the efficacy of the drug based on the analysis of BMD and the bone markers in both groups, and did not find any significant differences. They concluded that their effect is not conditioned by age or by the complexity of the patient. Although it was not a study that was methodologically focused on fractures, there were also no notable differences in this aspect at the two year follow-up (13 fractures in the group over 80 with low BMD and/or a history of a fracture versus 10 in those under 80 years of age with lower fracture risk).
In the well-known EFOS study,85 although it is an observational study, it analyses the incidence of clinical fractures, lumbar pain, and quality of life in women over the age of 75, in treatment with teriparatide for 18 months, and after this, with an extension for another 18 months, with positive results noted in all of the parameters studied. In another trial – this time randomized – which compared teriparatide against risedronate, after 26 weeks, an initial improvement in functional parameters was observed in patients (average age 77) treated with the bone growth drug after a pertrochanteric hip fracture.86 The extension of the treatment to 78 weeks87 showed a significant increase in bone mineral density in both the lumbar level and femoral neck in the patients treated with teriparatide. One of the conclusions of the study was that both drugs are safe when used in the period immediately following the fracture, because no problems of consolidation of the fractures was observed. Recently, another published trial, also randomized, known as the VERO trial,88 compares teriparatide and risedronate. Risedronate is an active comparator that reduces vertebral and non-vertebral fractures compared with placebo with a 60% relative reduction in the risk of hip fractures in elderly patients with osteoporosis and prevalent spine fractures in the HIP trial.93,89 Previous to this paper, no head-to-head studies have compared the effects of antiresorptives and bone-forming medications on reducing the risk of fractures as the primary outcome. Although only close to 20% of the patients included were older than 80 years, the results in the reduction of a new vertebral fractures were notable for the patients who received teriparatide, with an absolute risk reduction after two years of 6.6%. These data inform of the superior anti-fracture efficacy of this anabolic drug in the management of severe osteoporosis
Reviews of the efficacy and effectiveness of the most widely-used osteo-protective drugsSome reviews show, with high-grade evidence, that biphosphonates (alendronate, ibandronate, risedronate, zoledronic acid) denosumab and teriparatide reduce fractures in comparison with a placebo in post-menopausal women with fracture risk of 0.4–0.6 for vertebral fractures and 0.6 to 0.8 for non-vertebral fractures. The CPG of the American College of Physicians recommends giving these drugs to women with osteoporosis.15,17 There are no comparative studies between drugs, and several meta-analyses found no superiority of one drug over the other.15
In terms of secondary prevention, in the Saito review16 with 26 studies selected, they found that all of the drugs evaluated (biphosphonates and teriparatide) were able to prevent fractures (reduction of the relative risk of new fractures between 0.38 and 0.77), although teriparatide with lower NNT for vertebral fractures and biphosphonates for non-vertebral fractures. This meta-analysis did not include denosumab, because it had only a single trial, but they did mention the Palacios trial73 in which denosumab reduced secondary vertebral and non-vertebral fractures.
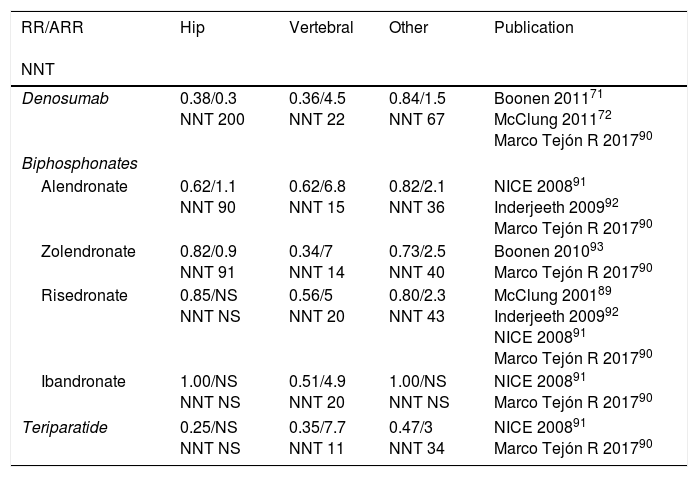
Table 5 shows the relative risk of the most widely-used treatments for each type of fracture and the reduction in absolute risk in comparison with a placebo, as well the NNT to prevent fragility fractures, according to several studies.
Relative risk, reduction of absolute risk of fractures under anti-osteoporotic treatment for secondary prevention of fractures.55,90
| RR/ARR NNT | Hip | Vertebral | Other | Publication |
|---|---|---|---|---|
| Denosumab | 0.38/0.3 NNT 200 | 0.36/4.5 NNT 22 | 0.84/1.5 NNT 67 | Boonen 201171 McClung 201172 Marco Tejón R 201790 |
| Biphosphonates | ||||
| Alendronate | 0.62/1.1 NNT 90 | 0.62/6.8 NNT 15 | 0.82/2.1 NNT 36 | NICE 200891 Inderjeeth 200992 Marco Tejón R 201790 |
| Zolendronate | 0.82/0.9 NNT 91 | 0.34/7 NNT 14 | 0.73/2.5 NNT 40 | Boonen 201093 Marco Tejón R 201790 |
| Risedronate | 0.85/NS NNT NS | 0.56/5 NNT 20 | 0.80/2.3 NNT 43 | McClung 200189 Inderjeeth 200992 NICE 200891 Marco Tejón R 201790 |
| Ibandronate | 1.00/NS NNT NS | 0.51/4.9 NNT 20 | 1.00/NS NNT NS | NICE 200891 Marco Tejón R 201790 |
| Teriparatide | 0.25/NS NNT NS | 0.35/7.7 NNT 11 | 0.47/3 NNT 34 | NICE 200891 Marco Tejón R 201790 |
RR, relative risk; ARR, absolute reduction of risk versus a placebo; NNT, number needed to treat for secondary prevention.
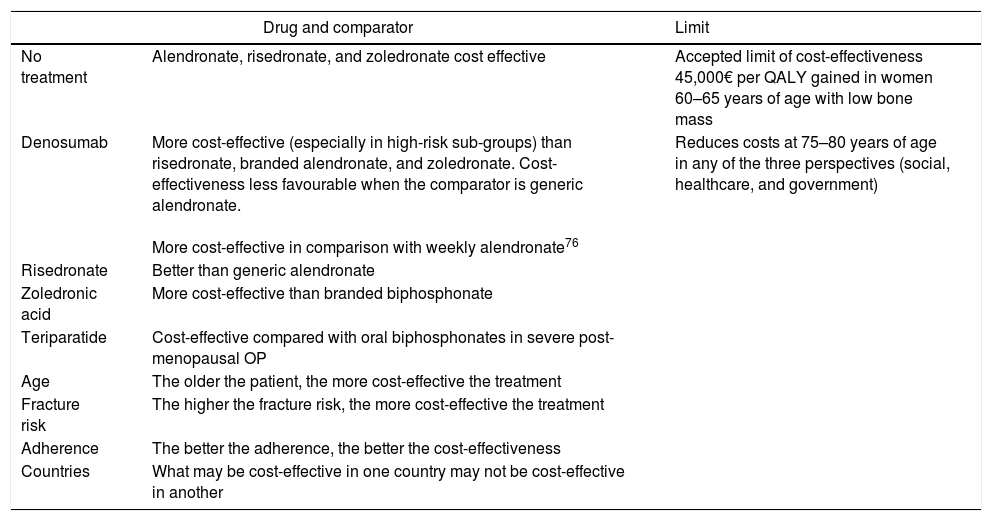
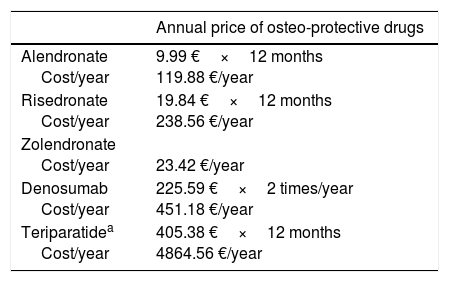
In terms of cost-effectiveness, this is determined by numerous factors and some of them are different in each country, such as the incidence of fracture and treatment cost (Table 6). Other factors, such as adherence, the drug used to compare, BMD, age, and life expectancy also influence effectiveness. When it is assumed that all patients have the same risk and the intervention thresholds are based on a limited number of clinical characteristics such as age and BMD, there is a risk of not applying an adequate treatment. In the economic evaluation of the drug, it is better to base intervention thresholds on the long-term probability of fracture and use data for diverse populations (with different risks), taking into account the regional factors (cost and epidemiology of fractures in the country).94Table 7 shows the current annual cost in Spain of the drugs covered in this analysis.
Factors that influence the cost-effectiveness of the different drugs.94
| Drug and comparator | Limit | |
|---|---|---|
| No treatment | Alendronate, risedronate, and zoledronate cost effective | Accepted limit of cost-effectiveness 45,000€ per QALY gained in women 60–65 years of age with low bone mass |
| Denosumab | More cost-effective (especially in high-risk sub-groups) than risedronate, branded alendronate, and zoledronate. Cost-effectiveness less favourable when the comparator is generic alendronate. More cost-effective in comparison with weekly alendronate76 | Reduces costs at 75–80 years of age in any of the three perspectives (social, healthcare, and government) |
| Risedronate | Better than generic alendronate | |
| Zoledronic acid | More cost-effective than branded biphosphonate | |
| Teriparatide | Cost-effective compared with oral biphosphonates in severe post-menopausal OP | |
| Age | The older the patient, the more cost-effective the treatment | |
| Fracture risk | The higher the fracture risk, the more cost-effective the treatment | |
| Adherence | The better the adherence, the better the cost-effectiveness | |
| Countries | What may be cost-effective in one country may not be cost-effective in another | |
Current annual cost of anti-osteoporotic drugs in Spain.
| Annual price of osteo-protective drugs | |
|---|---|
| Alendronate Cost/year | 9.99 €×12 months 119.88 €/year |
| Risedronate Cost/year | 19.84 €×12 months 238.56 €/year |
| Zolendronate Cost/year | 23.42 €/year |
| Denosumab Cost/year | 225.59 €×2 times/year 451.18 €/year |
| Teriparatidea Cost/year | 405.38 €×12 months 4864.56 €/year |
It should be noted that in a recent study done in the US on thousands of individuals with previous fractures (vertebral, radius, or hip), the risk of dying especially in subjects over the age of 85 with several comorbidities, was higher than the risk of suffering another fracture. In this study, it was estimated that the NNT for 5 years to prevent a second osteoporotic fracture would range between 8 and 65 and is similar to the NNT of other preventive strategies (aspirin, statins, and b blockers).95
For this reason, when deciding whether to prescribe anti-osteoporotics for the elderly, the patient's estimated survival (after the fracture that occurred or the risk of suffering a fracture) must be considered along with the effect of the treatments in terms of the prevention of new fractures and a possible reduction of mortality.
Effect of osteoprotective treatments on mortalityThe relationship between treatment of osteoporosis and mortality is a controversial topic, with several authors indicating the existence of a reduction in the risk of dying in patients who are administered osteoprotective treatments.96–102 In 2010, a meta-analysis102 was published with the objective of determining the degree to which the drugs that are effective in treating osteoporosis, in addition to reducing the incidence of new fractures, manage to reduce mortality in patients over the age of 50 with an established fracture (vertebral and non-vertebral). It includes 8 double-blind, placebo-controlled randomized clinical trials, with a 3-year follow-up, and it found a significant reduction of 11% in mortality, regardless of age and the incidence of fracture. It detected that the reduction in mortality is mainly observed in the studies done on the oldest, most fragile population, with a higher fracture risk and higher mortality rate. This study did not find differences in mortality depending on the type of drug analyzed (biphosphonates, strontium ranelate, or denosumab), although some authors indicate that the use of calcitonin could have less of an influence on mortality than other osteoprotectors.103
Most of the studies that analyze the relationship between osteoprotective treatment and mortality have been done on biphosphonates.
In 2011, Lyles98 carried out a double-blind placebo-controlled randomized trial, with an average follow-up of almost two years, which indicated a 28% reduction in death due to any cause after the administration of intravenous zoledronic acid in the first three months following a hip fracture. Cengiz104 found a reduction in mortality after one year of 20.2% in pertrochanteric fractures. It is not clear when the optimum moment for the administration of zoledronic acid is, but it appears that the beneficial effect on mortality is lower if it is administered in the first two weeks after the fracture.105
In 2012, Grey4 published a systematic review that indicated a reduction in the risk of dying after the use of biphosphonates between 24 and 66%. Other authors99 have found that treatment with these drugs is an independent factor for the reduction of mortality due to any cause, such that for each year of treatment with biphosphonates, the relative risk of dying is reduced by 63% in patients with hip fractures over the age of 50.
Center106 designed a study over the course of 18 years with the goal of analysing the association between biphosphonates and the risk of dying, independently from the type of fracture. It found a reduction in mortality in the group of women with and without fractures of 69%, with a reduction of 76% in the sub-group of women without fractures.
If we take into account that the treatment for osteoporosis, in addition to reducing the risk of FV and FNV mentioned earlier, reduces mortality by between 11% and 70%, this is one more reason to initiate the treatment in elderly patients with fractures.
There are several theories regarding how osteoprotective treatment could reduce mortality, although the specific mechanism is not precisely known. On one hand, there is the reduction of the risk of new fractures and the morbimortality associated with them if they do occur100 but it appears that the percentage of mortality justified for this reason is low, because most of the patients who die did not develop new fractures before dying.95,102,107 It has also been indicated that biphosphonates could have a protective effect on the mortality associated with the appearance of pneumonia and cardiovascular events such as acute myocardial infarction or arrhythmia,107–109 although it is not clear that they achieve a reduction in the incidence of these complications.98,106,110 It has been suggested that biphosphonates could have a protective effect in the atherogenic process.111 It is possible that the treatment for osteoporosis involves an improvement in the physiological reserve and in the situation of frailty of the patient, improving their capacity to respond to other diseases that may arise.107 This theory would be in line with the fact that a larger reduction in mortality has been observed in groups that were previously more frail.102
Factors to be taken into account to indicate the treatment of osteoporosis in the elderly- 1.
Fracture risk
- 2.
Time that it takes the treatment to be effective
- 3.
Prediction of mortality
- 4.
Method for the treatment of fractures and the secondary prevention of osteoporosis: FLS and Orthogeriatric Units.
Several factors related to osteoporotic fracture risk have been described and they are those that are evaluated in the screening scales such as the FRAX or Q-Fracture (age, gender, smoking and alcohol habits, diabetes, parents with osteoporosis or hip fractures, institutionalized, history of fragility fractures or falls, dementia, cancer, COPD/asthma, cardiopathy or CVA, liver or kidney disease, Parkinson's, rheumatoid arthritis, digestive malabsorption disease, endocrine issues, epilepsy, or anti-epileptic drugs, antidepressants, corticosteroids, or estrogens).112 In addition, therapeutic recommendations have been made based on the 10-year risk of suffering osteoporotic or hip fractures. But there are already several studies that relate some specific factors with imminent fracture risk, such as: advanced age, diseases with physical affectation or functional deterioration, specific comorbidities (psychosis, Alzheimer's and diseases of the CNS), previous falls or factors that contribute to them (customary treatment with psychotropics) and alterations of bone mineral metabolism and/or history of osteoporotic fractures.113 On the other hand, imminent risk of suffering a second major osteoporotic fracture increases with age, is more frequent in women, and is more likely immediately after the first fracture.114
Therefore, it is in this profile of a frail, aged woman, with a recent fracture in which the benefit of the prevention of fragility fractures must be evaluated. And the decision must be quick, due to the imminent risk and because the fracture is going to generate a dependency that is difficult to reverse, ultimately worsening her precarious functional situation.
2. Time that it takes the treatment to be effectiveThere is not much available bibliography that provides information on the treatment time needed to reduce the incidence of fractures. It has already been mentioned that in geriatric patients, there are many more variables that contribute to fractures, especially those related to falls, which generates much confusion when determining the effect of anti-osteoporotic treatment. In general, the authors take about a period that ranges from between 6 months to prevent vertebral fractures and 18–24 months to reduce the risk of new hip fractures, and of a half year.14,37,70,115,116 Adherence directly influences the efficacy of the drug and in this concept, denosumab and teriparatide are the drugs that achieve the greatest adherence.117
This detail, along with the time necessary for the drug to show its effectiveness, becomes especially important in the elderly population, for which we have to estimate survival longer than the value mentioned (6–12 months) before initiating treatment, if what we want to do is prevent new fractures.
3. Prediction of mortalityDespite the substantial risk of death after an osteoporotic fracture, the risk of a new fracture generally is high enough to justify a preventive treatment for osteoporosis,95 unless there is a very high expectation of mortality in the short term. It is therefore important to know and predict the increased mortality involved with these fractures, especially hip fractures, which is associated with the greatest risk.
In the study by Alonso,118 factors such as age, male gender, comorbidity, admittance in summer, low score on the Barthel Index upon admittance and discharge, or elevated levels of urea, creatinine, and sodium upon arrival at the emergency room significantly increase the risk of dying in the first year after the fracture. In addition to these baseline factors, it also found that patients who develop respiratory complications, delirium, malnutrition, or hydroelectrolytic alterations during admittance, as well as those who require re-hospitalization for any reason during the first month, have an increased risk of dying during the first year. Hu119 and Smith120 found similar preoperative factors in two meta-analyses that give a prediction of mortality with strong evidence.
In order to be able to calculate the individual mortality risk that a specific patient has after suffering a hip fracture, more than 25 predictive tests have been described.121,122 Of all the available scales, the Nottingham Hip Fracture Score (NHFS)123 appears to be the most suitable for determining the risk of dying in patients who suffer hip fractures.121 It has been validated in the British population in which it was designed to predict mortality within one month122–126 and one year127 of the fracture.
Knowing the factors associated with higher mortality after fragility fractures or the application of instruments such as the NHFS can help make clinical decisions for these patients, since they make it possible to adapt the therapeutic strategies based on the life prognosis of each individual.
But the most important reason to predict something as serious as mortality is the possibility of trying to avoid it. With regard to the factors associated with mortality after hip fracture, several have been identified that are modifiable, such as avoiding orthopaedic treatment and intervening as soon as possible, achieving maximum functional recovery by means of rehabilitation, early detection and treatment of perioperative complications, or, as indicated previously, the treatment of osteoporosis.118 It is in this group of interventions where the Orthogeriatric Units have demonstrated their benefits.
4. Method for the treatment of fractures and the secondary prevention of osteoporosis: FLS and Orthogeriatric UnitsCurrently, the principal CPGs recommend orthogeriatric collaboration in the treatment of patients with hip fractures,128 because it has demonstrated important benefits such as reduction in hospital complications, higher rate of surgical treatment, earlier surgery, shorter average hospital stay, improvement in the functional situation upon discharge and long term, greater prescription of treatment for osteoporosis upon discharge, better planning of the level of care after hospital discharge, lower rate of re-admittance and overall, a reduction in mortality.129–143 There are numerous studies that indicate that the multidisciplinary treatment of hip fractures improves the percentage of patients who receive treatment for osteoporosis.131–135 Fisher finds an increase in the prescription of 12% to 69% when switching from a system in which the geriatrics department only participated sporadically by means of interconsultation to a system of orthogeriatric collaboration with daily monitoring of patients.132 Sánchez134 obtained similar percentages after the application of a clinical method for the multidisciplinary treatment of hip fractures in their centre, with an increase in prescription from 14.8% to 76%.
Recently, fracture units (FLS) have also been implemented, where the cause is studied, patients are monitored, and secondary prevention of osteoporosis is undertaken, also achieving better adherence to the treatment, improvement in BMD, and a reduction in new fractures as well as mortality.137,144,145
Both methods, Orthogeriatric Units and FLS, must be considered as a work mode when the objective is the secondary prevention of fractures in geriatric patients.
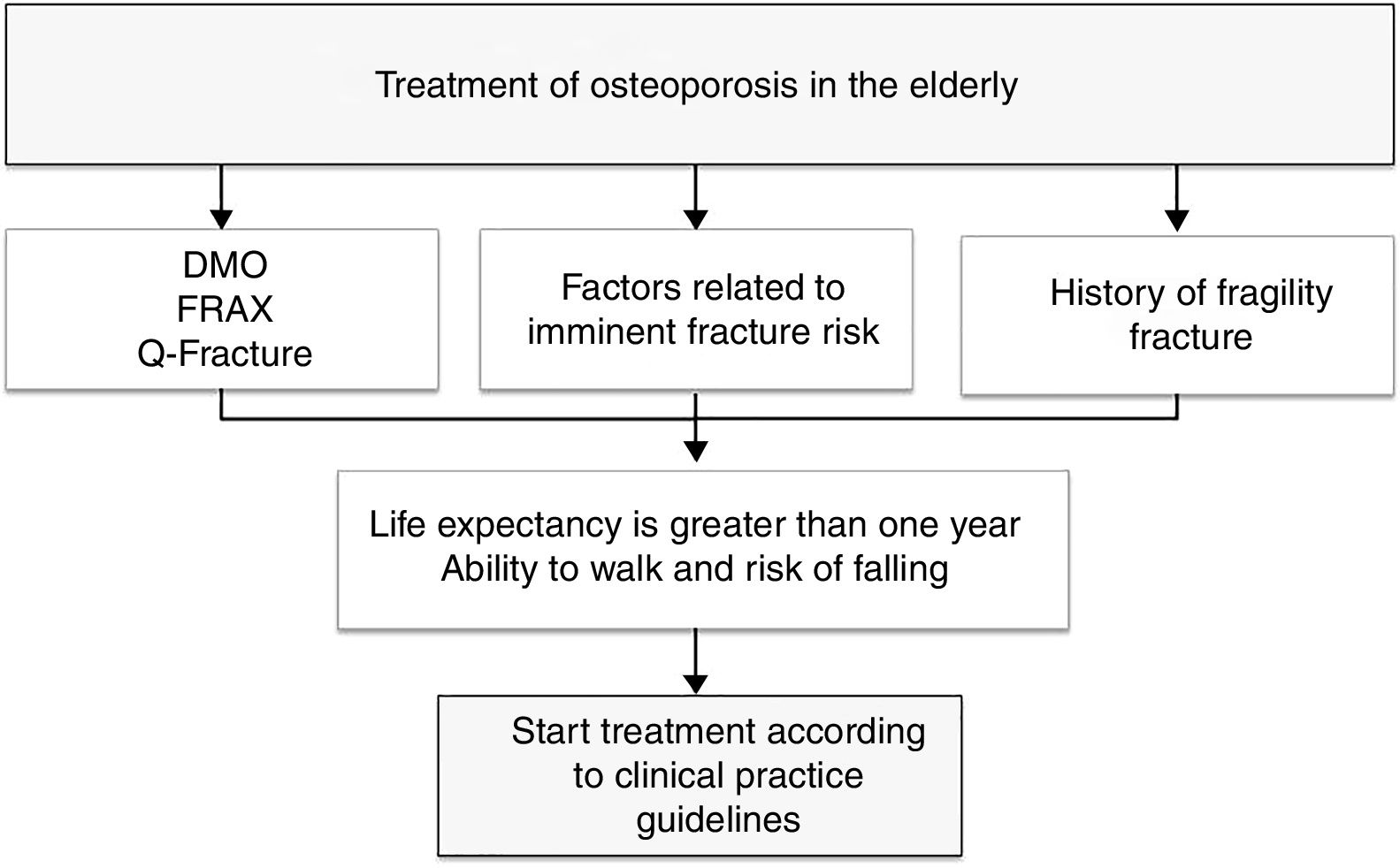
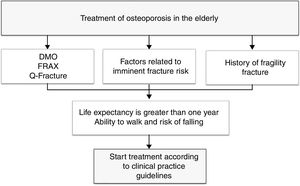
To provide a practical summary, the authors propose that in patients with high fracture risk (evaluated according to FRAX, Q fracture, densitometry, or with factors related to imminent fracture risk), that life expectancy be estimated using a method such as the NHFS, and if life expectancy is greater than one year and the patient maintains the ability to walk, the initiation of treatment for osteoporosis is recommended with the intention of reducing the risk of new fragility fractures (Fig. 1).
DiscussionIn this paper we have intended to do a review of different factors that need to be taken into account when managing specially elderly patients in risk of suffering a fracture. It is true that the vast majority of clinical essays and studies with higher clinical relevance are done with younger patients while the average age of most of the fractured patients is more elevated. This would be probably due to recruitment problems and follow up issues, being more expensive and difficult in patients in their eighties. But it is precisely in this age group where it is more interesting to contemplate some kind of treatment, as the risk of fracture is higher here. NNTs, costs and therefore efficiency are probably optimum in this type of patients, but we understand that even though it is information of high clinical value, it is more complicated to get it.
It is true that our work is not a “systematic review” that follows the data gathering protocols on a standardized way and it is not a meta-analysis either with which we could obtain conclusions with any degree of certainty. This could involve some degree of subjectivity in the analysis, but our main objective is above all to draw the attention to the elderly population which has more fracture risk and which is not generally well managed from the prevention's point of view. Chronological age is not a valid guideline anymore for making certain decisions, as life expectancy and its quality are more important, as it has been developed through this manuscript. It is true that in some patients clinically very deteriorated, the decision to take must be focused specially on the comfortableness of the future of these patients, but there are many others in which some strategies may reduce the probability of those aggressive fractures happening.
We have not wanted to deal with falls in our study. We are aware that a fracture is generally a consequence of a fall and that the approach for reducing fractures must be developed concurrently with a pharmacological intervention. We have not detailed it intentionally as we consider it a large enough subject as to appear on a publication specifically dedicated to it.
From the studied meta-analysis that have been studied,32,34,146–148 we can declare that we have effective treatments for fracture reduction, that alendronate and zolendronate, in their generic version, are probably the most cost-effective and that the effect of the drug does not depend on the age of the patients. Zolendronate has the inconvenience of being exclusively for intra-hospital treatment. We have effective “weapons”. The factors to take into consideration when indicating a treatment are many, but each patient is different and requires a personalized evaluation. We have expressed the ones we consider most relevant taking into account characteristics of the patient and the drug. An undeniable issue is that a drug, in order to be effective, it must be taken by the patient. Medication adherence in this type of processes, in which patients do not perceive their real importance, can usually be improved, with a clear decrease in treatments in a year time. Parenteral drugs have better adherence than oral ones, which depending on the type of patient, may have significance.117
Finally, we emphasize the need of a global treatment, not just for the fracture but also for the patient that has the fracture. Orthogeriatric models of care have demonstrated being the future for the elderly fractured patient, with a special interest in the organization of an additional estructure of secondary prevention that it is giving extraordinary results, the Fracture Liason Services.128,142–145
ConclusionsStudies have shown that the efficacy of the treatment of osteoporosis in the elderly population is similar to that of the adult population.
In terms of the effectiveness of these treatments in the elderly, especially after suffering a major fracture, they are more cost-effective than in adults. This is because of the higher risk of suffering new fractures and the serious functional, social and economic repercussions of those new fractures.
The cost-effectiveness of each drug varies in each country because it is influenced by factors such as the incidence of fractures and the price of drugs, which are different in each country. It also varies according to other factors related to the patient, such as adherence, the comparator that is used, and the rest of the clinical characteristics of the patient.
Before deciding on the therapeutic intervention of osteoporosis in the elderly, numerous factors must be taken into account, such as the individual fracture risk of the patient, their life expectancy, the risk of side effects or interactions and comorbidities, to make a suitable selection of the drug.
Orthogeriatric Units have demonstrated clinical benefits and improvements in the care of elderly patients with hip fractures, in both the acute and ambulatory follow-up. The implementation of FLS in coordination with the Orthogeriatric Units can improve the secondary prevention approach and the overall care of orthogeriatric patients.
Conflict of interestsPSL has been a speaker at a scientific event of Amgen. IEF has received speaker honoraria from Lilly. MPML has received speaker honoraria from Amgen and Lilly. The rest of the authors declares no conflict of interests.