The new coronavirus SARS-CoV-2, first identified in Wuhan, China in December, 2019, can cause Severe Acute Respiratory Syndrome (SARS) with massive alveolar damage and progressive respiratory failure. We present the relevant autopsy findings of the first patient known to have died from COVID19 pneumonia in Spain, carried out on the 14th of February, 2020, in our hospital (Hospital Arnau de Vilanova-Lliria, Valencia). Histological examination revealed typical changes of diffuse alveolar damage (DAD) in both the exudative and proliferative phase of acute lung injury. Intra-alveolar multinucleated giant cells, smudge cells and vascular thrombosis were present. The diagnosis was confirmed by reverse real-time PCR assay on a throat swab sample taken during the patient's admission. The positive result was reported fifteen days subsequent to autopsy.
El nuevo coronavirus SARS-CoV-2, identificado inicialmente en China en diciembre de 2019 puede cursar con un síndrome respiratorio agudo severo (SARS) con daño alveolar difuso y fracaso respiratorio progresivo. Presentamos los hallazgos más relevantes encontrados en la autopsia clínica efectuada en nuestro hospital (Hospital Arnau de Vilanova-Lliria de Valencia) a fecha de 14 de febrero de 2020, al primer paciente fallecido conocido en España por neumonía COVID-19. A nivel pulmonar, la autopsia revela cambios típicos de daño alveolar difuso (DAD) en fases exudativa y proliferativa. Se observan células multinucleadas gigantes, células tipo smudge intraalveolares y trombosis vasculares. El diagnóstico microbiológico confirmativo mediante PCR se realizó 15 días después de la autopsia sobre la muestra faríngea del enfermo tomada durante su ingreso.
In January 2020 a new subtype of coronavirus, named SARS-CoV-2, was identified as the causative agent of Severe Acute Respiratory Syndrome (SARS), which had occurred one month previously in the city of Wuhan, in the province of Hubei, China.1 The disease associated with this virus has been named COVID-19 and can cause only mild symptoms, such as those of an upper respiratory tract infection or develop into severe, acute respiratory disease. By February 2020, COVID-19 had spread to 26 countries and in March it was declared a pandemic by the World Health Organization (WHO).
To date, very few reports of the histopathological findings characteristic of this disease have been published. We present the principal findings found during the autopsy of the first patient known to have died of COVID-19 in Spain; the post-mortem was carried out by pathologists in the Hospial Arnau de Vilanova-Lliria, Valencia, on the 14th of February, 2020.
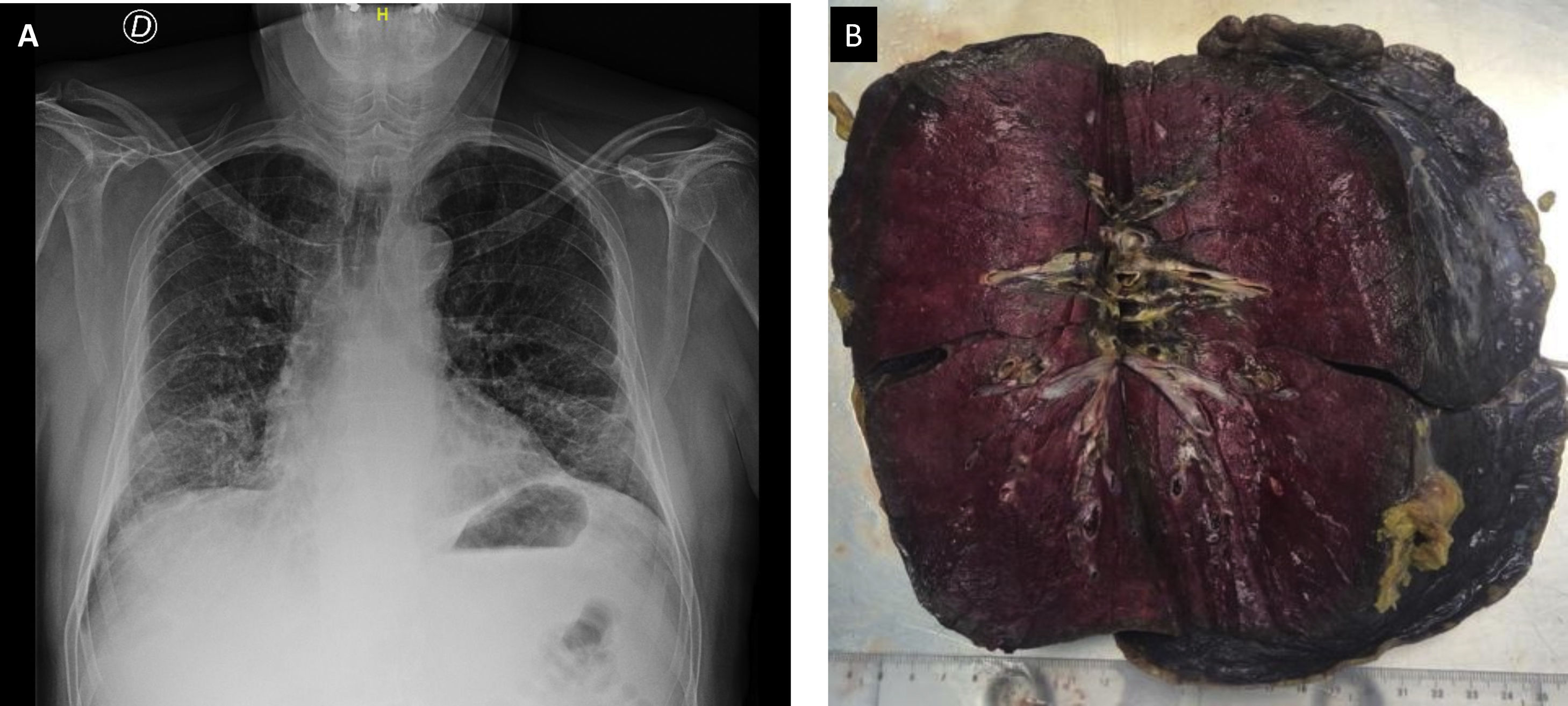
Case historyA 69-year-old male presented in the emergency department on the 11th of February, 2020, with fever (38°C), dyspnoea, cough and hypoxia without acidosis. He was transferred to the department of pneumology. He had attended the emergency department on a previous occasion and was diagnosed as suffering from a common cold and discharged with symptomatic treatment. He had visited Nepal, but without a stop-over in China, from the 19th to the 30th of December, 2019. In 2018 he had been diagnosed with low grade, non-invasive urothelial carcinoma of the bladder. On admission he had neither hypertension nor diabetes mellitus. A chest X-ray revealed a bilateral interstitial infiltrate with a ground glass appearance involving the inferior lobes (Fig. 1A). A CT angiogram ruled out pulmonary thromboembolism. Antibiotic treatment with Levofloxacin and Ceftriaxone was initiated but with no clinical improvement. As viral pneumonia was suspected, he underwent a panel of tests for respiratory viruses, all of which proved negative for: Influenza virus A and B, Influenza virus A H1N1, syncytial respiratory virus (SRV), Enterovirus, Adenovirus, Metapneumovirus, Bocavirus, Coronavirus type 229, Coronavirus NL63, Coronavirus OC43, Parainfluenza virus type I, II and II and Rhinovirus. Non-invasive mechanical ventilation up to 90% FiO2 was initiated.
Due to his poor evolution, he was transferred to the ICU on the 13th of February, 2020, where he died four hours later in shock with renal failure unresponsive to treatment.
Epidemiological criteria at the time did not require the patient to be tested for PCR COVID-19. The principal diagnosis was severe bilateral community acquired pneumonia and an autopsy was requested.
Macroscopically, the lungs were deep red in colour with increased weight and density (Fig. 1B). Pleural adhesions were present in posterior aspects of the right lung.
Mild stenosis of the aortic valve was seen, as well as a slight increase in the thickness of the left ventricle as well as dilation of both ventricles. No tumoral remnants were found in the bladder. All other organs showed generalized congestion. The skull was not opened.
Tissue samples were taken from each pulmonary lobe and all other organs for histopathology. They were fixed in formalin, stained with H&E and embedded in paraffin. Fresh samples were sent for microbiological analysis.
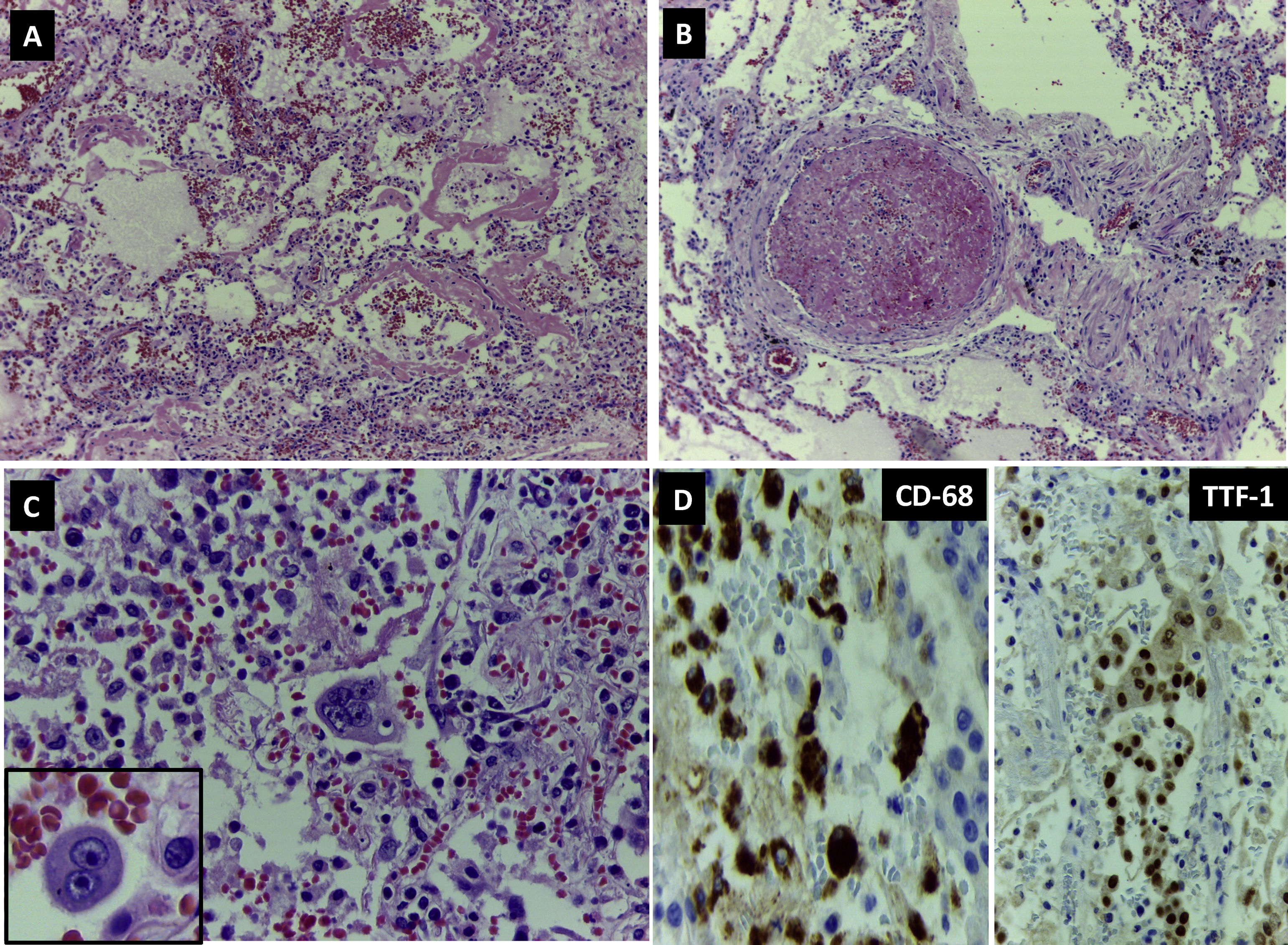
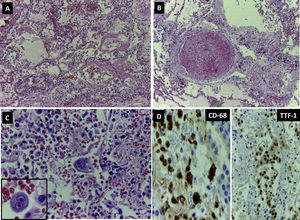
Microscopically, 80% of the pulmonary parenchyma examined had undergone pathological changes. Adjacent to large areas of oedema and intraalveolar haemorrhage were characteristic signs of diffuse alveolar damage such as desquamation of type II pneumocytes and hyaline membranes (Fig. 2A), as well as thrombi in the medium sized vessels (Fig. 2B).
DAD. (A) Lung parenchyma with haemorrhage, alveolar oedema and abundant hyaline membranes (H&E 100×). (B) Vascular thrombosis (H&E 200×). (C) Mono- and multinucleated cells with nuclear inclusions suggestive of viral cytopathic changes. (D) Immunohistochemistry: expression of CD68 in intraalveolar macrophages (400×) and of TTF1 in pneumocytes.
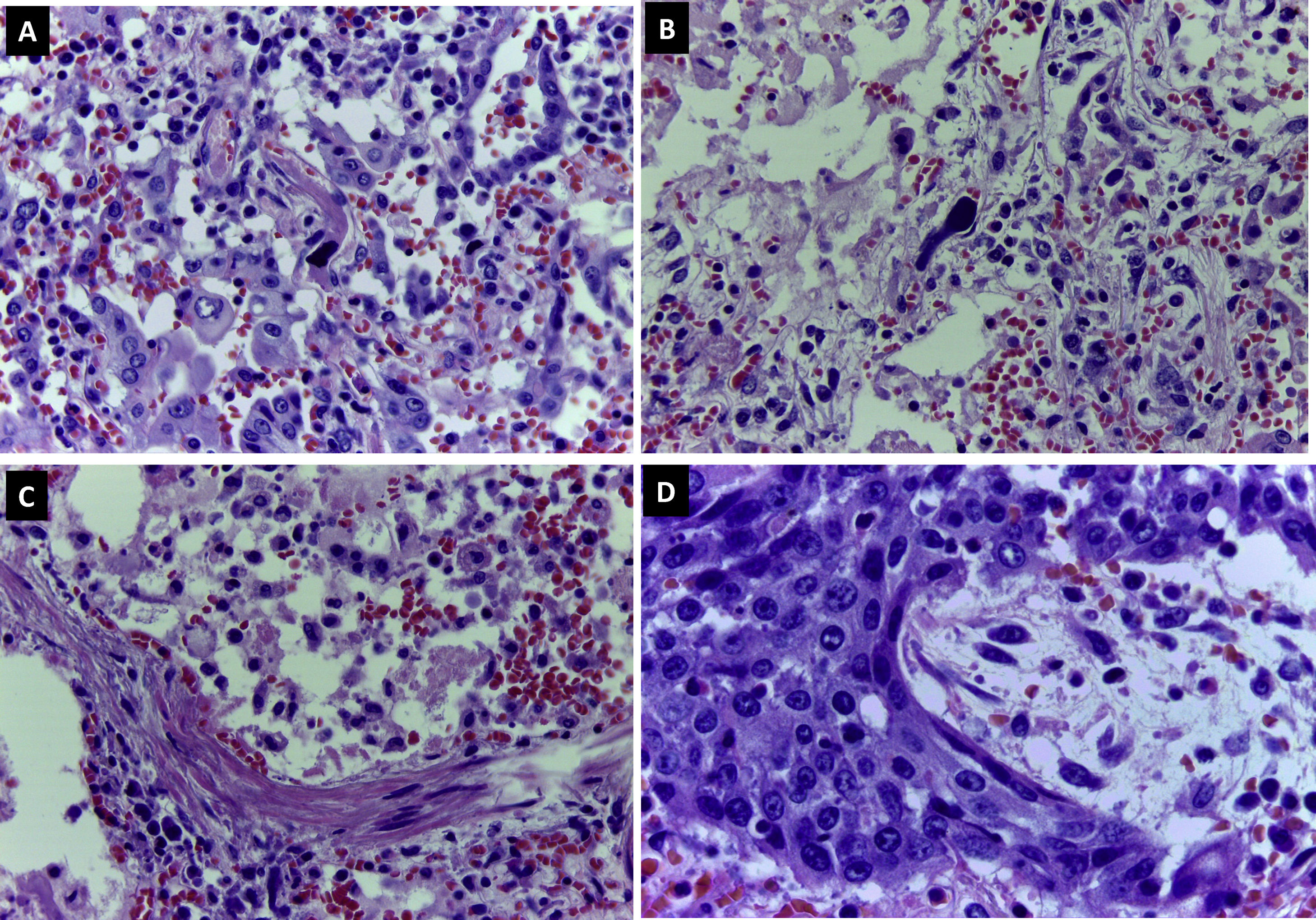
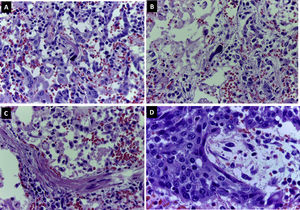
Abundant intraalveolar macrophages and occasional multinucleated giant cells were seen. A striking finding was the presence of vesicular nuclei with prominent nucleoli suggestive of viral cytopathic involvement (Fig. 2C). Immunohistochemistry for cytokeratin AE1/AE3, TTF-1 and CD68 revealed that these lesions were found in both pneumocytes and macrophages (Fig. 2D). Cells with large, hyperchromatic nuclei, similar to smudge cells described in adenovirus related pneumonitis, were also observed (Fig. 3A and B). No intraalveolar organized fibrin similar to that found organized acute fibrinous pneumonia was seen.
In approximately 30% of the pulmonary parenchyma findings were consistent with the proliferative phase of DAD. Pneumocytic hyperplasia and myofibroblastic proliferations contributed to widening of alveolar septa (Fig. 3C). No collagen fibrosis was present; however, squamous pneumocytic metaplasia was evident (Fig. 3D). The inflammatory component consisted of a mild lymphoid infiltrate with abundant macrophages. No neutrophilic or eosinophilic cells were observed. Areas of emphysema were found.
No extrapulmonary vascular thromboses were seen.
Additional immunohistochemical studies for herpes simplex virus, cytomegalovirus and Epstein-Barr virus proved negative.
DiscussionThere are few published reports of the morphological changes secondary to SARS-CoV-2 infection. The virus's high contagiousness and the need for urgent treatment in serious cases outweigh the need for biopsies or autopsies to confirm the diagnosis.
In the present case, the diagnosis of SARS-CoV-2 was confirmed subsequent to the autopsy, as the protocol on admission did not require the necessary testing.
The morphological findings on autopsy were consistent with histopathological patterns of DAD similar to those described in other viral pneumonias that clinically show acute respiratory distress syndrome/acute pulmonary damage (pneumonia, SARS, influenza and adenovirus).2
Tian et al.3 from the Wuhan Hospital, China, published the pathological findings from surgical specimens of COVID-19 positive patients undergoing surgery for pulmonary neoplasia. In contrast to the present case, they found no hyaline membranes or squamous metaplasia; the authors propose that their absence was due to the fact that the patients were asymptomatic and therefore in an early phase of the disease. However, Wang et al.,4 from the Centre for Infectious Diseases in Beijing, reported such changes in post-mortem biopsies of the lungs of a COVID-19 positive patient.
Morphological changes characteristic of DAD and organized acute fibrinous pneumonia, occurring alone or simultaneously, have been reported in cases of SARS caused by coronavirus5 and smudge cells have been described in pneumonia due to adenovirus.6 The intranuclear inclusions found in herpes pneumonia and confirmed by immunohistochemistry are key diagnostic features7 and pulmonary vascular thrombotic haemorrhage are predominant in influenza A H1N1 pneumonia.8
In the present case, no images compatible with acute fibrous pneumonia were seen and the microbiological studies ruled out coinfection. No indications of myocarditis or hepatitis, such as those described by Wang et al.4 were found.
To date, no specific histopathological changes permitting a diagnosis of DAD secondary to SARS-CoV-2 have been reported. Although research is ongoing, as yet, no immunohistochemical tests are available for the identification of the virus in tissue samples, making it necessary to test for the virus in patients’ secretions.3
To our knowledge, this is the first report of autopsy findings of a COVID-19 death in Europe, thus we wished to present a detailed description of our findings. The Spanish Society of Pathology has recently published recommendations for the handling of cadavers clinically suspected of being infected by COVID-19.9
Conflict of interestsAll authors declare that they have no conflict of interest.
Técnicos Superiores del Servicio de Anatomía Patológica, Hospital Arnau de Vilanova de Valencia.
Celadora de Autopsias del Servicio de Anatomía Patológica, Hospital Arnau de Vilanova de Valencia.
Servicio de Unidad de Cuidados Intensivos, Hospital Arnau de Vilanova de Valencia.
Dr Luis Alfaro Ferreres, Servicio de Anatomía Patológica, Hospital 9 de Octubre de Valencia.