The incidence of systemic infections by Saccharomyces cerevisiae has increased in recent years, especially among immunocompromised patients. Amphotericin B, voriconazole or echinocandins have been used with favorable outcome against systemic infections by this fungus. However, clinical experience is limited and no in vivo studies have been conducted.
AimsWe evaluated the in vitro activity of nine antifungal compounds against S.cerevisiae and the in vivo efficacy of those three antifungals showing the highest in vitro activity by using a murine model of systemic infection.
MethodsMinimal inhibitory concentrations (MICs) were determined by the microdilution method against three strains of S. cerevisiae. After intravenous infection with 5×107 CFUs, animals received liposomal amphotericin B (5mg/kg), voriconazole (25mg/kg) or anidulafungin (5mg/kg). Treatment efficacy was assessed by determining of CFUs/g in liver, kidney, brain, lung and spleen.
Results5-Fluorocytosine was the most in vitro active compound followed by amphotericin B, voriconazole and anidulafungin. The in vivo study showed that liposomal amphotericin B was the most effective drug driving highest fungal clearance.
ConclusionsAll treatments reduced the fungal load in comparison to the control group, being liposomal amphotericin B the most effective drug followed by anidulafungin and finally voriconazole.
La incidencia de infecciones sistémicas causadas por Saccharomyces cerevisiae ha aumentado en los últimos años, especialmente entre pacientes inmunodeprimidos. A pesar de que la anfotericina B, el voriconazol o las equinocandinas han dado buen resultado en infecciones sistémicas por este hongo, no se han establecido recomendaciones terapéuticas sólidas.
ObjetivosSe evaluó la actividad in vitro de nueve antifúngicos frente a S. cerevisiae y la eficacia in vivo de los tres fármacos con mayor actividad in vitro mediante un modelo murino de infección sistémica.
MétodosSe determinaron las concentraciones mínimas inhibitorias (CMIs) frente a tres cepas de S. cerevisiae por el método de microdilución. Después de la inoculación intravenosa con 5×107UFC, los ratones fueron tratados con anfotericina B liposomal (5mg/kg), voriconazol (25mg/kg) o anidulafungina (5mg/kg). La eficacia de los tratamientos se estableció basándose en la determinación de UFC/g en hígado, riñón, cerebro, pulmón y bazo.
ResultadosLa 5-fluorocitosina fue el compuesto más activo in vitro, seguido por la anfotericina B liposomal, el voriconazol y la anidulafungina. En el estudio in vivo, la anfotericina B liposomal fue el fármaco más eficaz en términos de reducción de la carga fúngica y esterilización de los órganos estudiados.
ConclusionesTodos los tratamientos redujeron la carga fúngica en comparación con el grupo control, y la anfotericina B liposomal fue el antifúngico más efectivo, seguido de la anidulafungina y el voriconazol.
Saccharomyces cerevisiae is a widely distributed yeast, commonly used in the production of food, alcoholic beverages and different biotechnological processes.1,3 Despite its beneficial applications, S. cerevisiae can also act as a human opportunistic pathogen causing a variety of infections in immunocompromised individuals ranging from localized, genitourinary infections, esophagitis, pneumonia, liver abscess or peritonitis, to systemic infections.12,18,21,23 The acquisition of infections by S. cerevisiae has been recently linked to the use of probiotics or dietary supplements, both of which could represent a source for the disease.11,16
According to the current guidelines, the recommended treatment for these infections consists of amphotericin B (AMB) or AMB plus 5-fluorocytosine (5FC) in the most severe cases,2 but few studies have evaluated the efficacy of other antifungal agents. Due to the good in vitro activity shown by posaconazole (PSC), voriconazole (VRC) and AMB against S. cerevisiae,7,14,20,26 these drugs could represent a therapeutic option, although few clinical experience exists. Similarly, echinocandins and fluconazole (FLC) in combination with AMB have shown favorable outcomes against S. cerevisiae infections.9,13,17,21,24
The aim of the present study was to evaluate the in vitro activity of AMB, FLC, PSC, VRC, anidulafungin (AFG), 5FC, itraconazole (ITC), caspofungin (CFG) and micafungin (MFG) against S. cerevisiae and to determine the time-kill kinetics as well as the in vivo efficacy of the most active compounds.
Three clinical strains of S. cerevisiae (FMR 13211, FMR 13212 and FMR 13213) isolated from patients with acute vulvovaginitis were included in the study. Species identification was confirmed by comparing the sequences of large-subunit ribosomal RNA gene of the used strains with those from the type species.
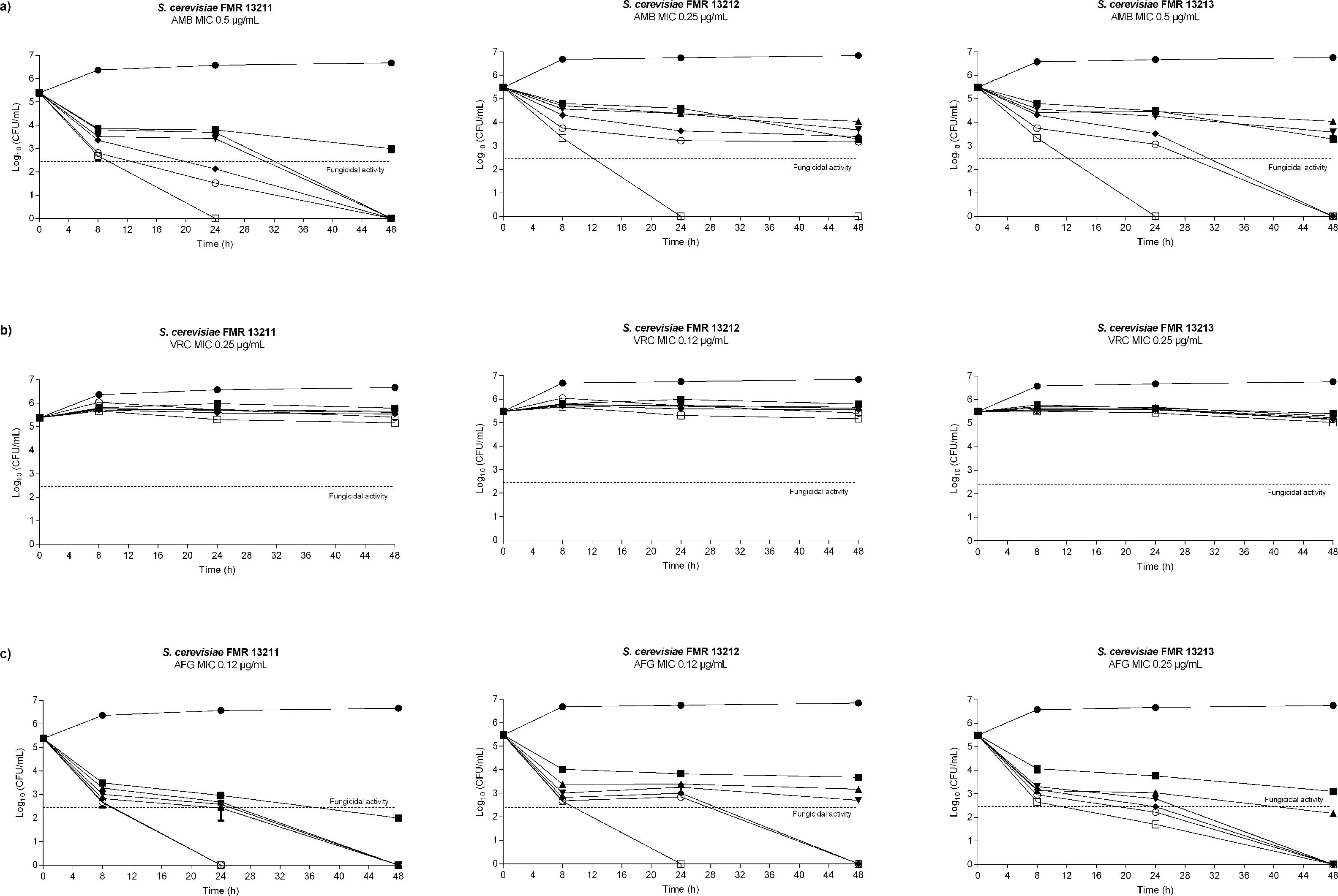
Antifungal susceptibility was assayed according to Clinical and Laboratory Standards Institute (CLSI) document M27-A3,10 and time-kill studies were performed as previously described6 by using four-fold serial dilutions in standard RPMI 1640 of AMB, VRC and AFG ranging from 0.06 to 32μg/ml. At predetermined time points (0, 8, 24 and 48h) aliquots of 100μl diluted in sterile water were placed onto PDA plates and incubated at 35°C for 48h in order to determine colony forming units (CFU)/ml. Strain ATCC 22019 of Candida parapsilosis was used as a quality control and all assays were carried out in duplicate. A fungicidal effect was defined as a reduction of ≥3log10 in viable colony counts in comparison with the starting inocula, whereas a reduction of <3log10 in colony counts was considered a fungistatic effect.15
For in vivo studies, male OF-1 mice weighing 30g (Charles River; Criffa S.A., Barcelona, Spain) were immunosuppressed with cyclophosphamide given intraperitoneally.8 Animals were housed under standard conditions and care procedures were supervised and approved by the Universitat Rovira i Virgili Animal Welfare Committee.
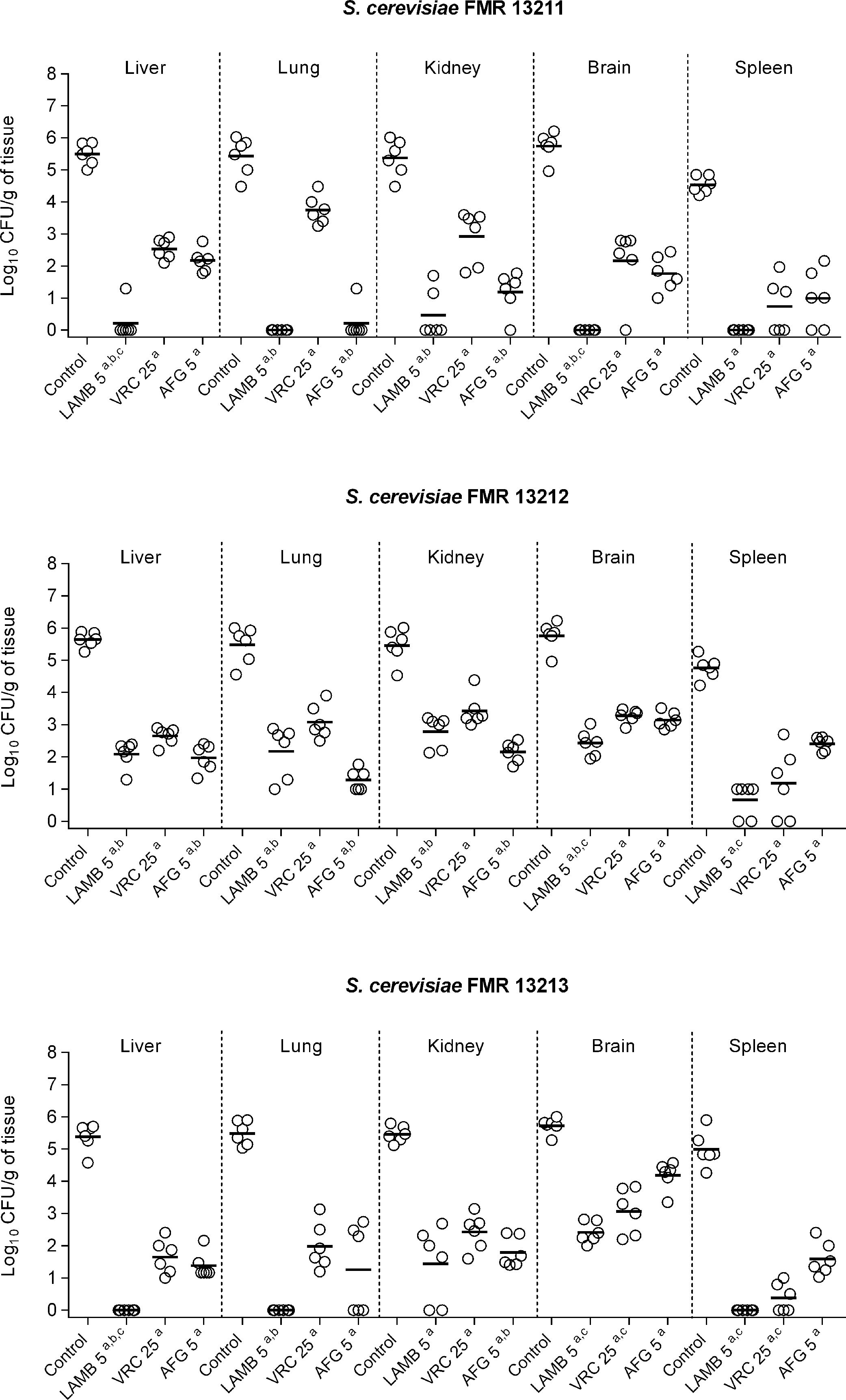
For each strain and drug assayed, 8 mice were included. Mice were infected intravenously (i.v.) via the lateral tail vein with 5×107 CFU in 0.2ml of 0.9% saline. Therapies consisted of liposomal AMB (LAMB) (AmBisome; Gilead Sciences S.A., Madrid, Spain) administered i.v. at 5mg/kg once daily (QD), VRC (Vfend, Pfizer S. A., Madrid, Spain) at 25mg/kg given orally by gavage (p.o.) QD or AFG (Ecalta, Pfizer S.A.) at 5mg/kg intraperitoneally (i.p.) QD. In vivo assayed drugs were chosen according to the in vitro results obtained, and doses were selected based on previous studies.5,27 From 3 days before infection, mice treated with VRC received grapefruit juice instead of water, as grapefruit juice is an inhibitor of cytochrome P450 enzymes, which display an extensive metabolism in mice resulting in elevated drug clearance.25 Control groups received no treatment. Drug efficacy was evaluated according to the fungal burden reduction in brain, liver, spleen, lungs and kidneys. Despite the high in vitro activity of 5FC, this drug was not included into the in vivo study due to its known toxicity.19 At day 8 post-infection animals were euthanized by CO2 inhalation and organs were aseptically removed, homogenized in 1ml of sterile saline, ten-fold diluted and placed onto PDA for CFU/g determination. Results from the tissue burden studies were analyzed using the Mann–Whitney U-test by Graph-Pad Prism 6.0 for Windows (GraphPad Software, San Diego California USA). A p value of ≤0.05 was considered statistically significant.
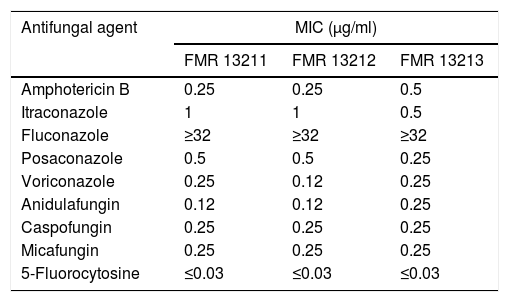
Despite FLC has been used in combination with AMB to treat systemic infections caused by Saccharomyces, this drug showed no in vitro activity (MIC≥32μg/ml) against the strains we tested. This has also been reported by others.7,20 The rest of the assayed antifungals showed greater activity (MIC≤1μg/ml), being 5FC the most active compound (MIC≤0.03μg/ml) (Table 1). In the time-killing assay, VRC displayed fungistatic activity while AMB and AFG showed fungicidal effect in a concentration dependent manner starting at a drug concentration of 0.06μg/ml at 8h in both cases (Fig. 1).
Results of antifungal susceptibility testing.
| Antifungal agent | MIC (μg/ml) | ||
|---|---|---|---|
| FMR 13211 | FMR 13212 | FMR 13213 | |
| Amphotericin B | 0.25 | 0.25 | 0.5 |
| Itraconazole | 1 | 1 | 0.5 |
| Fluconazole | ≥32 | ≥32 | ≥32 |
| Posaconazole | 0.5 | 0.5 | 0.25 |
| Voriconazole | 0.25 | 0.12 | 0.25 |
| Anidulafungin | 0.12 | 0.12 | 0.25 |
| Caspofungin | 0.25 | 0.25 | 0.25 |
| Micafungin | 0.25 | 0.25 | 0.25 |
| 5-Fluorocytosine | ≤0.03 | ≤0.03 | ≤0.03 |
MIC: minimal inhibitory concentration.
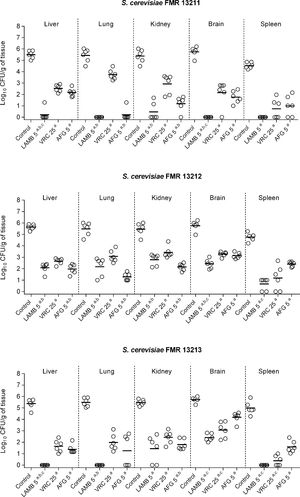
In the in vivo study, control animals showed high fungal load in all studied organs (ranging from 105 to 106CFU/g tissue) with the exception of the spleen, in which fungal load was slightly lower (104–105CFU/g) (Fig. 2). All assayed treatments were significantly effective in burden reduction from all organs in comparison with the control group regardless of the infecting strain (p≤0.0043). AMB, which is the recommended drug against Saccharomyces infections, showed fungicidal activity correlating with our in vivo results, as it displayed the highest efficacy against all strains with great clearance effect in its liposomal formulation, LAMB (Fig. 2). LAMB was especially effective against strains FMR 13211 and FMR 13213, for which fungicidal effect was observed at MIC concentration, resulting in undetectable CFUs from lung, kidney and spleen and in significant CFU reduction from liver and brain in comparison to VRC or AFG treatments (p=0.0022). The obtained results with LAMB sustain clinical reports that, although scarce, have shown efficacy of AMB-based therapies.13 In our case, AFG was the second most effective therapy corroborating previous observations of echinocandins efficacy against Saccharomyces in the clinical settings.9,17,21 Both drugs, LAMB and AFG exhibited similar efficacy against all assayed strains independently of the MIC and the kill-curves obtained. Finally, as previously reported,4,22 VRC displayed good activity although it showed lower efficacy than LAMB or AFG treatments.
Fungal load of immunosuppressed mice infected with S. cerevisiae FMR 13211, FMR 13212 and FMR 13213. LAMB 5, liposomal amphotericin B at 5mg/kg i.v. QD; VRC 25, voriconazole at 25mg/kg p.o. QD; AFG 5, anidulafungin at 5mg/kg i.p. QD. Horizontal lines indicate median values. ap≤0.05 versus control; bp≤0.05 versus VRC 25; cp≤0.05 versus AFG 5.
Guidelines for infections by S. cerevisiae recommend the use of LAMB and echinocandins, as well as the discontinuation of S. cerevisiae as probiotic, especially in vulnerable populations.2 Although a few strains have been tested, our study contributes with new evidence to prove LAMB treatment effectiveness against experimental invasive infection by S. cerevisiae, as well as the potential use of AFG (and in lesser extent, VRC) as alternative treatments for disseminated infections caused by this fungus.