The high sensitivity achieved in genetic analysis of forensic interest allows to obtain genetic profiles from minimal traces of biological material deposited on the evidence before or even subsequent to the events under investigation. Accidental contamination of biological evidence and erroneous interpretation of genetic findings have important implications with the consequent impact on the judicial process. Minimising and detecting the presence of accidental contamination that may be generated during some phases of collection of samples or genetic analysis are a priority for forensic genetics laboratories. This article reviews legislation and national and international standards applicable to field of forensic genetics, which aim to ensure the quality of expert evidence and the reliability of the conclusions reached in the expert reports.
La alta sensibilidad conseguida en los análisis genéticos de interés forense permite obtener perfiles genéticos procedentes de mínimas trazas de material biológico depositado sobre los indicios, antes o incluso después de los hechos investigados. La contaminación de los indicios biológicos de manera accidental y la interpretación errónea de los resultados genéticos tienen importantes consecuencias con la consiguiente repercusión en el proceso judicial. Minimizar y detectar la presencia de contaminaciones accidentales que se pueden generar durante algunas de las fases de recogida o análisis genético es una prioridad para los laboratorios de análisis genéticos.
El presente artículo revisa el marco normativo, así como los estándares de calidad nacionales e internacionales aplicables al ámbito de la genética forense que tienen como objetivo garantizar la calidad de la prueba pericial y la fiabilidad de las conclusiones emitidas en los informes periciales.
The technology and methodology that is currently used in laboratories that perform genetic analyses for identification purposes deliver a very high degree of sensitivity and a high discrimination power. Polymerase chain reaction (PCR) amplification [1] using latest-generation kits,1 which include improvements in primer designs and buffer compositions and thus deliver a better performance in the presence of inhibitors, the scant amount of necessary DNA (or the degradation thereof), the high sensitivity and precision of DNA quantification systems (real-time PCR), as well as the DNA sequencers that are currently in use, allow genetic profiles with a high identification value to be obtained from a few cells deposited on a biological vestige.
It is potentially possible to amplify and therefore obtain identification information from about 15–20 cells (approximately 100pg)2,3 thus achieving, thanks to the combination of just 21 short tandem repeat markers, discrimination values above 99.9999999%.4
However, paradoxically, the high sensitivity achieved may constitute a handicap with regard to the final outcome of the analysis. PCR reactions allow millions of DNA copies to be obtained from a scant number of initial molecules. However, the chemical reaction does not distinguish between the DNA from the cells specific to the liquid being investigated, and therefore related to the events investigated, and any DNA which, accidentally and in a way that is unrelated to the events, have been deposited on the evidence being studied. When the transfer of biological material onto the evidence takes place after the events, and once the expert investigation process begins, we are dealing with contamination,5 which may have different sources and can take place by means of different mechanisms.6
The results obtained as a consequence of contamination may lead to an erroneous interpretation by the laboratory, which will issue mistaken conclusions with significant repercussions on the course of the criminal investigation as well as the legal proceedings.7,8
This article reviews the most relevant aspects related to the phenomenon of contamination, the importance of the latter and the national and international regulations, legislation and standards that allow for the minimisation and detection of contamination.
Regulatory frameworkIn order to guarantee the quality, authenticity and certainty of the findings issued by laboratories performing genetic analyses in any European Union member state, the European Council approved the framework Decision 2009/905/JHA, of 30 November 2009,9 on the accreditation of forensic service providers carrying out laboratory activities. The objectives of the agreement include, among others, the accreditation, as per the EN ISO/IEC 1702510 standard, of laboratories engaged in forensic genetics, for which purpose the date of 30 November 2013 was set as the deadline for the fulfilment of the agreement.
Moreover, in Spain, the approval of Organic Law 10/2007, of 8 October, regulating the police database on identifiers obtained from DNA,11 heralded a watershed in the quality of genetic analysis expertise. Article 5 of said Law states that “DNA analyses for genetic identification purposes can only be performed by the laboratories accredited to that effect by the Spanish National Commission for the Forensic Use of DNA (CNUFADN) that pass the mandatory periodic quality controls”. Article 3 of Royal Decree 1977/2008 regulates, as one of the functions pertaining to the CNUFADN, “the accreditation of laboratories that are empowered to compare genetic profiles in the investigation and prosecution of offences and the identification of cadavers or missing persons”, whereas Article 8 thereof establishes the evaluation procedure for laboratories performing DNA analyses for forensic identification purposes.12
The agreement adopted by the CNUFADN on the accreditation and quality control of forensic genetics laboratories (approved on 21/07/2009)13 sets out two requirements to guarantee the quality and reliability of the genetic analyses performed by forensic genetics laboratories. On the one hand, it specifies the need to participate and pass some of the annual external inter-laboratory control exercises organised by international scientific bodies (Spanish and Portuguese-Speaking Working Group [GHEP] and the European Network Forensic Sciences Laboratories [ENFSI]) and, as a second requirement, the laboratory must be evaluated by the Spanish National Accreditation Body (ENAC) in order to substantiate its technical competence in accordance with the provisions of the EN ISO/IEC 17025 standard. Every year, the CNUFADN requests information about the accreditation status of all Spanish laboratories working in the forensic genetics field, issuing expert reports to the courts of law and, following the evaluation of the information provided, produces a catalogue of laboratories that fulfil the agreement, which is then published on the CNUFADN website, thus making these laboratories CNUFADN-accredited. In the latest report published, which corresponds to the year 2014,14 18 Spanish laboratories fulfilled the agreement. Most of them were from the public sphere (14) while four (4) were private sector organisations.
The concept of biological contamination and its repercussionsDNA can be transferred between people, people and objects, people and surfaces, objects and surfaces or between objects. This transfer may take place at different times, and the mechanisms and sources are also different.
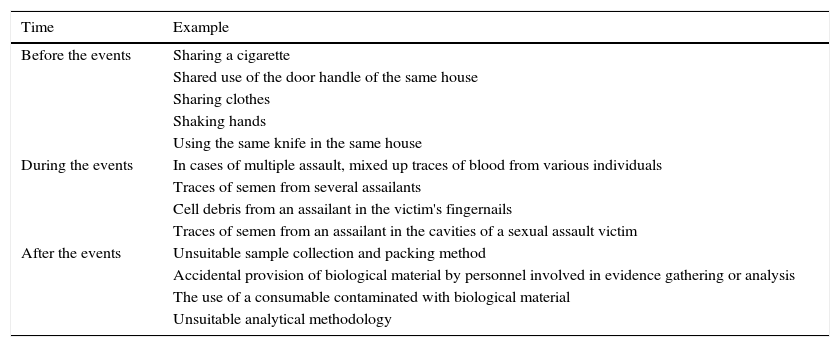
The transfer of genetic material can take place before, during or after the events under investigation. In the first two scenarios, DNA transfer takes place through a mechanism unrelated to the events or in a way that is inherent to the events under investigation, with such transfer therefore being inevitable. However, when the transfer takes place after the occurrence of the events under investigation, it is avoidable and usually occurs accidentally: in this case we are talking about contamination (Table 1). Contamination may occur at different times: during the evidence gathering phase, when the samples are in transit or while the analyses are being performed at the laboratory itself. The production mechanisms of contamination are also varied and range from direct contact through to transfer by means of aerosols or through the involvement of transmission agents (objects or persons)15–19 (Table 2).
Examples of processes that may lead to the mixture of genetic materials at different stages of the events under investigation.
| Time | Example |
|---|---|
| Before the events | Sharing a cigarette |
| Shared use of the door handle of the same house | |
| Sharing clothes | |
| Shaking hands | |
| Using the same knife in the same house | |
| During the events | In cases of multiple assault, mixed up traces of blood from various individuals |
| Traces of semen from several assailants | |
| Cell debris from an assailant in the victim's fingernails | |
| Traces of semen from an assailant in the cavities of a sexual assault victim | |
| After the events | Unsuitable sample collection and packing method |
| Accidental provision of biological material by personnel involved in evidence gathering or analysis | |
| The use of a consumable contaminated with biological material | |
| Unsuitable analytical methodology |
Examples of contamination mechanisms.
| Mechanism | Example |
|---|---|
| Direct contact | Touching evidence or laboratory material used in the analyses without gloves |
| Packing several items of evidence containing fluid in the same primary packaging | |
| Aerosols | Sneezing, coughing and even talking near evidence or the laboratory material used in the analyses |
| Transporting amplified material to pre-PCR areas | |
| Secondary transfer | Use of contaminated consumables in the manufacturing phase |
| Use of the same utensil or tool (gloves, scissors etc.) for processing different pieces of evidence |
Finally, the sources of transfer are similarly diverse and include the personnel involved in collecting and shipping samples, the personnel tasked with the analyses and the material used in the evidence gathering, shipping and analysis phase (packing, consumables or reagents).20 There is one other source that should not be overlooked, namely the accidental transfer of genetic material between DNA samples or extracts (DNA carry-over), which may occur in the laboratory in any of the analysis phases as a consequence of an unsuitable work flow procedure or in the handling of the samples.
From the practical standpoint, and for the purpose of interpretation, a DNA transfer may give rise to mixed profiles, which manifest through the appearance of more than two alleles in two or more short tandem repeat markers.21
The impact contamination may have on the final interpretation of genetic results, and therefore on the conclusions of an expert finding, is variable.22 Sometimes, if the amount of contaminating biological materials is scant, it may not be detected despite the low limit of sensitivity of the analytical devices currently used by forensic laboratories. In addition, when there is a major disproportion between the majority cell contributor deposited on the evidence and the contaminating material, the PCR reaction promotes the amplification of the majority component to the detriment of the minority one (contaminant),23 leading the latter to go completely unnoticed by the laboratory. In the situations described, the contaminating profile has no practical effect and there is therefore no real repercussion on the final conclusions after the assessment of the genetic profile. However, when exogenous material that is unrelated to the events is deposited on the evidence in a magnitude that is detectable by the laboratory, the consequences may be particularly relevant. Nevertheless, depending on the degree of contamination and on other laboratory-specific analytical variables (e.g. methodology used, kits used, sensitivity of the detection equipment, internal profile assessment criteria) the situations may be different.
Contamination renders the interpretation of a genetic profile difficult or impossible. However, there is one particularly serious scenario that should be underlined and which occurs mainly with regard to evidence containing a scant amount or quality of DNA. These are situations in which the genetic profile corresponding to the contaminant component clearly prevails over any other profile contained in the evidence. In such an eventuality, the laboratory may wrongly exclude the participation of a person under investigation due to the lack of their genetic contribution to the DNA profile obtained and, in turn, wrongly involve another participant (contaminant) in the events. This is known as the hidden perpetrator effect.5 On the other hand, recording these profiles, which are a result of contamination, in the DNA databases may yield incorrect associations that may have a significant impact on the investigation of the events.
In order to avoid and detect any type of contamination, working protocols and control measures must be established in order to guarantee the authenticity of the findings.
Technical quality standardsAt this moment in time, most of the laboratories that perform genetic analyses for forensic purposes in Europe and the USA are accredited according to the EN ISO/IEC 17025 standard. This establishes the general requirements for quality matters that allow us to guarantee the technical competence of test and calibration laboratories. The standard primarily includes two blocks of requirements: those pertaining to laboratory management matters (organisation, quality management system, request control, document control, etc.) and those related to technical aspects (personnel qualifications, accommodation and environmental conditions, test and calibration methods, method validation, equipment and devices, measurement traceability, sampling, the handling of test and calibration items, assuring the quality of test and calibration results and reporting the results).
The infrastructure and quality-related criteria that forensic genetics laboratories have implemented over the last decade may surpass that of many other forensic disciplines. The active participation of different scientific standardisation groups in Europe and the USA has made a decisive contribution to this work (European Network of Forensic Science Institutes [ENFSI], Forensic Science Regulator [FSR], Scientific Working Group on DNA Analysis Methods [SWGDAM], European DNA Profiling Group [EDNAP], Spanish and Portuguese-Speaking Working Group of the International Society of Forensic Genetics [GHEP-ISFG]). Over the last decade, numerous recommendations and guidelines have been issued, some of which are now quality standards.
Some of these recommendations focus on the biological sample and evidence gathering phase at the scene of the events,24 on the body of the victim or the exhumation operations,25 which undoubtedly constitutes the first step towards being able to establish the final guarantee of the genetic finding. Another set of recommendations develops the measures geared towards minimising and detecting contamination processes during any or some of the phases of the genetic analysis on samples in the laboratory, taking into account aspects such as laboratory design and layout, the analysis procedure and system, quality mechanism controls and contamination traceability.26,27
Moreover, the appearance of repetitive genetic profiles relating various, apparently unconnected cases in different countries across Europe, the USA and New Zealand, some of which enjoyed major media exposure (e.g. the Phantom of Heilbronn)28,29 led some scientific standardisation groups (ENFSI, SWGDAM and the Biology Specialist Advisory Group [BSAG]) to publish, in 2010, a document calling upon the manufacturers of consumables and reagents to implement measures that enable them to guarantee the absence of biological material traces in the products they manufacture and supply to forensic laboratories.30 In 2016, the International Organization for Standardization (ISO) approved a new standard (ISO 18385:2016),31 the objective of which is to minimise the risk of human contamination in products used for the gathering, maintenance, storage and analysis of biological material in forensic analyses.
Minimising and detecting the presence of accidental contaminations that may be generated during some of the collection phases or in the genetic analysis of evidence has become a priority for genetic analysis laboratories. This allows them to guarantee the quality of expert evidence, the authenticity of the final results and the accuracy of the conclusions issued in the court finding.
Minimising contamination- -
Training: Ensuring the training of the personnel involved in any of the genetic analysis phases (including the initial phases for recording and receiving evidence) in aspects pertaining to work procedures and mechanisms in the laboratory, as well as the impact and repercussion of unsuitable habits, is indispensable in preventing contamination and in guaranteeing the end results of the analysis.
- -
Laboratory layout and design: Proper laboratory design is a key element in minimising the risk of contamination in the laboratory. Laboratories pay particular attention to the physical separation between the post-PCR and pre-PCR area, avoiding the movement of samples and material from the post-PCR area to the pre-PCR area. Separate physical spaces for the processing and analysis of known and questioned samples must be guaranteed, and spatial or temporal simultaneity must also be avoided in the processing and analysis of samples with a high and low DNA content. Similarly, laboratories have clean areas where particularly critical and contamination-prone process operations are conducted (e.g. sample reception and recording, the extraction of critical samples – low DNA content or quality –, the preparation of PCR reactions, the preparation of reagents). All areas are equipped with the necessary material and equipment that are exclusive to these areas, and all staff working there wear personal protective equipment (lab coat, gloves, face mask).
- -
Access to the laboratory: Moreover, access to laboratories must be controlled and limited to the personnel working there, thus avoiding the accidental transfer onto the evidence of biological material from personnel who are not involved in the activities being carried out.
- -
Reagents and materials: The material used for packing and shipping samples to the laboratory, as well as the consumables, reagents or kits used, must comply with the provisions of the standard (ISO 18385:2016).
- -
Collaborative/intercomparison exercises: The laboratory's participation in collaborative or intercomparison exercises will help to ascertain the laboratory's work dynamic and, where applicable, will allow for the correction of any deficient or unsuitable aspects in the work flow.
- -
Automation: Finally, the automation of many of the processes performed in the forensic genetics laboratory constitutes an increasingly widespread trend that helps to reduce the risk of contamination, avoiding the minimal interaction between samples and the laboratory personnel. At the same time, automation helps to optimise resources and reduce execution times.32
Being able to trace and detect the origin of contamination, with a view to correct anomalies in the analysis methodology and to implement changes in the laboratory's work system, is just as important as preventing possible contamination. To that end, various actions are included:
- -
Importance of negative controls: The use of negative controls and blanks in the genetic material extraction and amplification phases, as well as the exhaustive and systematic evaluation thereof, permits the identification of contamination attributable to some of the reagents used, to deficiencies in sample handling or in the execution of the operating procedure.
- -
Use of exclusionary databases: Laboratories generate their own exclusionary databases containing the genetic profiles of laboratory personnel and other staff members that may access the work area (e.g. cleaning, maintenance or security services), as well as those of personnel that may somehow have come into contact with the samples. The use of exclusionary databases permits, by means of comparison, the identification of any detectable contamination caused by the transfer of biological material from the personnel included in such databases.33
- -
Computerised laboratory management: The use of IT program to manage the comprehensive processing of the data generated in the laboratory (e.g. Laboratory Information Management Systems [LIMS]) allows for the incorporation of algorithms to record the contamination observed in the laboratory and thus facilitate traceability with regard to origin and final impact. The historic log of the contaminations observed in the laboratory allows any possible sources to be addressed and also permits the implementation of corrective measures.
- -
Importance of the internal validation of the methods used: Validating the methods used enables the laboratory to ascertain the specific characteristics of its own equipment and instruments, as well as details pertaining to the technical procedure being used. This information may help to detect potential contaminations.
Various elements play a part in affording genetic evidence greater validity and rigour. Said elements are all complementary and allow the laboratories to guarantee the quality of the expert reports issued to the courts of law. Moreover, technological and scientific breakthroughs lead to greater sensitivity, speed and precision in genetic analyses, yielding a greater amount of genetic information from a small quantity of sample, and all in less time. In this regard, a continual and significant effort is being made by investigation groups and manufacturers all over the world. Furthermore, the establishment of technical and scientific standards (e.g. the genetic markers, nomenclature and methods used, etc.) constitutes another necessary part, helping to standardise scientific language and allowing forensic laboratories to compare results, irrespective of the laboratory where the analyses were performed, at a time when increasingly large quantities of genetic data are being compared due to the growth and interoperability of criminal DNA profile databases. Finally, in recent years, the Spanish laboratories that carry out forensic genetics analysis have gone to great lengths to implement an integrated quality system in accordance with the provisions of the EN ISO/IEC 17025 standard which, together with the other aforementioned aspects, guarantees reliability and assurance regarding the expertise obtained in Spain.
Authors’ noteThe ideas, comments and opinions expounded in this article are of a personal nature and do not necessarily reflect the point of view or positioning of the institutions to which the authors belong.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Crespillo Márquez M, García Fernández Ó, Paredes Herrera MR, Luque Gutiérrez JA. La importancia de garantizar la calidad y minimizar los riesgos de contaminación en el análisis genético forense. Rev Esp Med Legal. 2017;43:20–25.