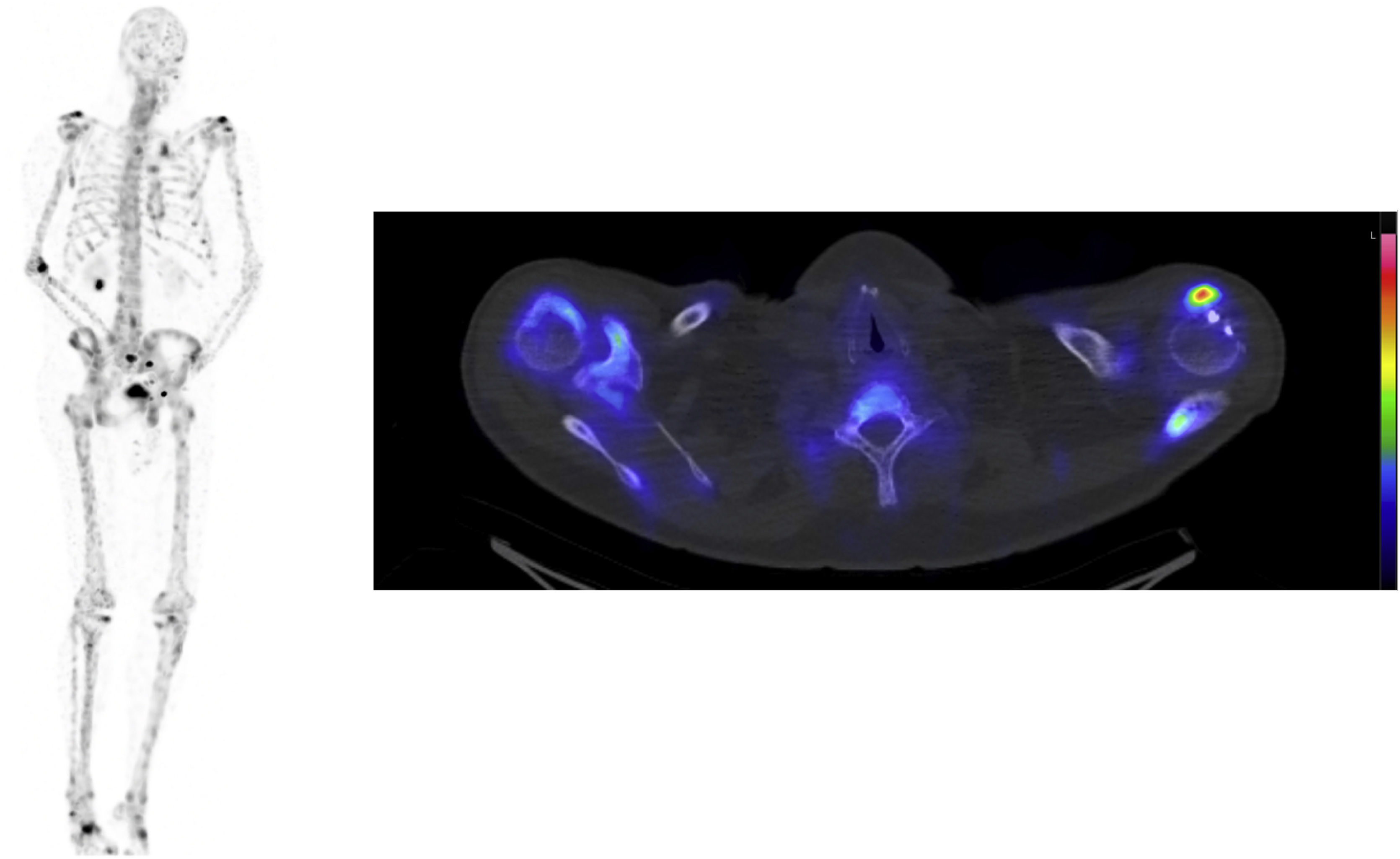
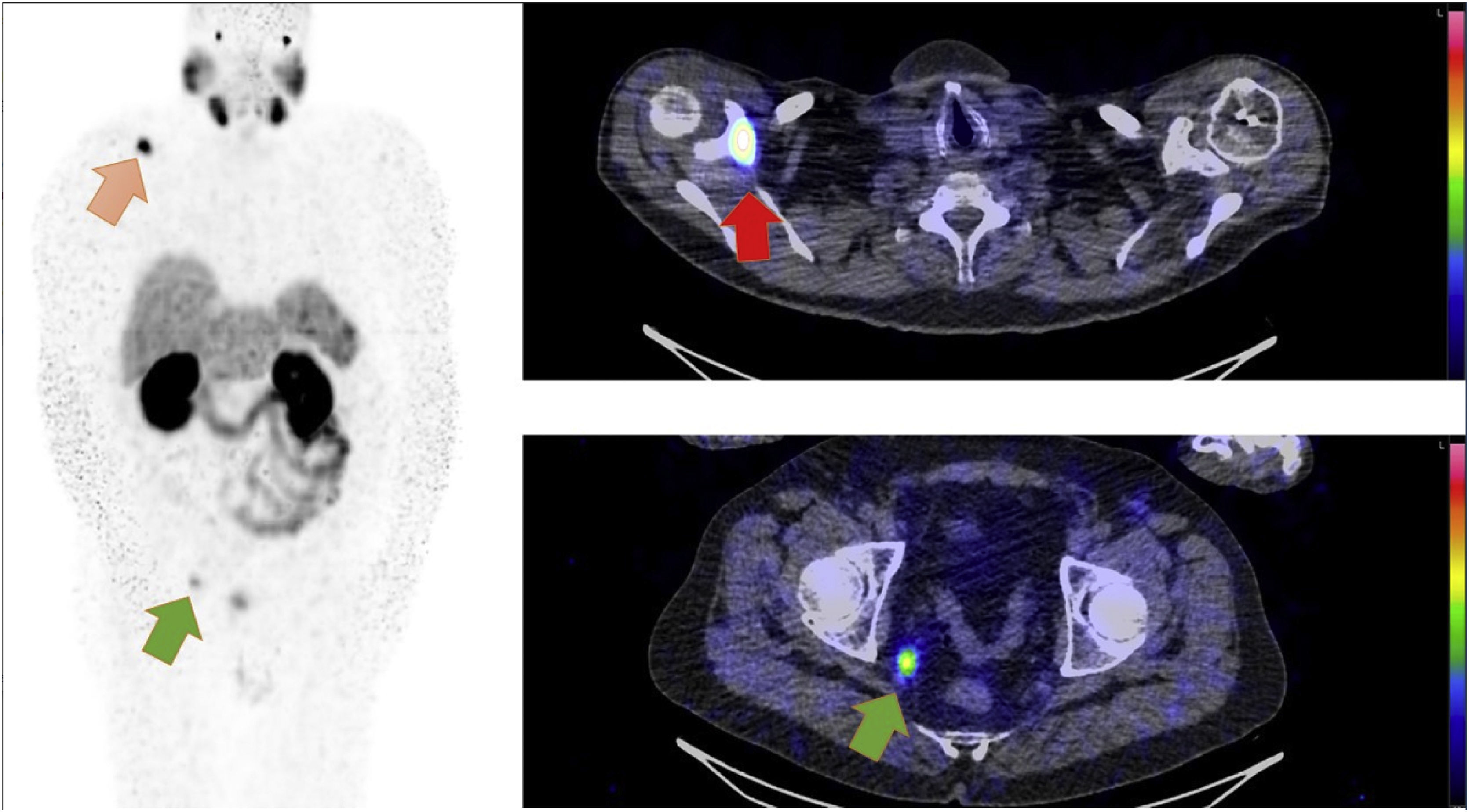
72-year-old male with prostate cancer cT3b, Gleason 7b and PSA level of 23 µg/l. Two weeks after diagnosis, a 99mTc-HDP whole body bone CZT SPECT/CT scan (Fig. 1) and a computed tomography (CT) scan (no image) were performed to investigate metastases, both scans were negative. Radical prostatectomy was performed and confirmed the finding of pT3b, Gleason score 7b with negative margins, but the PSA level after surgery remained elevated at 22 µg/l. A 68Ga-PSMA-11 PET/CT (Fig. 2) and a 99mTc-MIP-1404 CZT SPECT/CT (Fig. 3) scan were performed postoperatively to investigate possible metastasis and both scans reveal hypermetabolic activity in a 9 mm lymph node in the perirectal fat and in the right scapula with obvious clinical suspicion of metastasis. Previous studies have investigated 99mTc-MIP-1404 and conventional SPECT/CT for staging prostate cancer with promising results [1,2]. Furthermore, general-purpose CZT SPECT/CT cameras have shown high sensitivity and might be a good alternative to conventional cameras [3]. In this case, 68Ga-PSMA-11 PET/CT using a GE Discovery D710 (GE Healthcare; Milwaukee, WI, USA) and 99mTc-MIP-1404 CZT SPECT/CT using a CZT Veriton (Spectrum Dynamics, Caesaria, Israel) have shown comparable findings without inferiority with high lesion uptake in the metastatic lesions compared to the background, which raises the question of whether 99mTc-MIP-1404 CZT SPECT/CT could be an alternative to conventional imaging (99mTc-HDP whole body bone scan and CT) and possibly to 68Ga-PSMA-11 PET/CT for prostate cancer staging, emphasizing the need for further studies. To the authors knowledge this the first prostate cancer patient undergoing the combination of 99mTc-MIP-1404 and CZT SPECT/CT in the scientific literature.
Department of Urology and Department of Clinical Physiology, Linköping University Hospital, Region Östergötland. Faculty of Medicine and Health Sciences, LinkÖping University, Sweden.
Ethics approvalThe study was approved by the Swedish Ethical Review Authority (approval number 2021-01642) and the Swedish Medical Products Agency (EudraCT-number 2021-001059-15).
Consent to participateWritten consent was obtained from the patient.
Conflicts of interest/Competing interestsThe authors have no conflicts of interest to declare.
None.