Coronavirus disease 2019 (COVID-19) has spread worldwide, resulting in significant morbidity and mortality. Histopathological findings are essential in understanding its pathogenesis and we present our findings from postmortem core needle biopsies in an attempt to share information that may shed some light on this severe pandemic.
Different organ samples from four patients with PCR-confirmed COVID-19 at the Infanta Sofía Hospital (Madrid) were studied during the months of April and May, 2020 by six pathologists using routine stains, histochemistry and immunohistochemistry. Results were compared with other reported cases.
All patients had a clinical diagnosis of pneumonia and biopsies revealed lung damage in the majority. Heart, liver, spleen and kidney were also studied and abnormalities were found in all cases and are extensively described.
The histopathology of organs affected by COVID-19 is vital to the understanding of this disease and its sequelae.
La enfermedad de coronavirus 2019 (COVID-19) ha afectado de forma mundial causando intensa morbimortalidad. Los hallazgos patológicos son claves para entender su patogénesis. A través de biopsias con aguja gruesa postmortem, intentamos responder a las incógnitas que giran en torno a la severidad de esta infección.
Se enviaron muestras de cuatro pacientes COVID-19 positivos al servicio de Anatomía Patológica del Hospital Infanta Sofía (Madrid) en los meses de Abril y Mayo 2020. Se estudiaron a través de distintas técnicas y los resultados se compararon con la literatura, buscando similitudes y peculiaridades.
Todos los pacientes tenían un diagnóstico de neumonía. Las biopsias mostraron daño pulmonar en la mayoría. El resto de los órganos estudiados fueron: corazón, hígado, bazo y riñón. Se encontraron características distintivas en muchos, las cuales fueron descritas exhaustivamente.
En conclusión, el análisis microscópico de los órganos afectados por COVID-19 es importante para comprender ésta enfermedad y sus posibles consecuencias.
Spain has become one of the epicenters of an unprecedented pandemic caused by Coronavirus disease 2019 (COVID-19) and is one of the countries with a higher coronavirus mortality rate. Many hypotheses have been proposed to explain the increase in fatalities but so far it remains largely an enigma. The underlying factors responsible for worldwide variations in mortality are poorly understood but may be related to genetic predisposition,1 access and quality of healthcare and prevalence of comorbidities.
COVID-19 is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).2 It was first detected in December, 2019 in Wuhan (China) and declared a pandemic on March, 2020.3
Only a few autopsy results have been reported to date. However, it is vital that we improve our understanding of this disease from every possible angle. Histopathological analysis of postmortem core needle biopsies (PMCNB) of target organs may shed light on the pathogenesis of the microorganism.
The lungs and the heart are amongst the organs most affected by COVID-19.3 In the lung, the typical pattern of injury described is diffuse alveolar damage (DAD),3 a nonspecific pattern of interstitial pneumonia that evolves through phases (exudative, proliferative and fibrotic). It is characterized by hyaline membranes in the early stages and fibrosis in later stages.4 Interstitial lymphocytic infiltrates with predominant CD4-positive T cells4–6 are commonly reported. Although some authors propose that, in severe infections, “acute fibrinous and organizing pneumonia (AFOP)” is the main pattern found, where intra-alveolar fibrin balls are seen without hyaline membranes,7 this diagnosis should be reserved for large samples so that the absence of DAD features can be confirmed.8 An important common finding is the presence of microvascular thrombi6 and associated hemorrhage. Other findings include reactive pneumocytes and multinucleated giant cells (MGC). To our knowledge, no viral inclusions in association with this infection have been reported.4,9 In the heart, myocyte hypertrophy is a common finding, probably related to underlying conditions.10 A spectrum of mild interstitial chronic inflammation within the myocardium without necrosis to lymphocytic myocarditis with myonecrosis is also described.3 Further reports describe individual cell necrosis without associated inflammation.5
Other organs known to be involved in this disease are the liver, spleen and kidney. In the liver, frequent sinusoidal dilatation with congestion, as well as steatosis, have been described, in addition to patchy hepatocytic necrosis and microvascular thrombi.9,11 In the spleen, lymphocytic depletion of the white pulp with a decrease or absence of lymphoid follicles is usually found.12 Acute tubular necrosis (ATN) is generally present in the kidney,13 with glomerular capillary thrombi and vacuolization and dilatation of tubules10 also being reported.
Materials and methodsOnce the initial overloading of the hospital system due to the pandemic had abated, PMCNBs were performed on four cases of PCR-positive for COVID-19, between April and May 2020, at the Infanta Sofia Hospital in Madrid, Spain. The procedure was carried out with the previous consent of the first-degree relative of the deceased.
Six pathologists studied the samples using H&E, histochemical (Alcian blue and Masson for lungs; PAS-diastase, Masson, Reticulin and Perls Prussian blue for liver; PAS, Silver, Masson for kidney) and immunohistochemical stains (CD20, CD3, CD4 and CD8 for lungs; CD20, CD3 and CD23 for spleen). Lung tissue was sampled in all patients, as well as other organs such as heart, liver, spleen and kidney. Findings were compared with previously published reports.
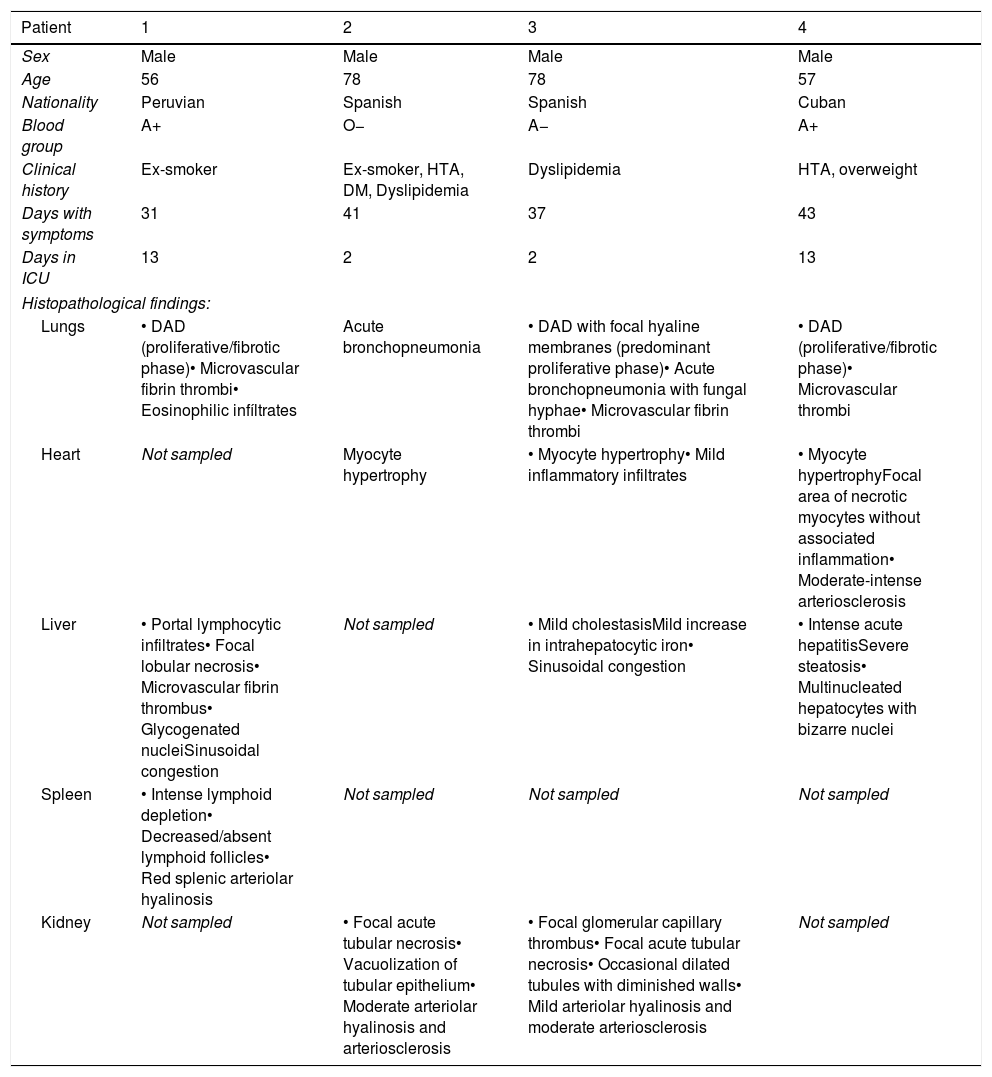
ResultsClinical data and pathologic findings of the patients are summarized in Table 1.
Clinical data and Pathological findings.
| Patient | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Sex | Male | Male | Male | Male |
| Age | 56 | 78 | 78 | 57 |
| Nationality | Peruvian | Spanish | Spanish | Cuban |
| Blood group | A+ | O− | A− | A+ |
| Clinical history | Ex-smoker | Ex-smoker, HTA, DM, Dyslipidemia | Dyslipidemia | HTA, overweight |
| Days with symptoms | 31 | 41 | 37 | 43 |
| Days in ICU | 13 | 2 | 2 | 13 |
| Histopathological findings: | ||||
| Lungs | • DAD (proliferative/fibrotic phase)• Microvascular fibrin thrombi• Eosinophilic infíltrates | Acute bronchopneumonia | • DAD with focal hyaline membranes (predominant proliferative phase)• Acute bronchopneumonia with fungal hyphae• Microvascular fibrin thrombi | • DAD (proliferative/fibrotic phase)• Microvascular thrombi |
| Heart | Not sampled | Myocyte hypertrophy | • Myocyte hypertrophy• Mild inflammatory infiltrates | • Myocyte hypertrophyFocal area of necrotic myocytes without associated inflammation• Moderate-intense arteriosclerosis |
| Liver | • Portal lymphocytic infiltrates• Focal lobular necrosis• Microvascular fibrin thrombus• Glycogenated nucleiSinusoidal congestion | Not sampled | • Mild cholestasisMild increase in intrahepatocytic iron• Sinusoidal congestion | • Intense acute hepatitisSevere steatosis• Multinucleated hepatocytes with bizarre nuclei |
| Spleen | • Intense lymphoid depletion• Decreased/absent lymphoid follicles• Red splenic arteriolar hyalinosis | Not sampled | Not sampled | Not sampled |
| Kidney | Not sampled | • Focal acute tubular necrosis• Vacuolization of tubular epithelium• Moderate arteriolar hyalinosis and arteriosclerosis | • Focal glomerular capillary thrombus• Focal acute tubular necrosis• Occasional dilated tubules with diminished walls• Mild arteriolar hyalinosis and moderate arteriosclerosis | Not sampled |
Patient 1(P1) was a 56-year-old male, native of Peru, ex-smoker with no concomitant diseases and with clinical diagnoses of COVID-19 pneumonia and respiratory failure. Lung, liver and spleen biopsies were sampled.
Lung parenchyma showed a DAD pattern in predominant proliferative/fibrotic phase, with many intra-alveolar fibrin balls. Moderate lymphocytic interstitial infiltrates with predominant CD8-positive T cells and frequent eosinophils were seen, both in the interstitial septa and inside the alveolar spaces. Numerous microvascular fibrin thrombi were noted, as well as reactive pneumocytes, MGC, intra-alveolar hemorrhage and edema.
Liver parenchyma showed moderate lymphocytic infiltrates in portal spaces and a microvascular fibrin thrombus. A focal area of lobular necrosis was also noted. Many hepatocytes had glycogenated nuclei and sinusoids were congested throughout.
Spleen parenchyma showed lymphocytic depletion of the white pulp with decreased/absent lymphoid follicles. Red splenic arteriolar hyalinosis was observed, with narrow lumens.
Patient 2(P2) was a 78-year-old male, hypertensive ex-smoker with type 2 diabetes mellitus, dyslipidemia, COPD and obstructive sleep apnea. His clinical diagnoses were COVID-19 pneumonia, respiratory infection by S. maltohphilia, bilateral pulmonary embolism, multi-organ failure and probable acute mesenteric ischemia (AMI). Lung, heart and kidney biopsies were sampled. Unfortunately, intestinal samples were not examined to rule out the clinical suspicion of AMI.
Lung parenchyma showed histologic signs of acute bronchopneumonia, with no features suggestive of DAD.
Heart parenchyma only showed myocyte hypertrophy.
Kidney parenchyma showed focal ATN and frequent vacuolization of the tubular epithelium. Vessels had moderate arteriolar hyalinosis and arteriosclerosis.
Patient 3(P3) was a 78-year-old man with a history of dyslipidemia. His clinical diagnoses were COVID-19 pneumonia, respiratory and renal failure and probable respiratory infection by A. fumigatus. Lung, heart, liver and kidney were sampled.
Lung parenchyma showed features of DAD predominantly in the proliferative phase, with focal hyaline membranes. Fibrin intra-alveolar balls and moderate interstitial lymphocytic infiltrates with a slight predominance of CD8-positive T cells were present. Microvascular fibrin thrombi, as well as intra-alveolar edema, hemorrhage, reactive pneumocytes and MGC were also found. Furthermore, acute bronchopneumonia with numerous intra-alveolar neutrophils and fungal hyphae positive by Grocott's silver stain, was diagnosed.
Heart parenchyma showed myocyte hypertrophy and mild patchy inflammatory mixed infiltrate, without associated necrosis.
Liver parenchyma showed hepatocytes with frequent glycogenated nuclei. Mild cholestasis, a slight increase in intrahepatocytic iron (detected using Perls Prussian blue) and widespread sinusoidal congestion were also found.
Kidney parenchyma showed a fibrin thrombus in a glomerular capillary. There were occasional dilated tubules with diminished wall thickness and focal ATN. Mild arteriolar hyalinosis and moderate arterial arteriosclerosis were also present.
Patient 4(P4) was a 57-year-old overweight, hypertensive male, native of Cuba, whose clinical diagnoses were COVID-19 pneumonia, respiratory and renal failure, catheter-related bacteriemia by S. epidermidis and candidemia by C. parapsilosis. Lung, heart and liver were sampled.
Lung parenchyma showed a DAD pattern predominantly in the proliferative/fibrotic phase, with frequent fibrin intra-alveolar balls. There were moderate foci of interstitial lymphocytic infiltrates with predominant CD8-positive T cells. Microvascular fibrin thrombi, common reactive pneumocytes, MGC and areas of alveolar hemorrhage were also present.
Heart parenchyma showed myocyte hypertrophy and focal areas of necrotic myocytes without associated inflammation. The sample included a medium-size artery with moderate to intense arteriosclerosis.
Liver parenchyma showed intense acute hepatitis, severe steatosis and widespread sinusoidal congestion. Some hepatocytes showed multinucleation and enlarged, bizarre nuclei.
DiscussionWe report the histopathological findings of PMCNBs of four cases of COVID-19. To date, available data are scarce, however, it is crucial that the reason for the elevated mortality associated with COVID-19 in Spain be investigated. Although we are aware that our study is limited by the small number of cases, we hope that it will contribute to a better understanding of the disease.
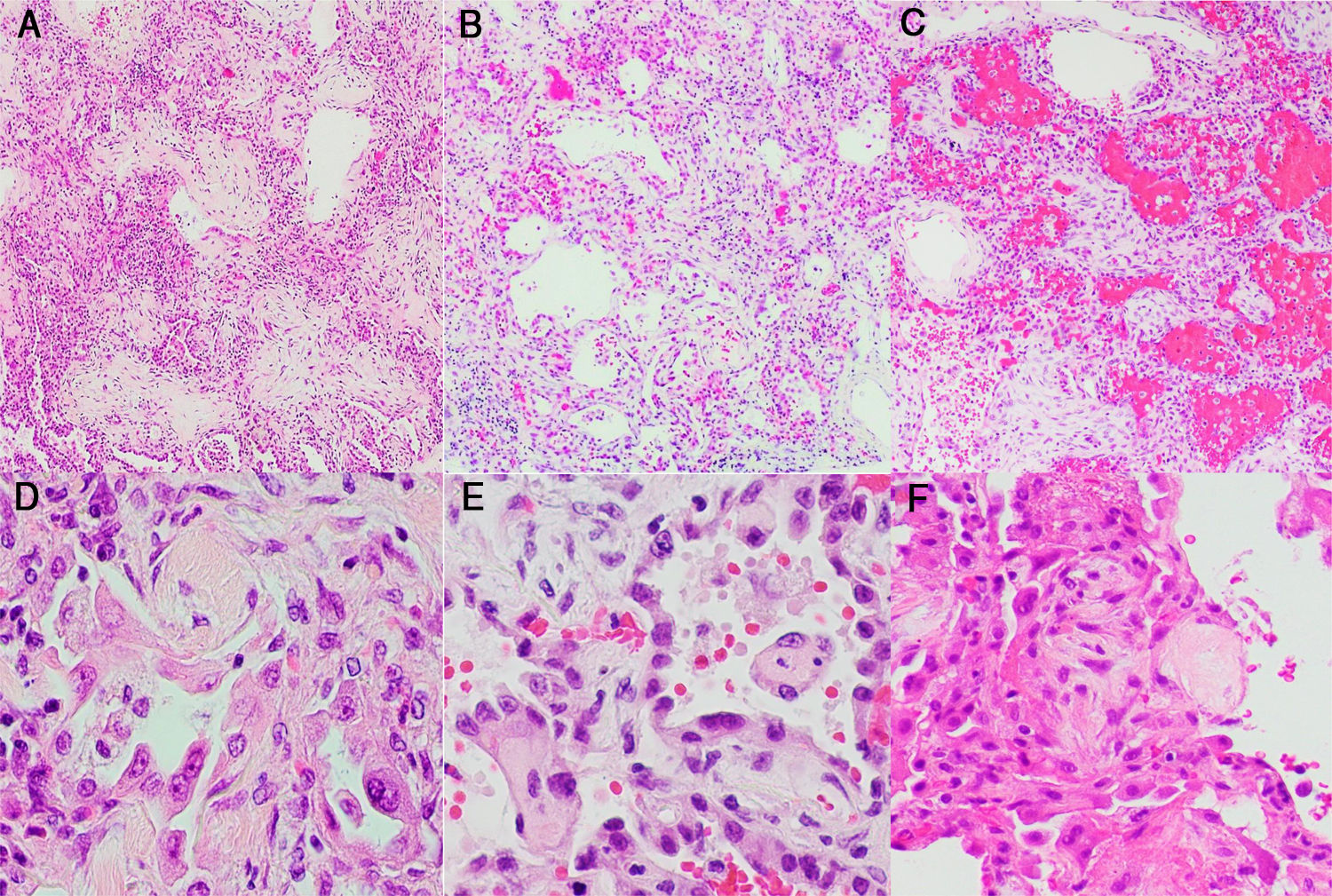
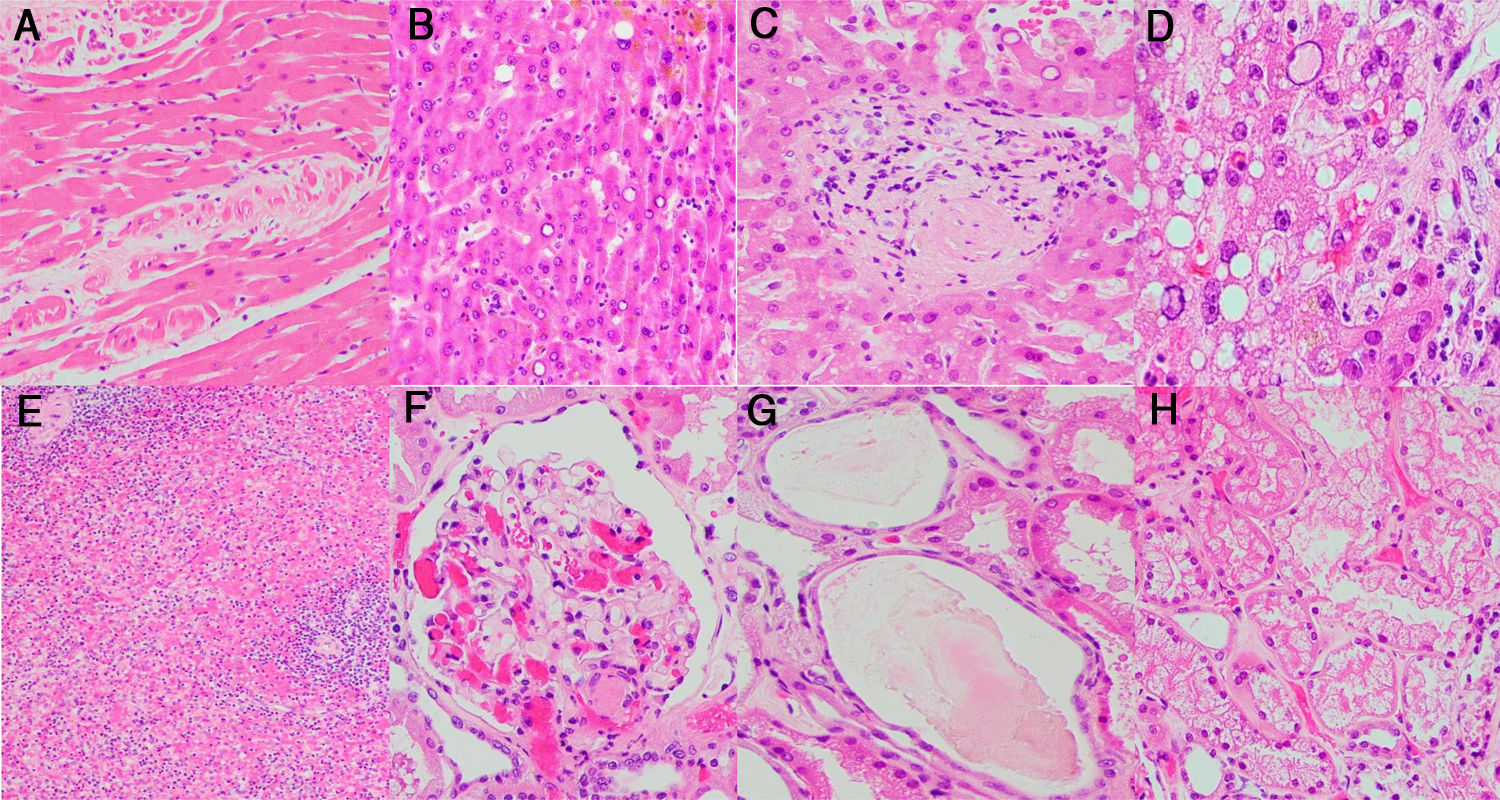
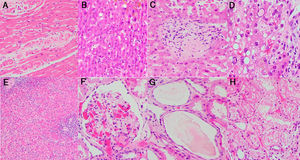
Morphological manifestations generally fitted the profile described in previous reports.13 Lung histology revealed a DAD pattern predominantly in a proliferative/fibrotic phase (Fig. 1a and b), with frequent intra-alveolar fibrin balls and focal hyaline membranes in only one case (P3). Reactive pneumocytes, MGC, interstitial lymphocytic infiltrates, alveolar hemorrhage and edema (Fig. 1c) were common, as well as microvascular fibrin thrombi (Fig. 1d–f). In all patients, cardiac histology showed some degree of myocyte hypertrophy, probably related to comorbidities, including hypertension. In one case (P3) there was mild interstitial chronic myocardial inflammation without associated necrosis, and in another case (P4) a focal area of acute myocardial infarct (Fig. 2a) without inflammatory infiltrates, as in prior reports.5 Liver histology showed diffuse sinusoidal congestion and frequent hepatocytes with glycogenated nuclei (Fig. 2b). One case (P1) had a small area of hepatocellular necrosis and a portal microvascular fibrin thrombus (Fig. 2c). Another case (P4) showed intense steatosis (Fig. 2d). In the only case in which spleen tissue had been biopsied (P1), histopathological findings similar to previous reports were seen, in the form of lymphocytic depletion of the white pulp and areas with diminished/absent lymphoid follicles12 (Fig. 2e). Kidney samples in one case (P3) showed vascular injury with a glomerular capillary thrombus (Fig. 2f), arteriolar hyalinosis and arteriosclerosis. Tubular injury was also frequently noted, with ATN in two cases (P2, P3) and widened tubular lumina with flattened epithelium in one case (P3) (Fig. 2g).
Histological study of the lung: (a and b) lung parenchyma with diffuse thickening of alveolar walls by inflammatory infiltrate and fibrosis (DAD pattern, proliferative/fibrotic phase) (H&E stain, 2×); (c) lung parenchyma with DAD pattern and frequent associated alveolar hemorrhage (H&E stain, 2×); (d–f) Alveolar walls coated by reactive pneumocytes and thickened by increased cellularity and fibrosis. Note the numerous vascular microthrombi (head of arrow) (H&E stain, 20×).
Histological study of other organs: (a) heart myocardium with necrotic myocytes (H&E stain, 20×); (b) hepatocytes with frequent glycogenated nuclei (H&E stain, 20×); (c) portal vascular microthrombus (H&E stain, 20×); (d) Hepatocytes with intense steatosis and enlarged nuclei (H&E stain, 40×); (e) Spleen with lymphocytic depletion of the white pulp and diminished lymphoid follicles (H&E stain, 4×); (f) Glomerular capillary microthrombus (H&E stain, 20×); (g) Tubular dilatation with flattened epithelium (H&E stain, 20×); (h) Vacuolization of tubular epithelium (H&E stain, 20×).
Abnormalities were found in most of the organs sampled, mainly in the lungs. In contrast to previous reports, interstitial lymphocytic infiltrates were predominantly CD8-positive.4–6 One case (P1) showed frequent eosinophilic infiltrates. Although some authors report the presence of a few eosinophils,4 to the best of our knowledge, these cells are not a frequent finding in COVID-19 lungs. One case (P2) did not have a DAD pattern, but acute bronchopneumonia, probably related to S. maltohphilia. Another case (P3) had, as well as DAD, concomitant acute bronchopneumonia, probably related to A.fumigatus. In the liver, one case (P4) showed acute hepatitis, not typically associated with this disease and probably secondary to drug cytotoxicity. The same patient showed hepatocytes with multinucleation and enlarged bizarre nuclei (Fig. 2d); to our knowledge, an unreported feature. Kidney histology showed vacuolization of tubular epithelium in one case (P2) (Fig. 2h), also probably in relation to drug therapy.
The present study also revealed two interesting clinical aspects. Both of the younger patients (P1, 56 and P4, 57 years-old) were Latin Americans (Peruvian and Cuban, respectively). Two previous studies have linked blood group A with a higher susceptibility to COVID-19 in comparison to blood group O, and suggested that this former blood group may be associated with coagulopathies related to this disease.14 Three of our four patients had blood group A (P1: A+, P3: A− and P4: A+). Only one patient had blood group O- (P2)15,16 and, interestingly, he had no features of DAD.
ConclusionAlthough the study of PMCNB of patients dying of COVID-19 is not as complete as a full autopsy, it will further the understanding of the histopathology related to this highly lethal virus. Our study, albeit a small one with only four cases, correlates well with previously published reports, and highlights some interesting pathological findings. Therefore, we hope it will contribute in shedding some light on the nature of this microorganism, although further and larger histopathological series are urgently required.
Conflict of interestThe authors have no conflict of interest to declare regarding this study.
The authors would like to thank the head of the ICU service of Hospital Infanta Sofía, Miguel Angel González, for performing the PMCNBs and thus making this study possible, as well as Dr Alejandro Ayala from the University of Miami (USA) for his significant contribution.