Anaplastic lymphoma kinase (ALK) rearrangement located on the short arm of chromosome 2, region 2 and band 3 is frequent in lung cancer patients who respond to targeted therapies with ALK inhibitors Therefore, their identification has become a standard diagnostic test in patients with advanced NSCLS, as such chromosomal alterations may lead to the activation of important signalling pathways involved in cell survival and proliferation.
MethodsTo investigate the ALK gene status, we performed FISH and IHC assays in 18 lung adenocarcinoma patients, 12 women and 6 men, aged between 29 and 85 years. Paraffin-embedded samples were analyzed in the Pathology Department of the Hospital Universitario San Ignacio.
ResultsResults between the two techniques in 5 patients showed discordant patterns, being positive for FISH and negative for IHC. The borderline to define ALK positivity was set at 15%, These results present experimental evidence that the techniques differ in specific situations.
ConclusionsOur findings show that it is advisable to investigate the ALK gene status in patients with suspected lung cancer using both FISH and IHC in combination.
La reorganización de la (anaplastic lymphoma kinase) ALK ubicada en el brazo corto del cromosoma 2, región 2 y banda 3 es frecuente en los pacientes con cáncer de pulmón que responden a terapias dirigidas con inhibidores de la ALK. Por ello, su identificación se ha establecido como una prueba diagnóstica estándar en pacientes con CPCNP, ya que dichas alteraciones cromosómicas puedan determinar la activación de importantes vías de señalización implicadas en la supervivencia y proliferación celulares.
MétodosPara determinar el estatus de gen ALK se realizaron pruebas FISH e IHC en 18 pacientes con adenocarcinoma pulmonar, 12 mujeres y 6 varones, con edades comprendidas entre 29 y 85 años. Las muestras fueron analizadas en el Departamento de Anatomía Patológica del Hospital Universitario San Ignacio.
ResultadosLos resultados entre ambas técnicas mostraron patrones discordantes en 5 pacientes, con positividad de FISH y negatividad con IHC. El límite para definir la positividad de ALK se estableció en el 15%. Estos resultados muestran evidencia experimental que dichas técnicas difieren en situaciones específicas.
ConclusionesEste estudio recomienda la investigación del estatus del gen ALK en los pacientes con sospecha de cáncer de pulmón, mediante la combinación de FISH e IHC.
Lung cancer can be classified into two histopathological types: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Approximately 85% of cases correspond to NSCLC and 15% to SCLC.1 Histologically, the main types of NSCLC are adenocarcinoma and squamous cell carcinoma.2
According to the Global Cancer Observatory of 2020, lung cancer ranks third in incidence but first in mortality, due to late diagnosis, as the initial stages of the tumour process are asymptomatic causing delay in treatment.3,4
Biomarkers commonly used for the diagnosis of lung cancer are KRAS, EGFR, MET, BRAF, RET, ROS1 and ALK. They respond to the necessity of early diagnosis and follow-up of lung cancer treatment and are directly linked to the specific deregulation of molecular pathways.5 The ALK gene is located on the short arm of chromosome 2, region 2 band 3 and encodes a protein with receptor functions called anaplastic lymphoma kinase, composed of an extracellular domain, a transmembrane domain and an intracellular domain, which contains the tyrosine kinase region. The rearrangements in ALK gene can lead to the anomalous activation of this receptor, generating its constitutive dimerization.6,7
In NSCLC, the principal activation of ALK occurs by the formation of fusion genes. The EML4-ALK rearrangement is the most common fusion, with a prevalence of 3–5% in NSCLC, in which the predominant histological type is adenocarcinoma.8 Some other fusion genes, although present in smaller proportions, have also been reported in the literature, such as KIF5B-ALK and TFG-ALK, indicating the therapeutic potential of ALK inhibition by analogy with EML4-ALK fusions. The approval of crizotinib as a tyrosine kinase inhibitor was a product of the discovery of the EML4-ALK fusion gene. It has been established that patients treated with this inhibitor have a higher response rate and progression-free survival than with cytotoxic chemotherapy.8
The effector action of ALK includes the activation of important cell signalling pathways, such as RAS-RAF-MEK-ERK, PLCγ-DAG-PKC, and PI3K-AKT-FOXO, involved with cell proliferation and apoptosis.9,10 Those patients with advanced NSCLC who receive treatment with crizotinib instead of chemotherapy have a better prognosis in terms of overall response and survival due to its function as a first-generation ALK inhibitor. Entities monitoring patients with lung adenocarcinoma or mixed lung cancer with an adenocarcinoma component such as the College of American Pathologists, the International Association for the Study of Lung Cancer and the Association for Molecular Pathology recommend that ALK gene testing should be performed.11
FISH is one of the commonly used methods for the identification of ALK rearrangements, using break-apart probes. The use of this technique has been useful for the implementation of crizotinib treatment, a small molecule inhibitor of the tyrosine kinases ALK, ROS1, and MET.12 However, for reasons of cost-effectiveness, some pathology laboratories have implemented the use of IHC as an alternative screening technique.
The diagnostic algorithm for ALK states that all positive and equivocal cases by IHC must be confirmed by FISH.11 However, discordances between the results obtained by FISH and IHC in routine clinical practice have been reported,11 causing problems in therapeutic decision-making. In this study, we evaluated the presence of discordant patterns between ALK gene rearrangements detected by FISH and ALK protein expression detected by IHC in tumour tissue from patients diagnosed with lung adenocarcinoma in a Colombian hospital.
Materials and methodsStudy populationThis study included 18 paraffin-embedded samples of tumour tissue from patients with lung adenocarcinoma (12 women and 6 men, with an age range between 29 and 85 years) collected between October 2019 and June 2022 from the Pathology Department of the Hospital Universitario San Ignacio (Bogota, Colombia). This study was conducted with the approval of the Ethics Committee of the Hospital Universitario San Ignacio and the Pontificia Universidad Javeriana (FM-CIE-0411-20).
Fluorescence in situ hybridizationIt was performed on 2-mm sections of samples contained in paraffin blocks using Break Apart probe for ALK (Vysis LSI ALK Dual Color, Break Apart Rearrangement Probe; Abbott Molecular), according to the protocol for FISH of the Hospital Universitario San Ignacio and the Pontificia Universidad Javeriana, following the technical instructions and the manufacturer's standard of interpretation.
The sections were placed on positively charged slides and left at 60°C overnight. Slides were deparaffinized, dehydrated, and immersed for 20min in 0.2N HCl and 1 hour in pH 6.0 citric acid at 82°C. Slides were washed in 2× SSC and H2O and proteins were digested with 0.5% pepsin solution at 37°C for 30min. Subsequently, slides were washed with 2× SSC and 0.01M HCl at room temperature and dehydrated with 70%, 80%, and 100% ethanol. 4μL of probe (REF:06N38) was applied to each slide, which was codenatured together with sample DNA at 73°C for 3min and hybridized at 37°C overnight. Finally, it was washed with 0.4× SSC for 2min at 72°C and 2× SSC for 30s. The dried slides were counterstained with diamidino-2-phenylindole (DAPI) and stored in the dark at −20°C previous to microscopic examination.
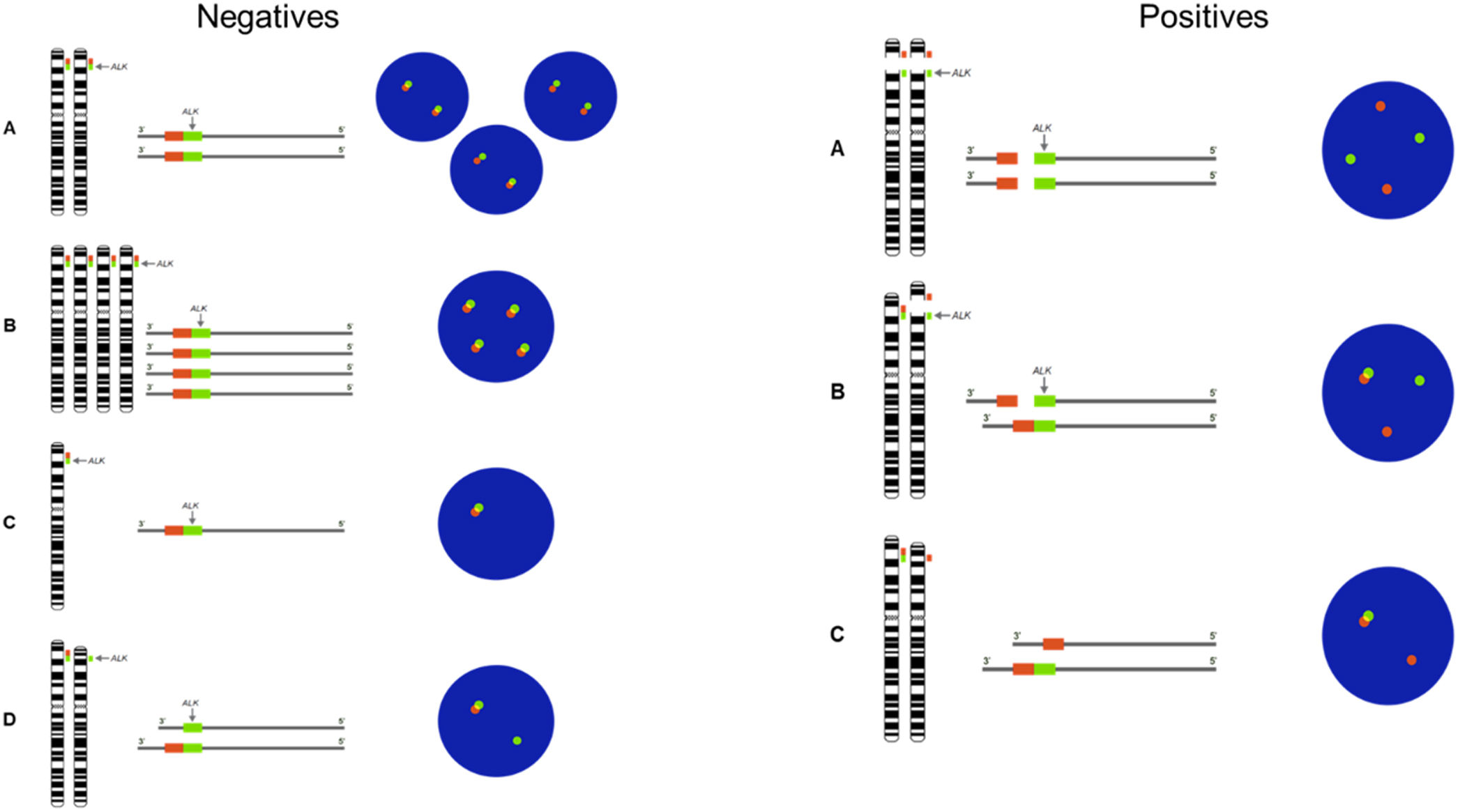
ALK gene rearrangement analysis was performed on a minimum of 100 nuclei per patient, observed under a ZEISS Axio Scope.A1 fluorescence microscope at 100X. The description of chromosomal alterations was performed according to the International System of Cytogenetic Nomenclature 2020 (ISCN) standards.13 Cells with green and red signals separated by more than 2 diameters or the presence of an isolated 3′ red signal were considered positive for rearrangement, as shown in Supplementary Fig. 1.
ALK immunohistochemistryIHC was performed on formalin-fixed, paraffin-embedded, 3mm thick tissue sections using Roche's monoclonal rabbit ALK D5F3 primary antibody (Ventana anti-ALK D5F3) on the Ventana BenchMark GX automated slide processing system (Ventana Medical Systems Inc). The process consisted of deparaffinization, cell conditioning, primary inhibition of endogenous peroxidase and the addition of the antibody with incubation for 20min. OptiView DAB IHC detection kit and OptiView amplification kit were used according to the manufacturer's instructions. Tissues were counterstained with eosin and haematoxylin and blue reagent. Finally, washes were performed with conventional liquid soap to remove excess LCD oil, followed by immersion in 96% alcohol and three immersions in xylol. Mounting and sealing the samples were done by adding a drop of resin.
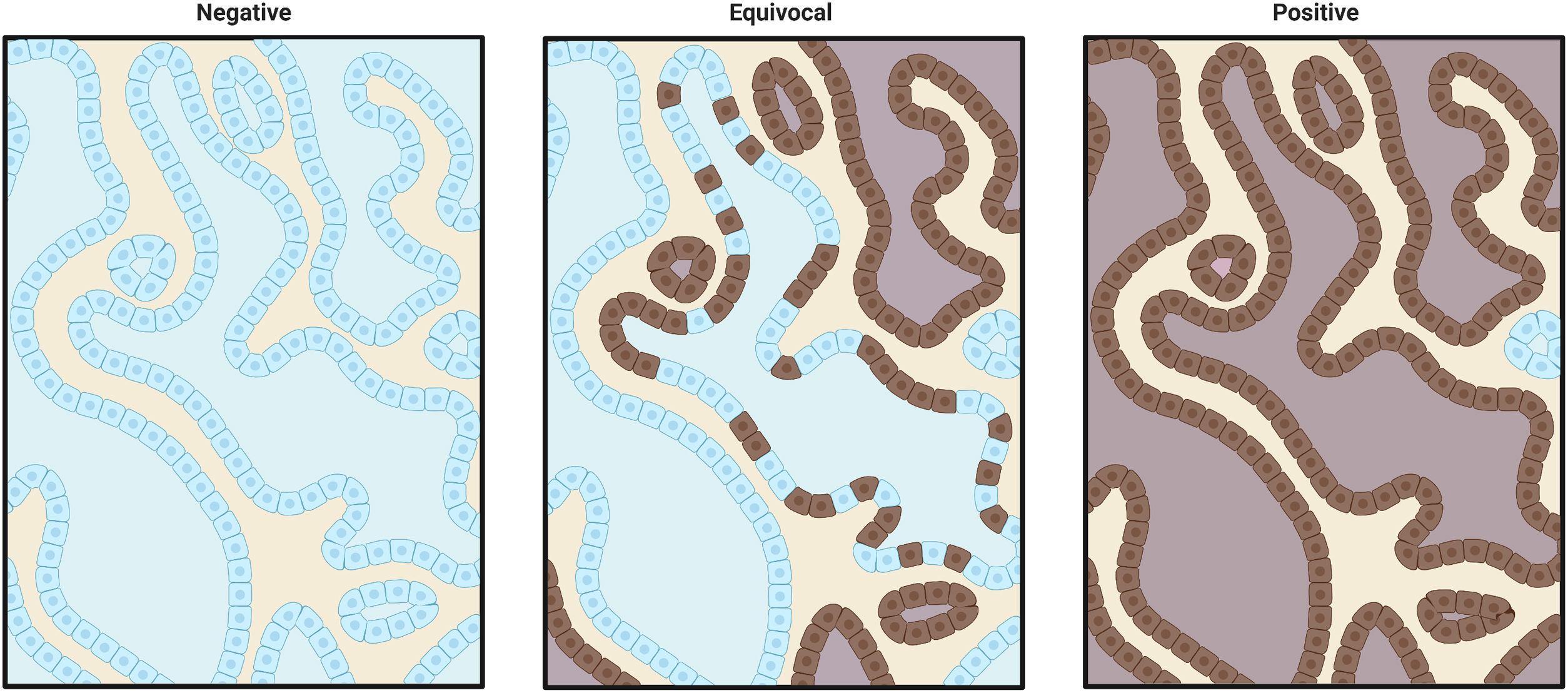
The appendix was used as a positive and negative control, where ganglion cells stained positive. The evaluation of the results was carried out by the pathology department of the Hospital Universitario San Ignacio using an OLYMPUS BX43 trinocular brightfield microscope at 10X and 40X. For this case, a strong granular cytoplasmic brown staining was considered positive and a homogeneous blue colour was classified as negative (Supplementary Fig. 2).
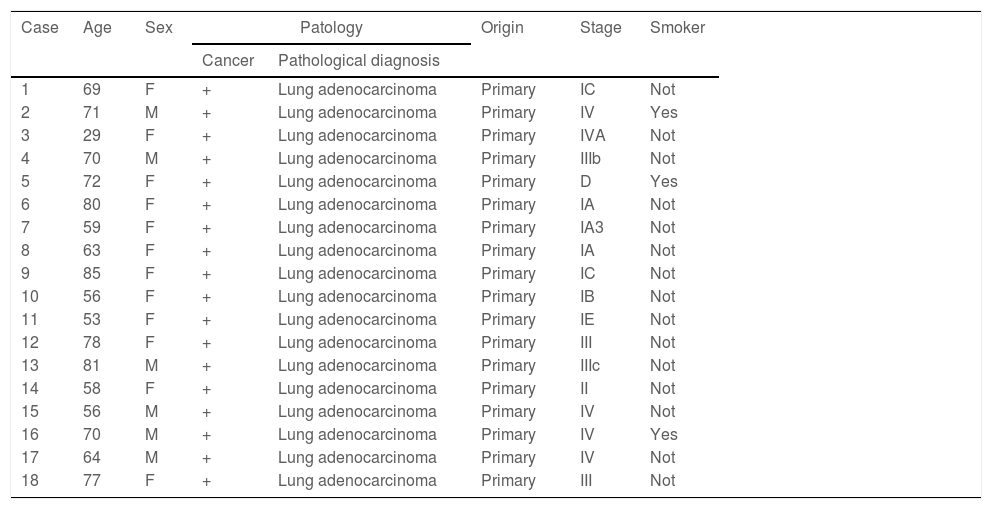
ResultsA total of 18 cases of lung adenocarcinoma, confirmed by the Pathology Department of the Hospital Universitario San Ignacio, were included in this study. Table 1 describes the clinical variables.
Clinical characteristics of all cancer patients.
| Case | Age | Sex | Patology | Origin | Stage | Smoker | |
|---|---|---|---|---|---|---|---|
| Cancer | Pathological diagnosis | ||||||
| 1 | 69 | F | + | Lung adenocarcinoma | Primary | IC | Not |
| 2 | 71 | M | + | Lung adenocarcinoma | Primary | IV | Yes |
| 3 | 29 | F | + | Lung adenocarcinoma | Primary | IVA | Not |
| 4 | 70 | M | + | Lung adenocarcinoma | Primary | IIIb | Not |
| 5 | 72 | F | + | Lung adenocarcinoma | Primary | D | Yes |
| 6 | 80 | F | + | Lung adenocarcinoma | Primary | IA | Not |
| 7 | 59 | F | + | Lung adenocarcinoma | Primary | IA3 | Not |
| 8 | 63 | F | + | Lung adenocarcinoma | Primary | IA | Not |
| 9 | 85 | F | + | Lung adenocarcinoma | Primary | IC | Not |
| 10 | 56 | F | + | Lung adenocarcinoma | Primary | IB | Not |
| 11 | 53 | F | + | Lung adenocarcinoma | Primary | IE | Not |
| 12 | 78 | F | + | Lung adenocarcinoma | Primary | III | Not |
| 13 | 81 | M | + | Lung adenocarcinoma | Primary | IIIc | Not |
| 14 | 58 | F | + | Lung adenocarcinoma | Primary | II | Not |
| 15 | 56 | M | + | Lung adenocarcinoma | Primary | IV | Not |
| 16 | 70 | M | + | Lung adenocarcinoma | Primary | IV | Yes |
| 17 | 64 | M | + | Lung adenocarcinoma | Primary | IV | Not |
| 18 | 77 | F | + | Lung adenocarcinoma | Primary | III | Not |
F: Female; M: Male; D: Unknown.
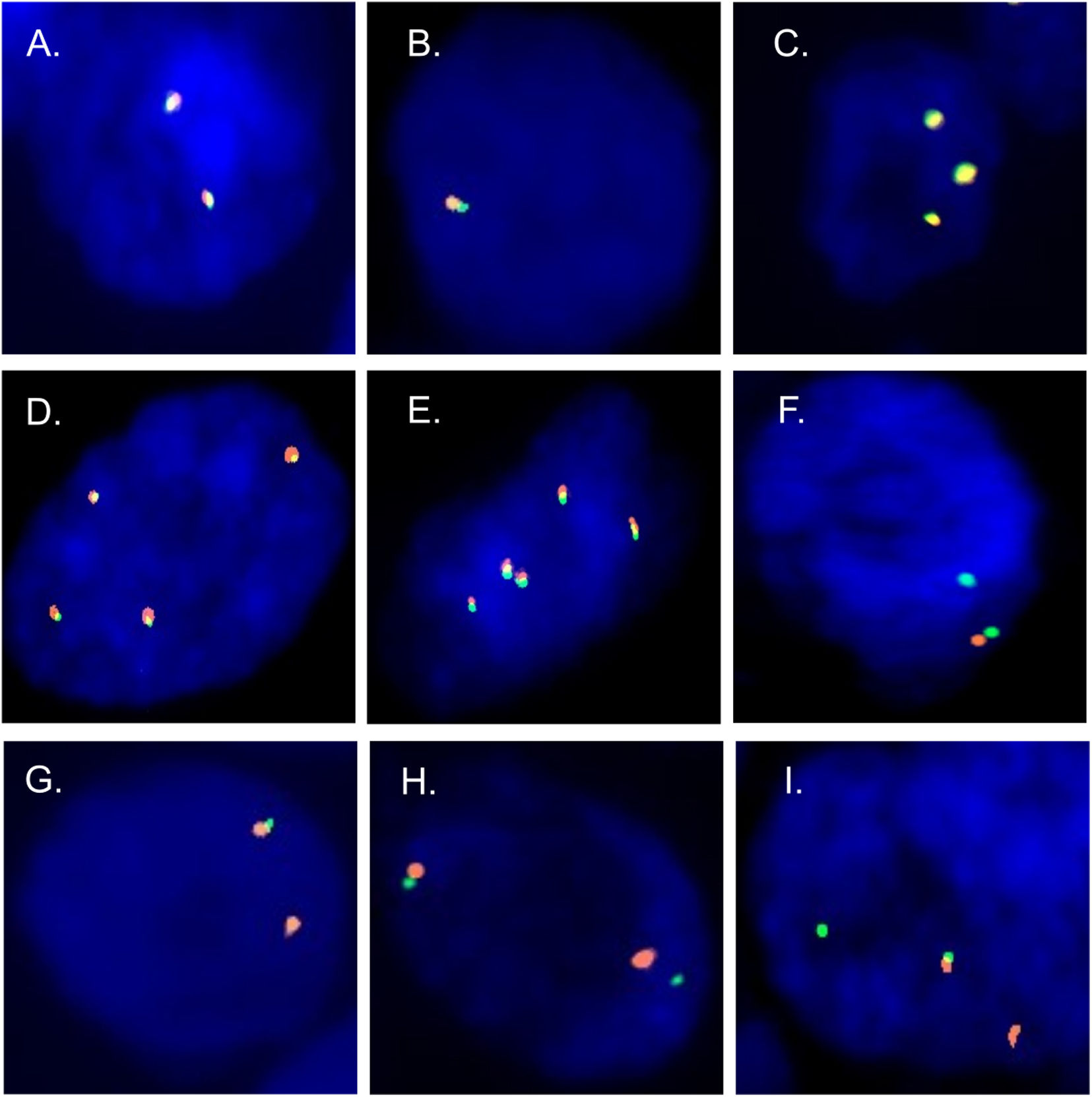
To evaluate the presence of ALK rearrangement, FISH assays were performed in tumour cells of patients diagnosed with lung adenocarcinoma. 6 negative and 2 positive patterns were found (Fig. 1).
Patterns negatives and positives by FISH. Negative patterns for the presence of rearrangements. (A) Two fusions. (B) Deletion of the gene in one of the homologs. (C–E) Copy number gain. (F) Deletion of the 3′ extreme. Positive patterns for the presence of rearrangements. (G) Deletion of the 5′ extreme. (H and I) Short and long splits of the 3′ and 5′ extremes in one of the homologs.
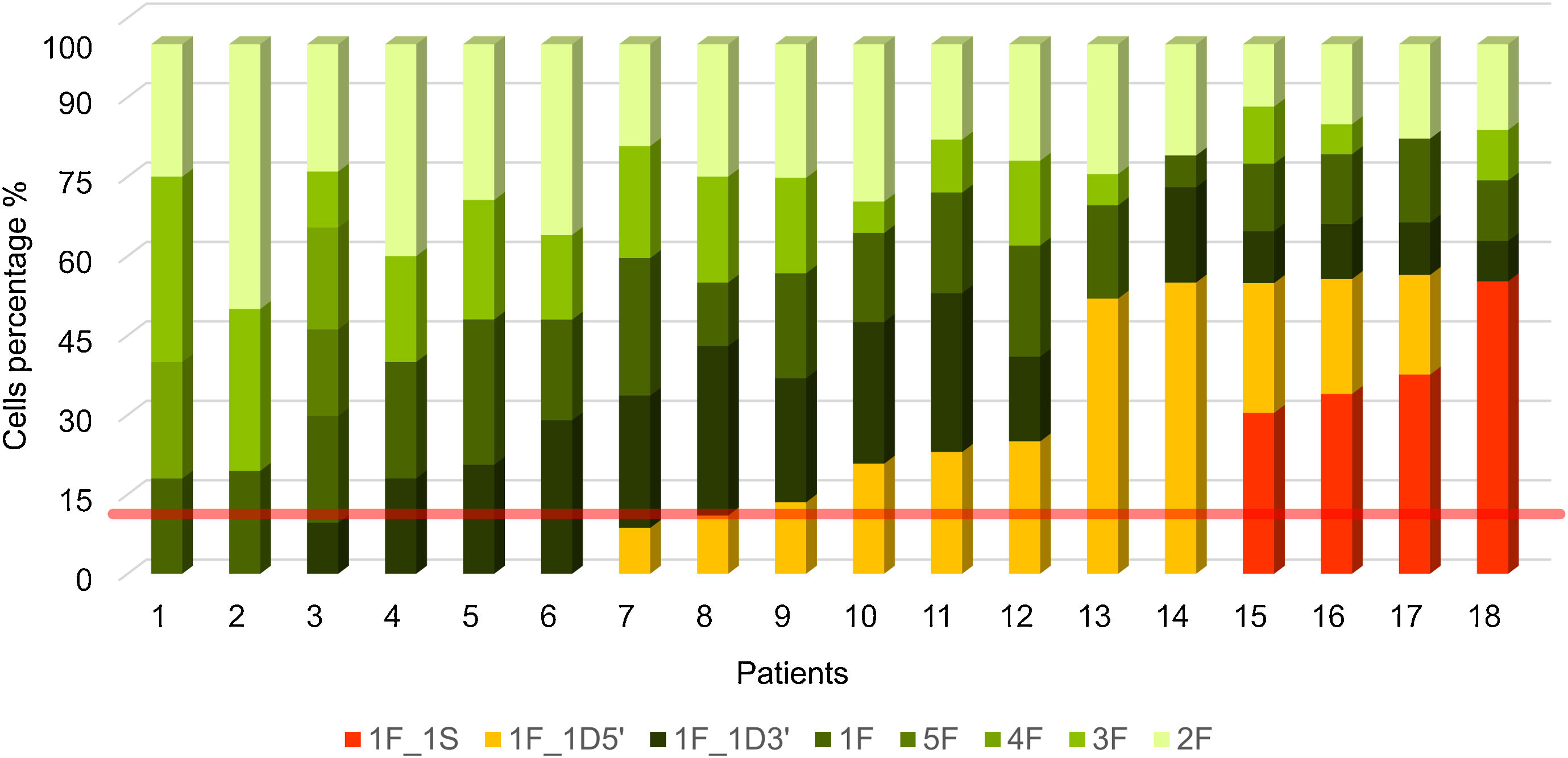
Fig. 2 shows the percentage of patterns found in at least 100 nuclei evaluated per patient. Patients coded from 1 to 9 have a negative FISH, while those from 10 to 18 have a positive FISH. The criterion for considering a result positive was the presence of patterns with rearrangements in more than 15% of the nuclei analyzed (red line). To rule out false-positive or false-negative results in an analysis of ALK gene rearrangements, those individuals with percentages of positivity between 10% and 20% were studied in detail. Only 2 of our cases (8 and 9) were in this range or borderline (Fig. 2).
Percentage of cells per hybridization pattern in each patient. Negative patterns in shades of green. (1F) Deletion of the gene in one of the homologs. (3F, 4F and 5F) Copy number gain. (2F) Two fusions. The positive pattern is in yellow. (1F_1D5′) One fusion and one deletion of the 5′ extreme. The positive pattern is in red. (1F_1S) A fusion and a split of the 3′ and 5′ extremes in one of the homologs.
On the other hand, in supplementary Table 1, the total percentage of cells with positive and negative patterns is recorded for each of the cases evaluated and the overall result of the patient and the nomenclature are indicated.
Despite the limitations related to the sample size, the statistical association analyses performed with the clinical variables and the percentage of positivity (supplementary Table 2) did not show significant differences.
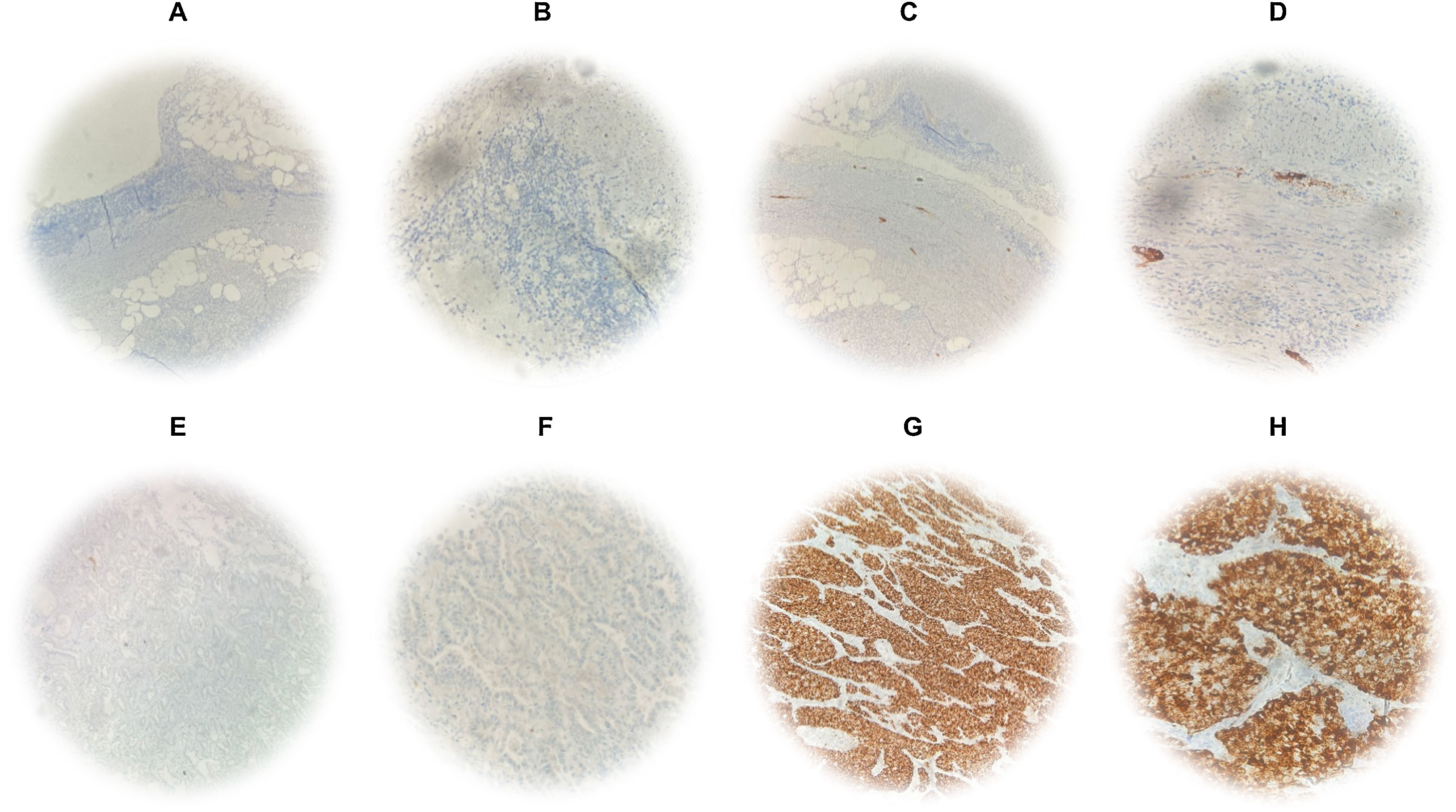
Detection of ALK protein expression in patients diagnosed with lung adenocarcinomaALK protein expression was evaluated by IHC using the D5F3 antibody, which is highly sensitive and specific, recognizing the carboxyl end of the protein. We found 4 positive and 14 negative patients, therefore 22.22% of the patients had the expression of the tyrosine kinase domain belonging to the receptor (Fig. 3).
Protein expression by IHC. Technical controls of immunostaining in appendix specimens with mixed positive and negative patterns. (A and B) Immunostaining of non-specific antibodies at 10× and 40× optical resolution. (C and D) Immunostaining with D5F3 antibody at 10× and 40× optical resolution. ALK protein expression in lung adenocarcinoma with specific immunostaining by D5F3 antibody. (E and F) Negative cytoplasmic immunostaining at 10× and 40× optical resolution. (G and H) Positive cytoplasmic immunostaining at 10× and 40× optical resolution.
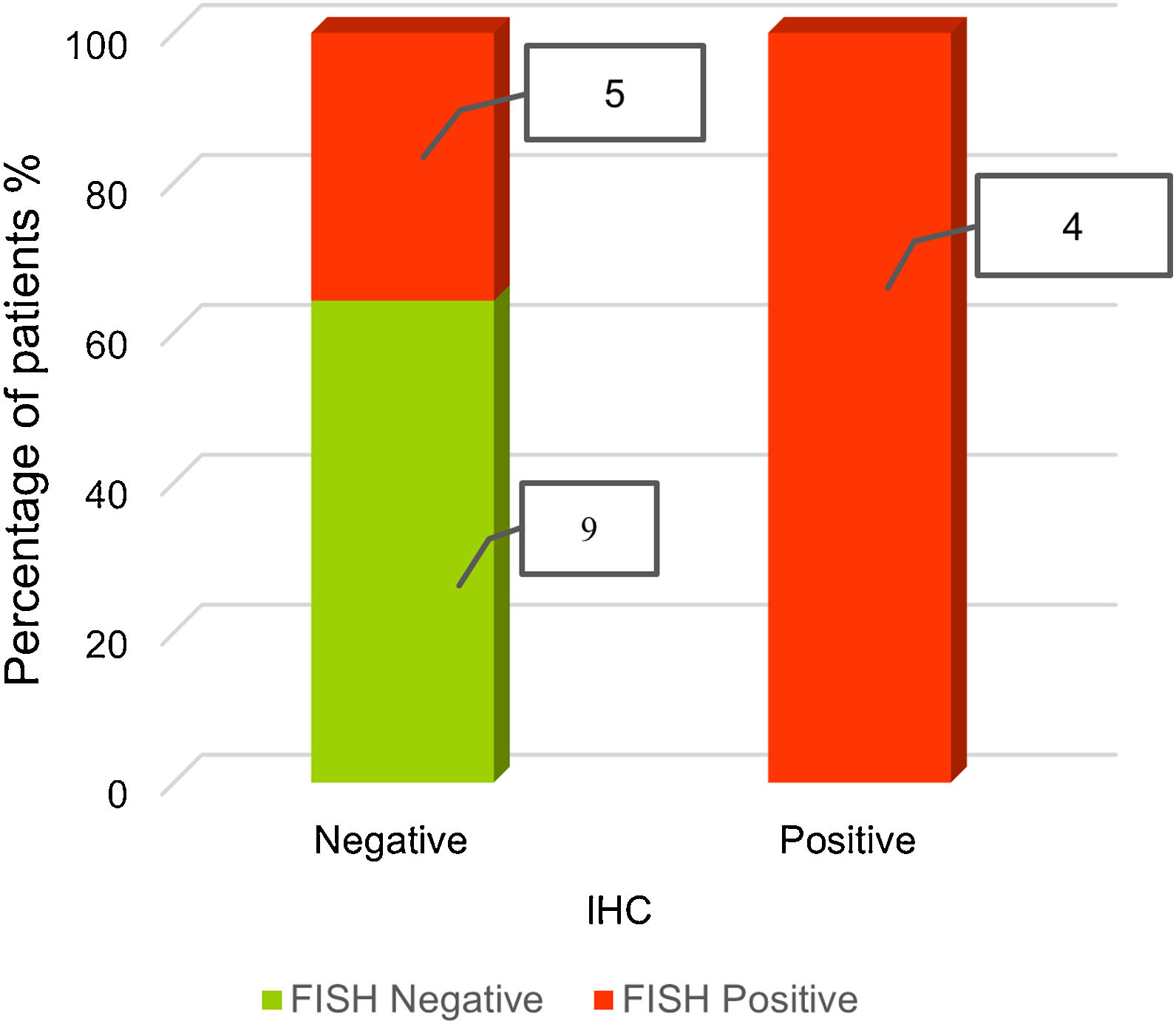
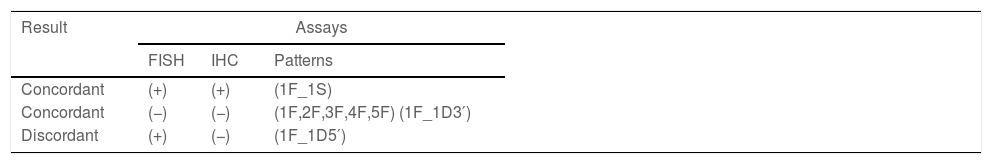
We analyzed the concordance between FISH and IHC for ALK finding two concordant and one discordant patterns (Table 2). The concordant patterns included those in which the predominant FISH and IHC patterns were jointly either negative or positive. The discordant pattern was obtained by the presence of a positive FISH pattern (deletion extreme 5′) in more than 15% of the nuclei analyzed and a negative IHC pattern.
Fig. 4 shows the relation between positive-negative FISH results and negative and positive IHC cases. Negative FISH and immunohistochemistry were observed for 9 patients, positive FISH and negative immunohistochemistry for 5 patients, and positive FISH and immunohistochemistry for 4 patients (Fig. 4).
DiscussionIdentification of ALK gene rearrangement has become a routine test in patients with advanced NSCLC.14-16 In 2011, the FDA approved crizotinib as a new ALK inhibitor for advanced-stage ALK-positive lung cancer. Kwak et al.17 demonstrated that patients with ALK rearrangement treated with crizotinib had a 72% progression-free survival (PFS) greater than 6 months and showed an overall response rate of 57%. Therefore, the detection of predictive biomarkers for the diagnosis and treatment of lung cancer is essential in the use of targeted molecular therapies that improve quality of life.
FISH using the Break apart probe is considered the main reference method for the diagnosis of ALK rearrangements, but results depend on operator-mediated interpretation, the number of nuclei analyzed per patient, preanalytical factors that may affect sample quality and, in some cases, loss of probe signals due to the location of target genes on the chromosome.18 Another routine method for diagnosis is the VENTANA ALK assay by IHC using the D5F3 antibody for the detection of aberrant receptor expression. To date, it is not clear which is the best method to determine ALK status in suspected lung cancer and cases have been reported with discordance between the findings detected by FISH and IHC,19 which may jeopardize the choice of the optimum treatment.
In our study, the rate of cases with ALK FISH-positive NSCLC was higher (50%) than the frequency reported in the literature (1–13%).20 Regarding ALK-positive FISH patterns, we had cases with a single 5′ end deletion pattern, a single signal separation rearrangement pattern (short or long) or a combination of both. Short separations are associated with inversions, for example, a paracentric inversion leading to ALK-EML4 fusion, and long separations have been related to translocations. Short split patterns are those in which there is a significant separation between the red and green signals of at least 2 diameters, while for long split patterns the distance is more than 2 diameters.21 The percentages of positivity found above the borderline point of ≥15% ranged from 20.8% to 56.4%. This indicates that our results are due to true positives, as opposed to false positives due to artefacts of the technique.
We found 2 cases located at the borderline (10–20%). Both cases were considered negative for FISH and were characterized by the presence of a pattern of positivity in which the 5′ end is deleted. This finding agrees with other studies, indicating that if the positive pattern does not correspond to a split signal, it is most likely not a true positive.20
On the contrary, ALK protein expression was visualized only in tumour cells with a cytoplasmic staining pattern, without equivocal results (Fig. 3) and the number of ALK IHC-positive cases (22.22%) was similar to previous studies.22 Usually, ALK-positive patients are young, in contrast to our findings23 where they were older, predominantly female and with only one cigarette-smoker. These demographic variables are not sufficient to identify patients with gene rearrangements; therefore, a diagnostic test to determine ALK status is indispensable, and it is possible that due to the small sample size of this study, such associations could not be corroborated.
We analyzed tumour stage with the percentage of positivity by FISH and demonstrated that statistically there is no significant relationship; however, the majority of patients with signal separation patterns were in advanced stages, similar to previous reports.24 Furthermore, we studied variables directly related to ALK gene expressions such as metastasis, EGFR gene mutation, stage, cellular pattern, and PDL1 expression and negative prognostic indicator variables such as pleural invasion, microvascular invasion, presence of necrosis, STAS, presence of lymphocytes and pleural involvement. However, as no significant differences were found, a larger study group is needed in order to corroborate these relationships.
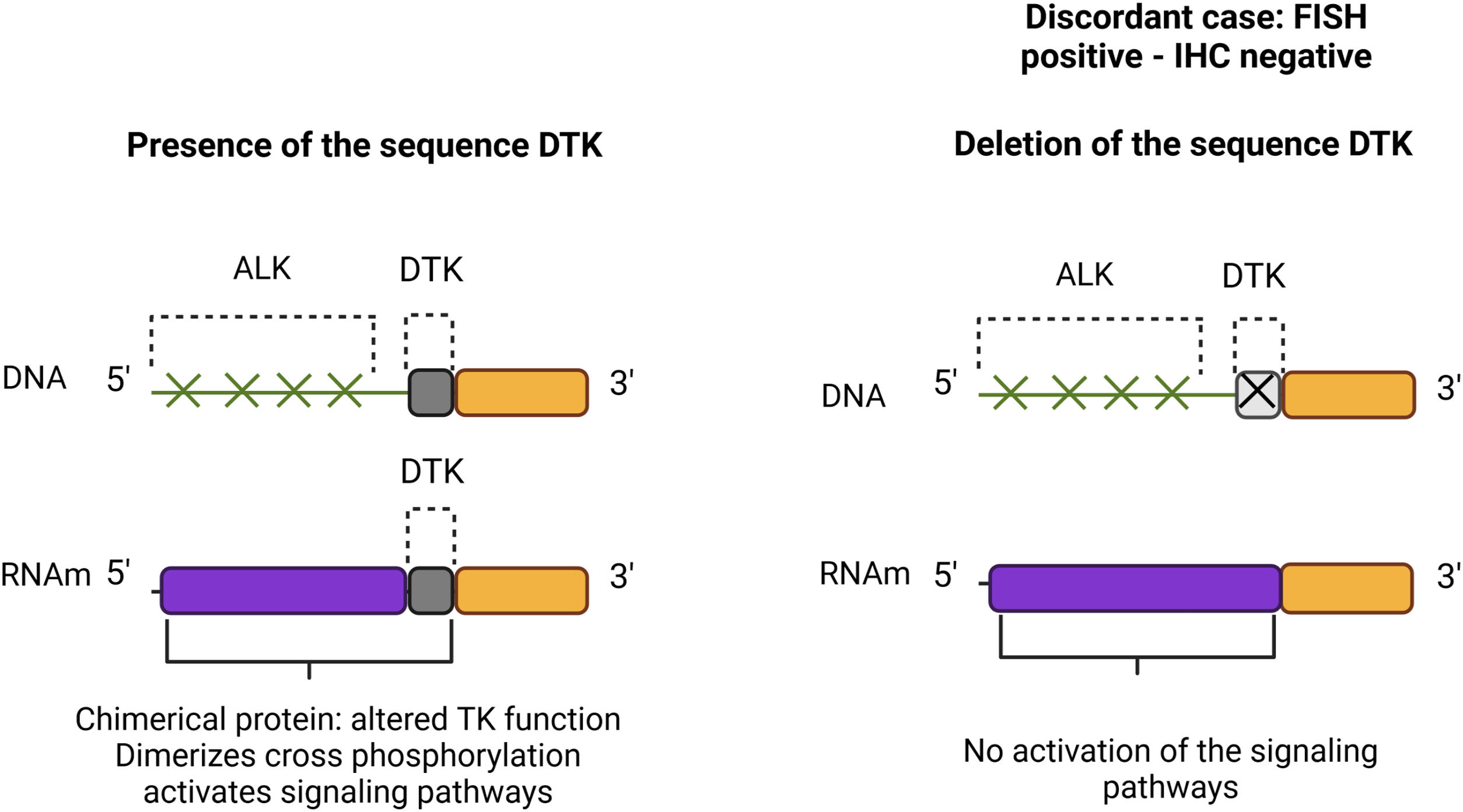
In discordant cases, a 5′ end deletion pattern has been detected by FISH analysis, which is considered positive because it is presumed that the sequence of the tyrosine kinase domain remains attached to the 3′ end, and the 5′ end is replaced by another gene, such as EML4, TFG, KIF5B, KLC1, among others; generating a fusion gene that causes activation of signalling pathways. However, in the discordant cases, it is presumed that green fluorochrome and the tyrosine kinase domain sequence are lost, therefore a signalling cascade is not activated (Supplementary Fig. 3).25
The discrepancies observed between IHC and FISH data could be caused by biological events such as the generation of microRNA, protein-likes, epigenetic alterations and variations in the tumour microenvironment, among others; with a potential impact on the therapeutic outcome.26 Variations in the tumour microenvironment can generate clonal diversity intratumorally, so it is important to evaluate the period before and after therapeutic intervention in future studies, to establish whether some patterns are related to resistance to treatment or increased clonality.
We had no cases with positive cells by signal separation below 15% in conjunction with a fusion. Future studies could identify whether there is a relationship between these cases and positive or negative IHC. If the IHC is negative, a follow-up should be performed to determine whether treatment is warranted prior to the increase in positivity, which would confer an advantage to FISH over IHC.
Although our number of cases is small, these results demonstrate the potential of an alternative diagnostic strategy to improve quality of life. To date, FISH provides pathologists with a better opportunity to identify patients eligible for treatment.
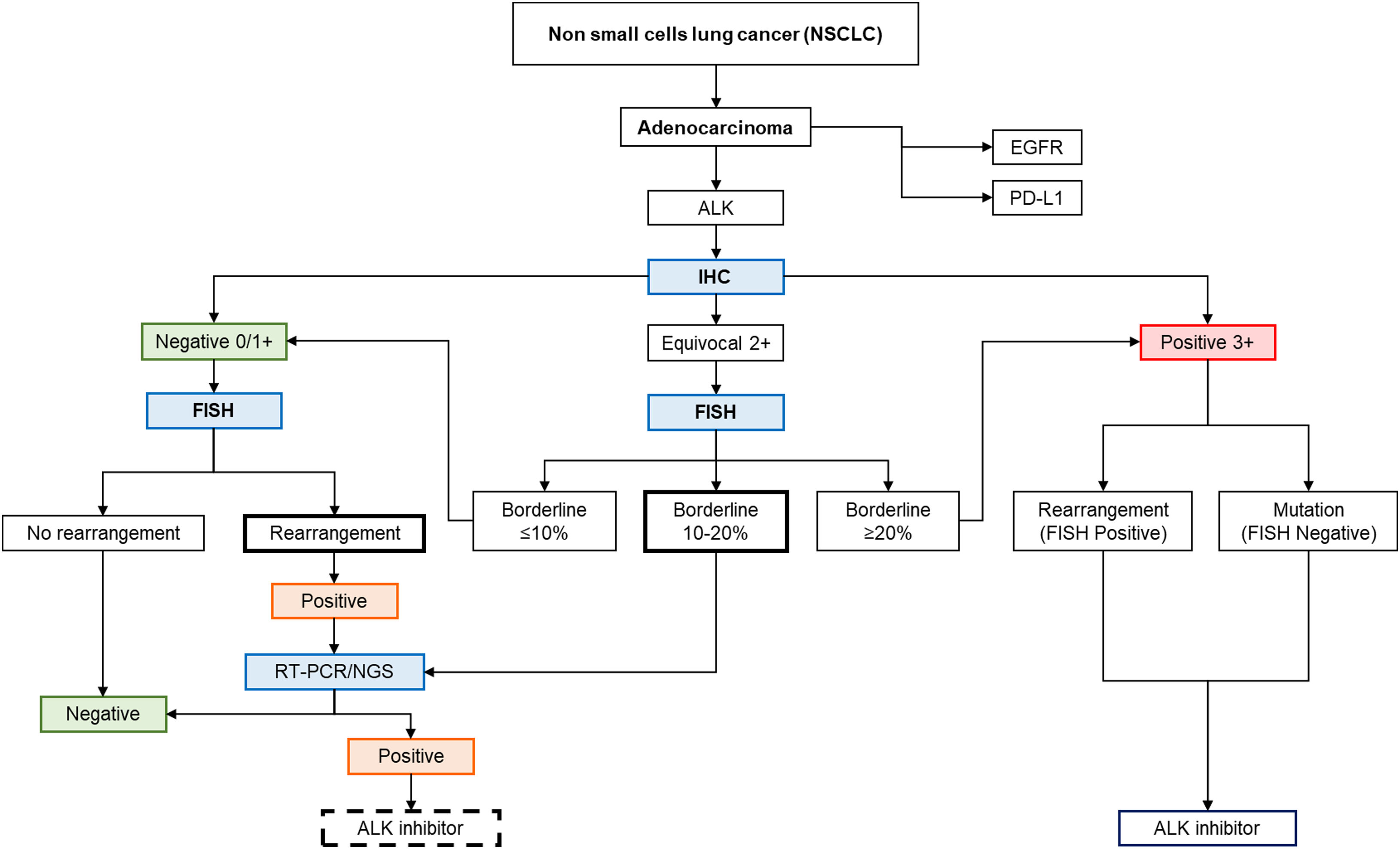
Previous reports of ALK IHC-negative and FISH-positive cases have reported poor response to treatment with ALK inhibitor crizotinib.27 Ilie et al.28 suggest that this finding could be related to poorly studied and complex biological mechanisms or artefacts (false positives). In these cases, it would be advisable to perform additional techniques such as reverse transcriptase polymerase chain reaction (RT-PCR) or next-generation sequencing (NGS),19 which would be beneficial as confirmatory tests before considering ALK-targeted therapy.29 With the established findings, a possible algorithm for patients with ALK FISH+ NSCLC with discrepant results (IHC−) is proposed, allowing for much more individualized diagnostic and therapeutic guidance (Fig. 5).
The diagnostic algorithm suggested ALK gene rearrangement in lung adenocarcinoma patients. Modified from Accurate and Economical Detection of ALK Positive Lung Adenocarcinoma with Semiquantitative Immunohistochemical Screening.14
The identification of different types of ALK gene rearrangements in the Colombian population is key for the implementation of targeted therapies with the inhibitor crizotinib offering an opportunity to improve patients’ quality of life. The overexpression of the tyrosine kinase domain was identified by means of the D5F3 clone, evidencing a high sensitivity and specificity.
Three guidelines were found from the results by FISH and IHC, where we obtained one discordant and two concordant patterns. The discordant cases with negative IHC, but positive FISH, may be caused by different biological events. However, a larger sample size is necessary, both to confirm this hypothesis and to evaluate the efficiency of the techniques and study the algorithm proposed for the management of patients with discordant results. The complementary techniques RT-PCR and NGS should be assessed based on the results of FISH and IHC in future clinical studies.
Although FISH is often considered the gold standard for the detection of ALK-positive patients, the use of IHC, as a complementary method, remains indispensable for the determination of treatment.
Authors’ contributionsConceptualization: AR, AV, OM, JR; Formal analysis: AR, AV, OM, LS, JR; Funding acquisition: AR, JR; Investigation: LS, AV, OM, AV, JR; Methodology: LS, AC, JN, AV, OM, AV, JR; Project administration: AR, JR; Supervision: AR, JR; Writing – review & editing: LS, AV, AR.
Availability of data and materialsAll relevant data can be acquired by contacting the correspondent author.
FundingThis work was supported by grant from Pontificia Universidad Javeriana (Grant No. 20451).
Conflict of interestThe authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the results reported in this manuscript.
Not applicable.