Xeric forests dominated by two tree species, Scutia buxifolia (Rhamnaceae) and Celtis tala (Ulmacea), are temperate, semi-deciduous wooded communities that represent the most abundant woodlands on the eastern plains of Buenos Aires Province, Argentina. The district of Magdalena has one of the most well-preserved native-forest areas, with an environmental heterogeneity that gives rise to the wide variability in the vegetation present.
AimsThe aim of this study was to analyze the species composition, diversity, seasonal variations, and substrate specificity of anamorphic fungi (Ascomycota) on the green leaves and in the leaf litter of native forests dominated by Scutia buxifolia and Celtis tala from Magdalena, Buenos Aires, Argentina.
MethodsIn order to obtain the mycobiota of decomposition, seasonal samples of green leaves and leaf litter from both types of trees were collected over a two-year period. In the laboratory, the leaves were placed in a moist chamber and incubated at room temperature.
ResultsA total of 100 species of anamorphic Ascomycota were identified in both forests. No significant variations were observed in the richness, diversity, or evenness of the fungal communities of the green leaves and leaf litter of both forests between seasons.
ConclusionsThe species that characterized the fungal communities in the leaves of each of the trees were found to be different. The type of substrate had a stronger influence in determining the composition of the fungal community in both types of forests.
Los bosques xerófilos dominados por las especies arbóreas Scutia buxifolia (Rhamnaceae) y Celtis tala (Ulmaceae) conforman comunidades boscosas semicaducas y templadas que constituyen los bosques nativos más abundantes de la provincia de Buenos Aires, Argentina. En el distrito de Magdalena constituyen una de las comunidades naturales de este tipo mejor conservada, con una heterogeneidad ambiental que da lugar a la gran variabilidad en la composición de la vegetación presente.
ObjetivosEl objetivo del presente trabajo fue analizar las distribución de especies, la diversidad, las variaciones estacionales y la especificidad por el sustrato de hongos anamórficos (Ascomycota) presentes en las hojas verdes y en la hojarasca de los bosques nativos xerófilos dominados por las especies arbóreas Scutia buxifolia y Celtis tala en el partido de Magdalena, provincia de Buenos Aires, Argentina.
MétodosPara obtener la micobiota descomponedora se tomaron muestras estacionales de hojas verdes y hojarasca de los 2 tipos de árboles durante un período de 2 años. En el laboratorio, las hojas se colocaron en cámara húmeda y se incubaron a temperatura ambiente.
ResultadosSe identificaron 100 especies de anamorfos de la división Ascomycota en las hojas de ambos tipos de árboles. No se observaron diferencias en cuanto a la diversidad, equidad y riqueza de especies entre ambos tipos de hojas.
ConclusionesLas especies que componen la comunidad fúngica de las hojas de Scutia buxifolia y Celtis tala es diferente. El tipo de sustrato que representan las hojas de cada especie arbórea condiciona la composición de la comunidad de anamorfos de Ascomycota presentes en ambos tipos de hoja.
In a forest, dead leaves constitute the main source of nutrients for the fauna and microorganisms with about 80% being degraded by microorganisms, mainly fungi.31 As the dead leaves are decomposed, the associated fungal communities undergo sequential alterations in their composition and substrate-degradation potential as a result of changes in the availability of nutrients, moisture, pH, and oxygen tension.
The litter and humus layers that overlie the mineral horizons of soils harbor various types of fungal communities. The litter is a transitional phase between the living plant community and the soil; thus, the mycotic composition of litter is closely linked to that of the plant community and contains many plant-inhabiting species as well as a distinctive assemblage of fungal taxa that are largely restricted to decaying plant material per se.14
The species composition and density of propagules are clearly affected by the sequence of changes occurring through the fragmentation, decomposition, and humidification of the plant detritus. Stable organic compounds such as waxes, lignins, and phenols—that may persist for hundreds of years—become incorporated in the mineral soil horizons and are, in turn, colonized by characteristic groups of fungal species.5
The phyllosphere fungi, that is the first group to colonize, have the advantage of gaining access to readily available organic compounds in freshly fallen leaves, before subsequent colonizing fungi alter the litter fall.33 A sterilization of the leaves and consequent exclusion of the phyllosphere fungi was found to reduce the decomposition rate of the leaves42; this change might thereafter alter the substrate use by succeeding fungal decomposers,32 thus suggesting the potential relevance of prior colonization by phyllosphere fungi to the initial and subsequent stages of decomposition and fungal succession of a decaying leaf fall.
Many studies have been published on the ecology of fungi in forest litter and the identification of specific fungal communities.36 Other investigations have focused on a possible nutritional specialization among saprotrophs.15,20,23,25–28,30,34,35,40,41 Several mycological studies have assessed the soil fungi6,10,11,13 and the mycorrhizae17–19 present in the native forests of Scutia buxifolia Reissek (coronillo) and Celtis tala Gillies ex Planch. (tala) in Magdalena (Buenos Aires province, Argentina). However, there are no studies of fungal communities present on green leaves and litter in both types of forest.
The aim of this investigation was to describe the composition, diversity, seasonal variations, and substrate specificity of the anamorphic saprotrophic fungi (Ascomycota) on the green leaves and in the leaf litter of native forests dominated by S. buxifolia and C. tala from Magdalena, Buenos Aires, Argentina (Biosphere Reserve, MAB-UNESCO).
Materials and methodsStudy areaThe study area (35°11′ S, 57°17′ W) is located in the Magdalena district, 20km southeast of Magdalena town in the province of Buenos Aires, Argentina. Xeric forests dominated by two tree species, S. buxifolia (Rhamnaceae) and C. tala (Ulmaceae), are temperate, semi-deciduous wooded communities that represent the most abundant woodlands on the eastern plains of Buenos Aires province, ranging from the banks of the Paraná River in the northwest to the suburbs of Mar Chiquita town in the southeast. The district of Magdalena has one of the most well-preserved native-forest areas, with an environmental heterogeneity that gives rise to the wide variability in the vegetation present. Wooded ranges run in parallel to La Plata River, where the forest grows on highly calcareous substrates derived from the sea's incursions and withdrawals during the Quaternary Period.37
The climate of the region includes an annual mean temperature of 15.9°C and annual mean precipitation of 885mm, with peaks of rainfall during autumn and spring. The characteristics of each tree species provide contrasting features in each forest: C. tala is a deciduous species, whose senescent leaves begin to drop at the end of the summer, reaching the peak of litter fall in the autumn. These forests contain an abundant development of herbaceous vegetation. In contrast, S. buxifolia is an evergreen, with periods of leaf fall through replacement in both the autumn and spring. Thus, during these seasons, with their greater contribution of leaf litter, the forest has an appearance different from that of the C. tala forest, featuring constant shade and sparse herbaceous vegetation.4 Considered together, the leaves of these two species of trees contribute more than 90% of the total amount of leaf fall.
SamplingTwo areas were studied: one of pure S. buxifolia forest and the other of pure C. tala forest. A pure forest was defined as one consisting of 90% of the dominant tree species.3 Samples were collected seasonally during a two-year period: in autumn (May 2004, 2005), in winter (August 2004, 2005), in spring (October 2004, 2005), and in summer (February 2005, 2006).
In order to obtain the mycobiota of decomposition, 150 leaves were collected directly from the plant and another 150 leaves from the litter layer of each forest during each season. In the laboratory, the leaves were placed in a moist chamber (10 leaves in a 90-mm Petri dish) and incubated at room temperature.12 The presence of each taxon on each leaf was recorded by means of the direct observation of both leaf surfaces. The taxonomic determination and preservation of the fungal species recorded were performed by isolation in plates with agar-containing media (malt-extract, potato-dextrose, corn-meal, or S. buxifolia and C. tala leaf litter extract agar).
Determination of substrate specificityIn order to evaluate whether the characteristics of the substrate consisting in the leaves of a given species of tree per se (here, the leaf litter from S. buxifolia or C. tala) was responsible for the species composition of the fungal communities rather than an effect at the forest level of the dominant species of tree present, 150 dead leaves of S. buxifolia in a pure C. tala forest and 150 dead leaves of C. tala in a pure S. buxifolia forest were collected seasonally. In the laboratory, the leaves were placed in the moist chambers described above and incubated at room temperature. Weekly observations were made and the taxa present on each leaf recorded.
Data analysisA leaf was considered as a natural sampling unit. After direct observation of both leaf surfaces, the taxa present on each leaf were recorded and their relative frequency determined as follows: (number of leaves where one species was recorded/total number of leaves analyzed)×100. These data were used to calculate the Shannon diversity index (H′), species richness (S), and species evenness (E).6,29 The differences in the values of these indices between seasons were evaluated through the analysis of variance (ANOVA). A principal-components analysis (PCA)9 was used to evaluate differences in composition between the fungal communities on the green leaves and on the leaf litter for the two forests. In order to determine whether there were differences between the fungal community developing on the C. tala leaves collected in the S. buxifolia forest and the one developing on the S. buxifolia leaves collected in the C. tala forest and also between the fungal communities found in the homogeneous leaf litter of both forests (i.e., the S. buxifolia leaf litter in the S. buxifolia forest and the C. tala leaf litter in the C. tala forest), we applied a PCA using the data of species frequencies from the different sampling occasions.
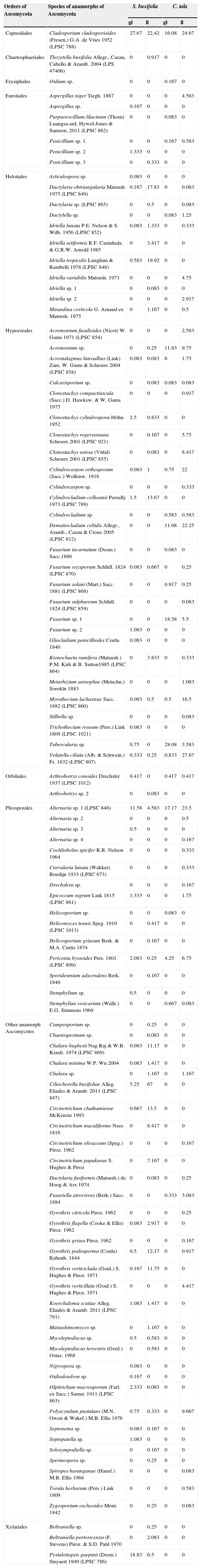
ResultsA total of 100 species of anamorphic Ascomycota were identified by traditional taxonomy on the green leaves and in the leaf litter from the S. buxifolia and C. tala forests studied (Table 1), with 25 of these species being recorded in the leaf litter from both forests.
Frequency of fungal species isolated from green leaves (gl) and leaf litter (ll) from the forest of S. buxifolia and C. tala during the 2004–2005 period. Species are ordered alphabetically within each order of Ascomycota.
| Orders of Ascomycota | Species of anamorphs of Ascomycota | S. buxifolia | C. tala | ||
|---|---|---|---|---|---|
| gl | ll | gl | ll | ||
| Capnodiales | Cladosporium cladosporioides (Fresen.) G.A. de Vries 1952 (LPSC 788) | 27.67 | 22.42 | 16.08 | 24.67 |
| Chaetosphaeriales | Thozetella buxifolia Allegr., Cazau, Cabello & Aramb. 2004 (LPS 47406) | 0 | 0.917 | 0 | 0 |
| Erysiphales | Oidium sp. | 0 | 0 | 0.167 | 0 |
| Eurotiales | Aspergillus niger Tiegh. 1867 | 0 | 0 | 0 | 4.583 |
| Aspergillus sp. | 0.167 | 0 | 0 | 0 | |
| Purpureocillium lilacinum (Thom) Luangsa-ard, Hywel-Jones & Samson, 2011 (LPSC 862) | 0 | 0 | 0.083 | 0 | |
| Penicillium sp. 1 | 0 | 0 | 0.167 | 0.583 | |
| Penicillium sp. 2 | 1.333 | 0 | 0 | 0 | |
| Penicillium sp. 3 | 0 | 0.333 | 0 | 0 | |
| Helotiales | Articulospora sp. | 0.083 | 0 | 0 | 0 |
| Dactylaria obtriangularia Matsush. 1975 (LPSC 849) | 0.167 | 17.83 | 0 | 0.083 | |
| Dactylaria sp. (LPSC 865) | 0 | 0.5 | 0 | 0.083 | |
| Dactylella sp. | 0 | 0 | 0.083 | 1.25 | |
| Idriella lunata P.E. Nelson & S. Wilh. 1956 (LPSC 852) | 0.083 | 1.333 | 0 | 0.333 | |
| Idriella setiformis R.F. Castañeda & G.R.W. Arnold 1985 | 0 | 3.417 | 0 | 0 | |
| Idriella tropicalis Lunghini & Rambelli 1978 (LPSC 848) | 0.583 | 19.92 | 0 | 0 | |
| Idriella variabilis Matsush. 1971 | 0 | 0 | 0 | 4.75 | |
| Idriella sp. 1 | 0 | 0.083 | 0 | 0 | |
| Idriella sp. 2 | 0 | 0 | 0 | 2.917 | |
| Mirandina corticola G. Arnaud ex Matsush. 1975 | 0 | 1.167 | 0 | 0.5 | |
| Hypocreales | Acremonium fusidioides (Nicot) W. Gams 1971 (LPSC 854) | 0 | 0 | 0 | 2.583 |
| Acremonium sp. | 0 | 0.25 | 11.83 | 9.75 | |
| Acrostalagmus luteoalbus (Link) Zare, W. Gams & Schroers 2004 (LPSC 858) | 0.083 | 0.083 | 6 | 1.75 | |
| Calcarisporium sp. | 0 | 0.083 | 0.083 | 0.083 | |
| Clonostachys compactiuscula (Sacc.) D. Hawksw. & W. Gams 1975 | 0 | 0 | 0 | 0.917 | |
| Clonostachys cylindrospora Höhn. 1952 | 2.5 | 0.833 | 0 | 0 | |
| Clonostachys rogersoniana Schroers 2001 (LPSC 921) | 0 | 0.167 | 0 | 5.75 | |
| Clonostachys setosa (Vittal) Schroers 2001 (LPSC 855) | 0 | 0.083 | 0 | 8.417 | |
| Cylindrocarpon orthosporum (Sacc.) Wollenw. 1916 | 0.083 | 1 | 0.75 | 22 | |
| Cylindrocarpon sp. | 0 | 0 | 0 | 0.333 | |
| Cylindrocladium colhounii Peerally 1973 (LPSC 789) | 1.5 | 13.67 | 0 | 0 | |
| Cylindrocladium sp. | 0 | 0 | 0.583 | 0.583 | |
| Dematiocladium celtidis Allegr., Aramb., Cazau & Crous 2005 (LPSC 812) | 0 | 0 | 11.08 | 22.25 | |
| Fusarium incarnatum (Desm.) Sacc.1886 | 0 | 0 | 0.083 | 0 | |
| Fusarium oxysporum Schltdl. 1824 (LPSC 870) | 0.083 | 0.667 | 0 | 0.25 | |
| Fusarium solani (Mart.) Sacc. 1881 (LPSC 868) | 0 | 0 | 0.917 | 0.25 | |
| Fusarium sulphureum Schltdl. 1824 (LPSC 859) | 0 | 0 | 0 | 0.083 | |
| Fusarium sp. 1 | 0 | 0 | 18.58 | 5.5 | |
| Fusarium sp. 2 | 1.083 | 0 | 0 | 0 | |
| Gliocladium penicilliodes Corda 1840 | 0.083 | 0 | 0 | 0 | |
| Kionochaeta ramifera (Matsush.) P.M. Kirk & B. Sutton1985 (LPSC 864) | 0 | 3.833 | 0 | 0.333 | |
| Metarhizium anisopliae (Metschn.) Sorokīn 1883 | 0 | 0 | 0 | 1.083 | |
| Myrothecium lachastrae Sacc. 1882 (LPSC 860) | 0.083 | 0.5 | 0.5 | 16.5 | |
| Stilbella sp. | 0 | 0 | 0 | 0.083 | |
| Trichothecium roseum (Pers.) Link 1809 (LPSC 1021) | 0.083 | 0 | 0 | 0 | |
| Tubercularia sp. | 0.75 | 0 | 28.08 | 3.583 | |
| Volutella ciliata (Alb. & Schwein.) Fr. 1832 (LPSC 807) | 0.333 | 0.25 | 0.833 | 27.67 | |
| Orbiliales | Arthrobotrys conoides Drechsler 1937 (LPSC 1012) | 0.417 | 0 | 0.417 | 0.417 |
| Arthrobotrys sp. 2 | 0 | 0.083 | 0 | 0 | |
| Pleosporales | Alternaria sp. 1 (LPSC 846) | 11.58 | 4.583 | 17.17 | 23.5 |
| Alternaria sp. 2 | 0 | 0 | 0 | 0.5 | |
| Alternaria sp. 3 | 0.5 | 0 | 0 | 0 | |
| Alternaria sp. 4 | 0 | 0 | 0 | 0.167 | |
| Cochliobolus spicifer R.R. Nelson 1964 | 0 | 0 | 0 | 0.333 | |
| Curvularia lunata (Wakker) Boedijn 1933 (LPSC 873) | 0 | 0 | 0 | 0.333 | |
| Drechslera sp. | 0 | 0 | 0 | 0.167 | |
| Epicoccum nigrum Link 1815 (LPSC 861) | 1.333 | 0 | 0 | 1.75 | |
| Helicosporium sp. | 0 | 0 | 0.083 | 0 | |
| Helicomyces tenuis Speg. 1910 (LPSC 1013) | 0 | 0.417 | 0 | 0 | |
| Helicosporium griseum Berk. & M.A. Curtis 1874 | 0 | 0.167 | 0 | 0 | |
| Periconia byssoides Pers. 1801 (LPSC 809) | 2.083 | 0.25 | 4.25 | 6.75 | |
| Sporidesmium adscendens Berk. 1840 | 0 | 0.167 | 0 | 0 | |
| Stemphylium sp. | 0.5 | 0 | 0 | 0 | |
| Stemphyliun vesicarium (Wallr.) E.G. Simmons 1969 | 0 | 0 | 0.667 | 0.083 | |
| Other anamorph Ascomycetes | Camposporium sp. | 0 | 0.25 | 0 | 0 |
| Chaetospermum sp. | 0 | 0.083 | 0 | 0 | |
| Chalara hughesii Nag Raj & W.B. Kendr. 1974 (LPSC 869) | 0.083 | 11.17 | 0 | 0 | |
| Chalara minima W.P. Wu 2004 | 0.083 | 1.417 | 0 | 0 | |
| Chalara sp. | 0 | 1.167 | 0 | 1.167 | |
| Ciliochorella buxifoliae Alleg. Eliades & Aramb. 2011 (LPSC 847) | 5.25 | 67 | 0 | 0 | |
| Circinotrichum chathamiense McKenzie 1993 | 0.667 | 13.5 | 0 | 0 | |
| Circinotrichum maculiforme Nees 1816 | 0 | 8.417 | 0 | 0 | |
| Circinotrichum olivaceum (Speg.) Piroz. 1962 | 0 | 0 | 0 | 0.167 | |
| Circinotrichum papakurae S. Hughes & Piroz | 0 | 7.167 | 0 | 0 | |
| Dactylaria fusiformis (Matsush.) de Hoog & Arx 1974 | 0 | 0.083 | 0 | 0.25 | |
| Fusariella atrovirens (Berk.) Sacc. 1884 | 0 | 0 | 0.333 | 3.083 | |
| Gyrothrix citricola Piroz. 1962 | 0 | 0 | 0 | 0.25 | |
| Gyrothrix flagella (Cooke & Ellis) Piroz. 1962 | 0.083 | 2.917 | 0 | 0 | |
| Gyrothrix grisea Piroz. 1962 | 0 | 0 | 0 | 0.167 | |
| Gyrothrix podosperma (Corda) Rabenh. 1844 | 0.5 | 12.17 | 0 | 0.917 | |
| Gyrothrix verticiclada (Goid.) S. Hughes & Piroz. 1971 | 0.167 | 11.75 | 0 | 0 | |
| Gyrothrix verticillata (Goid.) S. Hughes & Piroz. 1971 | 0 | 0 | 0 | 4.417 | |
| Koorchaloma scutiae Alleg. Eliades & Aramb. 2011 (LPSC 791) | 1.083 | 1.417 | 0 | 0 | |
| Matsushimomyces sp. | 0 | 1.167 | 0 | 0 | |
| Mycoleptodiscus sp. | 0.5 | 0.583 | 0 | 0 | |
| Mycoleptodiscus terrestris (Gerd.) Ostaz. 1968 | 0 | 0.583 | 0 | 0 | |
| Nigrospora sp. | 0.083 | 0 | 0 | 0 | |
| Oidiodendron sp. | 0.167 | 0 | 0 | 0 | |
| Olpitrichum macrosporum (Farl. ex Sacc.) Sumst. 1911 (LPSC 863) | 2.333 | 0.083 | 0 | 0 | |
| Polyscytalum pustulans (M.N. Owen & Wakef.) M.B. Ellis 1976 | 0.75 | 0.333 | 0 | 9.667 | |
| Septonema sp. | 0.083 | 0.167 | 0 | 0 | |
| Septopatella sp. | 1.083 | 0 | 0 | 0 | |
| Solosympodiella sp. | 0 | 0.167 | 0 | 0 | |
| Spermospora sp. | 0 | 0.25 | 0 | 0 | |
| Spiropes harunganae (Hansf.) M.B. Ellis 1968 | 0 | 0 | 0 | 0.083 | |
| Torula herbarum (Pers.) Link 1809 | 0 | 0 | 0 | 0.583 | |
| Zygosporium oscheoides Mont. 1842 | 0 | 0.25 | 0 | 0.083 | |
| Xylariales | Beltraniella sp. | 0 | 0.25 | 0 | 0 |
| Beltraniella portoricensis (F. Stevens) Piroz. & S.D. Patil 1970 | 0 | 2.083 | 0 | 0 | |
| Pestalotiopsis guepinii (Desm.) Steyaert 1949 (LPSC 786) | 18.83 | 6.5 | 0 | 0 | |
LPSC: number Culture Collection of Instituto Spegazzini.
The species identified belonged to the following orders: Hypocreales (27 taxa), Pleosporales (15 taxa), Helotiales (11 taxa), Xylariales (3 taxa), Eurotiales (6 taxa), Capnodiales (1 taxon), Chaetosphaeriales (1 taxon), Erysiphales (1 taxon), Orbiliales (2 taxa), and 33 species of anamorphs of uncertain position within the Ascomycota.
Cultures of these isolates were maintained in selective medium, being then deposited in the culture collection of the Instituto Spegazzini, UNLP, La Plata, Argentina (LPSC) (Table 1).
A total of 69 and 57 taxa were recorded in the S. buxifolia and C. tala forest respectively on the green leaves and in the leaf litter (Table 1).
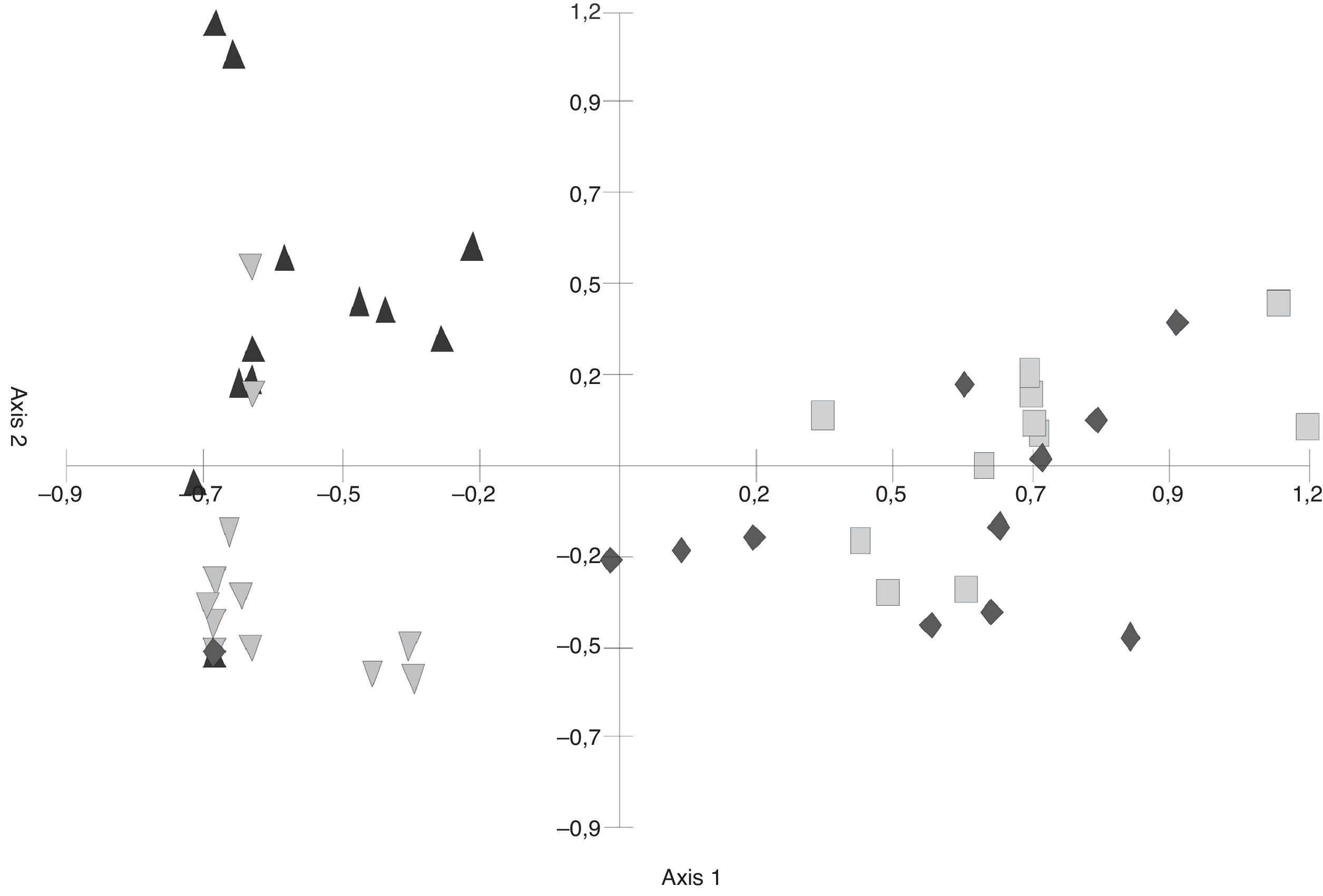
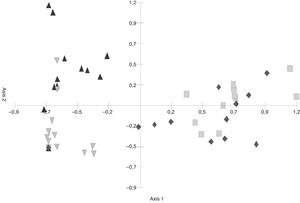
In the S. buxifolia–forest data, axis 1 and axis 2 of the PCA (Fig. 1) explained a total of 57.78% of the total variability (at 41.34% and 16.43%, respectively). The first axis discriminated between the green-leaf and the leaf-litter data, with the former being located in the negative end and the latter in the positive end of the axis.
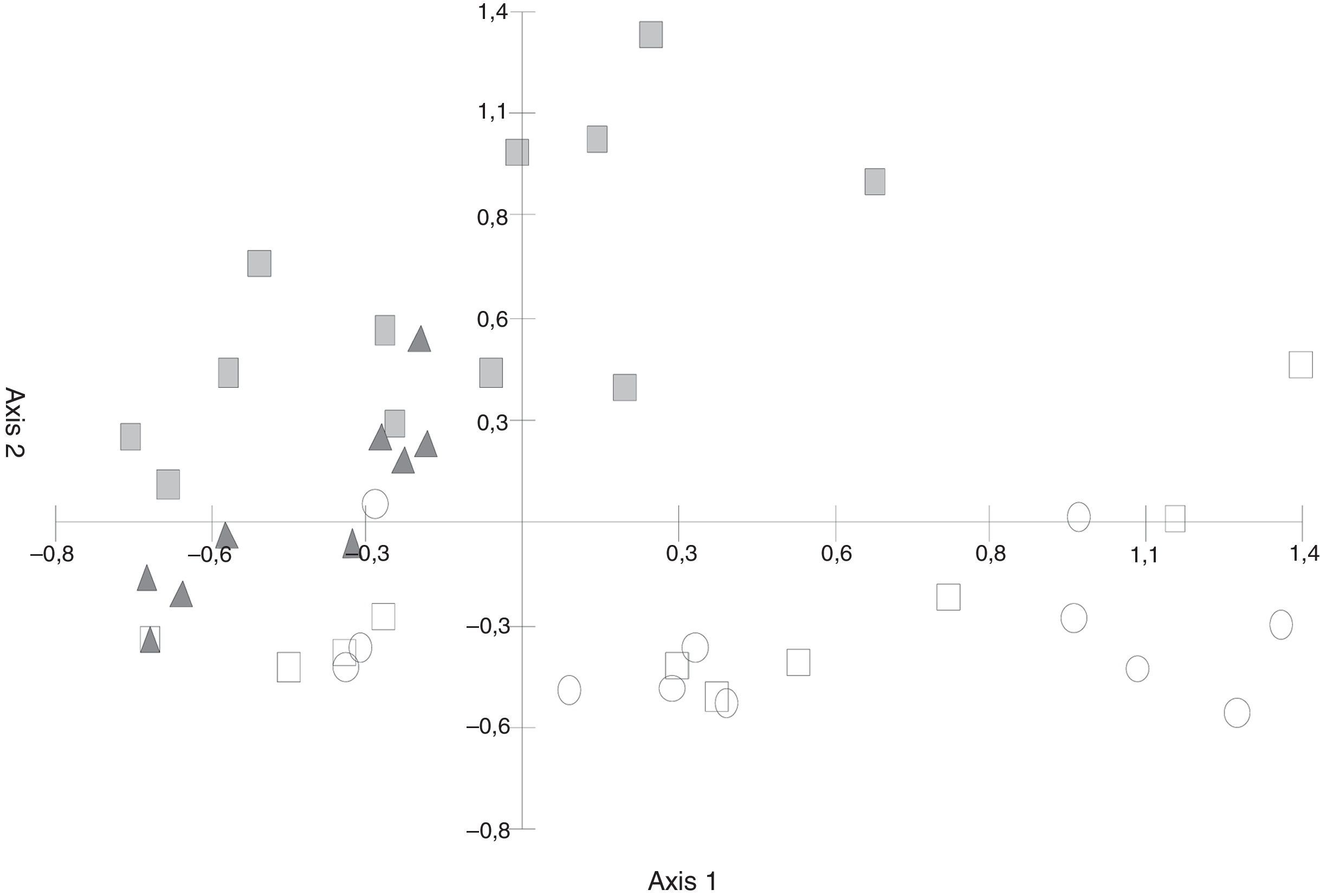
In the C. tala–forest data, the PCA diagram (Fig. 2) showed that both axis 1 (31.98%) and axis 2 (17.34%) together explained 49.32% of the cumulative variance. The second axis separated the samples of green leaf and leaf litter since species characteristic of green leaves such as Tubercularia sp., Fusarium sp., and Acremonium sp. could be observed, and their frequency were higher than that seen in leaf litter.
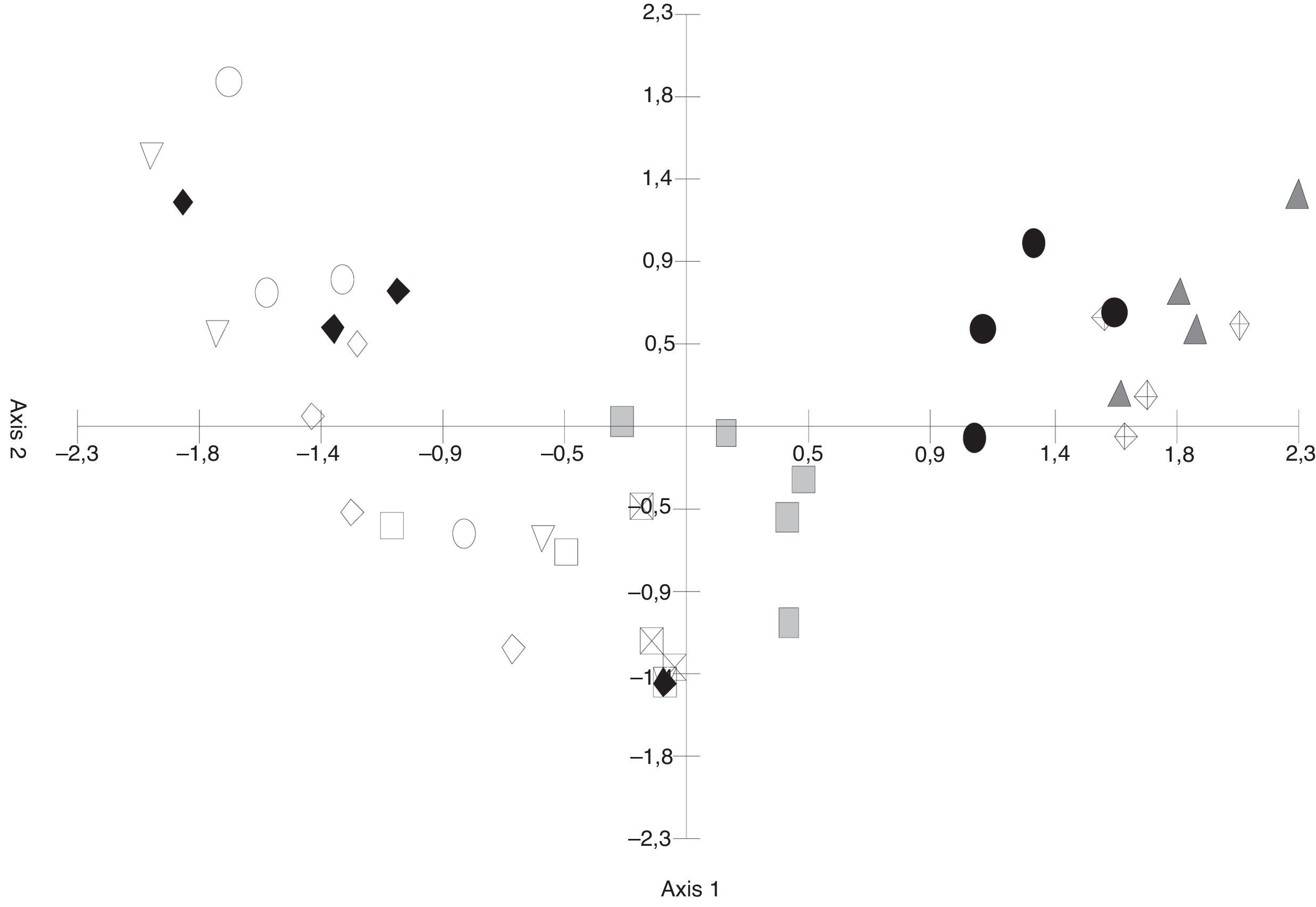
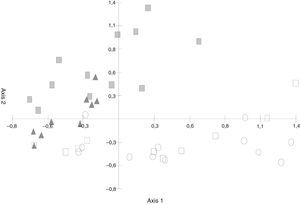
The PCA (Fig. 3) discriminated the S. buxifolia samples (of both the green and the dead leaves) from the C. tala samples. The first two axes explained 54.82% of the total variation, with the first component being the one that better separated the two forest types. Toward the positive end, the species on the S. buxifolia dead leaves were characterized by Ciliochorella buxifoliae, Idriella tropicalis, Dactylaria obtriangularia, Circinotrichum chathamiense, Gyrothrix verticiclada, Gyrothrix podosperma, Cylindrocladium colhounii, Chalara hughesii, Circinotrichum maculiforme, and Pestalotiopsis guepinii, whereas toward the negative end, the taxa on the green and dead leaves of C. tala featured Dematiocladium celtidis, Volutella ciliata, Tubercularia sp., Acremonium exigenum, Fusarium sp., Alternaria sp. 1, and Cylindrocarpon orthosporum.
Principal Component Analysis (PCA) ordination of green leaves and leaf litter samples of S. buxifolia and C. tala forest (Abbreviations:
The greatest difference in fungal composition was observed among the leaf-litter samples from both species of forest, whose data tended to be located at opposite ends of the axes. In the C. tala forest, the data from the green leaves and those from the dead leaves were less separated from each other than were those two data sets from the S. buxifolia forest. Moreover, the data from the green leaves of both species tended to be located in the intermediate positions of axis 1, indicating a smaller difference in composition than that observed among the samples of leaf litter.
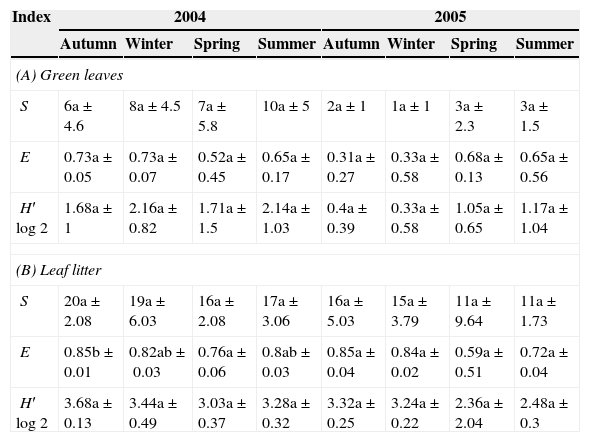
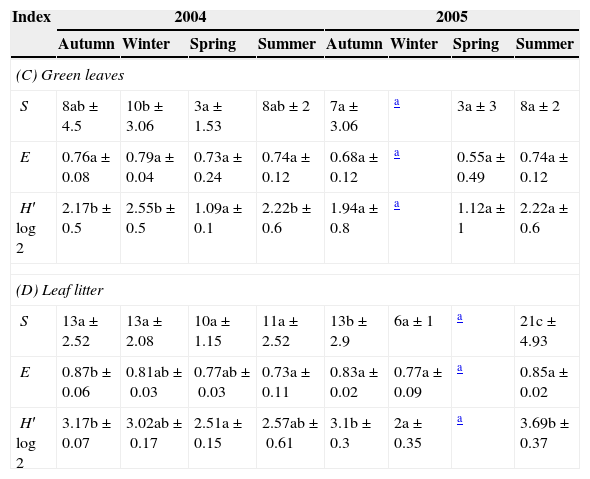
The richness (S), evenness (E), and diversity (Shannon–Wiener H′log2) of both forest types did not show significant differences between seasons (P>0.05, Tables 2A, B and 3C, D). The values of these indices, however, were higher for the leaf litter than for the green leaves in both the years 2004 and 2005.
Values of richness (S), evenness (E), and Shannon–Wiener diversity (H′log2) during the seasons (mean of three samples±SD) in Scutia buxifolia green leaves and leaf litter (A and B).
| Index | 2004 | 2005 | ||||||
|---|---|---|---|---|---|---|---|---|
| Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | |
| (A) Green leaves | ||||||||
| S | 6a±4.6 | 8a±4.5 | 7a±5.8 | 10a±5 | 2a±1 | 1a±1 | 3a±2.3 | 3a±1.5 |
| E | 0.73a±0.05 | 0.73a±0.07 | 0.52a±0.45 | 0.65a±0.17 | 0.31a±0.27 | 0.33a±0.58 | 0.68a±0.13 | 0.65a±0.56 |
| H′log2 | 1.68a±1 | 2.16a±0.82 | 1.71a±1.5 | 2.14a±1.03 | 0.4a±0.39 | 0.33a±0.58 | 1.05a±0.65 | 1.17a±1.04 |
| (B) Leaf litter | ||||||||
| S | 20a±2.08 | 19a±6.03 | 16a±2.08 | 17a±3.06 | 16a±5.03 | 15a±3.79 | 11a±9.64 | 11a±1.73 |
| E | 0.85b±0.01 | 0.82ab±0.03 | 0.76a±0.06 | 0.8ab±0.03 | 0.85a±0.04 | 0.84a±0.02 | 0.59a±0.51 | 0.72a±0.04 |
| H′log2 | 3.68a±0.13 | 3.44a±0.49 | 3.03a±0.37 | 3.28a±0.32 | 3.32a±0.25 | 3.24a±0.22 | 2.36a±2.04 | 2.48a±0.3 |
Same letter indicates that the values do not differ significantly (P>0.05) between the seasons.
Values of richness (S), evenness (E), and Shannon–Wiener diversity (H′log2) during the seasons (mean of three samples±SD) in Celtis tala green leaves and leaf litter (C and D).
| Index | 2004 | 2005 | ||||||
|---|---|---|---|---|---|---|---|---|
| Autumn | Winter | Spring | Summer | Autumn | Winter | Spring | Summer | |
| (C) Green leaves | ||||||||
| S | 8ab±4.5 | 10b±3.06 | 3a±1.53 | 8ab±2 | 7a±3.06 | a | 3a±3 | 8a±2 |
| E | 0.76a±0.08 | 0.79a±0.04 | 0.73a±0.24 | 0.74a±0.12 | 0.68a±0.12 | a | 0.55a±0.49 | 0.74a±0.12 |
| H′log2 | 2.17b±0.5 | 2.55b±0.5 | 1.09a±0.1 | 2.22b±0.6 | 1.94a±0.8 | a | 1.12a±1 | 2.22a±0.6 |
| (D) Leaf litter | ||||||||
| S | 13a±2.52 | 13a±2.08 | 10a±1.15 | 11a±2.52 | 13b±2.9 | 6a±1 | a | 21c±4.93 |
| E | 0.87b±0.06 | 0.81ab±0.03 | 0.77ab±0.03 | 0.73a±0.11 | 0.83a±0.02 | 0.77a±0.09 | a | 0.85a±0.02 |
| H′log2 | 3.17b±0.07 | 3.02ab±0.17 | 2.51a±0.15 | 2.57ab±0.61 | 3.1b±0.3 | 2a±0.35 | a | 3.69b±0.37 |
Same letter indicates that the values do not differ significantly (P>0.05) between the seasons.
The variations in the diversity index were related to the variability of species richness. The evenness remained constant, indicating that changes in the species composition were present, but not in the distribution of the species in the samples.
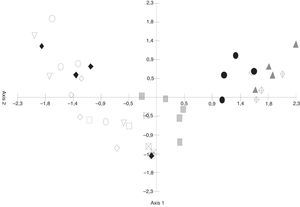
The differences in the frequencies observed between the fungal species registered in samples of S. buxifolia and C. tala leaf litter collected from the opposite forest type and those recorded in the two leaf-litter samples removed from the same respective forest type were compared by means of PCA. The first component absorbed 35.24% of the total variation (Fig. 3) and was an informative discriminant of samples of S. buxifolia and C. tala. This discrimination allowed the separation of two groups in all instances. The first was located at the negative end of the axis, contained C. tala green leaves and leaf litter along with C. tala leaf litter in a forest of S. buxifolia, and was characterized throughout by the following species: D. celtidis, V. ciliata, Fusarium sp., Tubercularia sp., Alternaria sp. 1, and Acremonium exiguum; the second was found at the positive end of the axis, consisted in the S. buxifolia green leaves and leaf litter along with S. buxifolia leaf litter in a forest of C. tala, and featured throughout C. buxifoliae, D. obtriangularia, I. tropicalis, C. chathamiense, G. verticiclada, G. podosperma, C. hughesii, Circinotrichum papakurae, and C. colhounii.
DiscussionThe species that characterized the fungal communities of the forest of S. buxifolia were different from those of the forest of C. tala. Three taxa were recorded as new species in the leaf litter of S. buxifolia: Thozetella buxifolia,1C. buxifoliae, and Koorchaloma scutiae,2 along with one new genus in the C. tala litter, represented by the species D. celtidis.8 According to Cai et al.,7 differences like these may indicate that the habitat and/or substrate could play a decisive role in the composition of these species, either through stimulating or inhibiting the growth of specific fungal species. The presence of different fungal communities is the result of the differing initial colonization mechanisms on the part of the species involved, and of the ability of those species both to explore the substrate and to compete for available resources.
The values of biodiversity indices were higher for the leaf litter than for the green leaves in both years. This result agrees with previous observations,22 where it was found that senescent leaves were significantly more densely colonized by endophytes when compared with young leaves. It was suggested that the increased density of colonization of older leaves was due to repeated reinfection of the leaf over time, probably from air-borne inoculum.
The assemblage of plant-associated species usually intergrades with the assemblages of soil species at the humus–soil interface.14 The leaf litter presented species that had reached that stage from the original green leaves. These species thus came from propagules that had already been present on the green leaves, together with other species that were able to colonize and decompose the leaf litter.24 Accordingly, in S. buxifolia, 26 out of the 69 species (38%), and in C. tala 19 out of the 57 species (33%) recorded, had already been present on the green leaves. Species such as Cladosporium cladosporioides, Alternaria sp. 1, and Periconia byssoides were among the most frequent, having been recorded in all the green-leaf and leaf-litter samples of both forests. In this study, we found no significant seasonal variations in species richness or diversity between the two forests studied.
Litter degradation by the soil microbiota is dependent on the substrate composition in terms of organic as well as inorganic compounds and/or the availability of nutrients.16,21,38 With respect to nutrient requirements, the levels in organic-matter needed for its fungal degradation are also a function of the microbial species associated with the habitat cum substrate, with the latter being also influenced by conditions such as the soil, the vegetation, and the climate.39 The PCA applied to the characteristic of substrate specificity separated the samples into two groups: those from S. buxifolia and those from C. tala, thus indicating that the type of substrate was a more influential determinant of the composition of the fungal community present than either the differences in the environmental conditions between the two forest types or the availability of fungal species within the environment. Rambelli et al.,36 assumed that in environments where conditions are optimal for microfungal development (e.g., in terms of humidity and temperature), saprotrophic specialization is related primarily to nutritional characteristics and secondarily to the chemistry of the substrata.
In conclusion, the nature and amount of nutrients along with the physical characteristics of the substrate, the availability of water, the humidity, and the temperature determine the success, the degree of colonization, and the ultimate survival of anamorphic individuals that will constitute the eventual fungal-species composition of the community.
Conflict of interestThe authors declare no conflict of interest.
This research is part of 11/N651 UNLP project. This work was performed with the financial support of CIC and CONICET. The authors thank Dr. Donald F. Haggerty, a retired career investigator and native English speaker, for editing the final version of the manuscript